Abstract
The optimal management in transplant recipients with coronavirus disease 2019 (COVID-19) remains uncertain. The main concern is the ability of immunosuppressed patients to generate sufficient immunity for antiviral protection. Here, we report on immune monitoring facilitating a successful outcome of severe severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-associated pneumonia, meningoencephalitis, gastroenteritis, and acute kidney and pancreas graft failure in a pancreas-kidney transplant recipient. Despite the very low numbers of circulating B, NK, and T cells identified in follow-up, a strong SARS-CoV-2 reactive T cell response was observed. Importantly, we detected T cells reactive to Spike, Membrane, and Nucleocapsid proteins of SARS-CoV-2 with majority of T cells showing polyfunctional proinflammatory Th1 phenotype at all analyzed time points. Antibodies against Spike protein were also detected with increasing titers in follow-up. Neutralization tests confirmed their antiviral protection. A correlation between cellular and humoral immunity was observed underscoring the specificity of demonstrated data. We conclude that analyzing the kinetics of nonspecific and SARS-CoV-2-reactive cellular and humoral immunity can facilitate the clinical decision on immunosuppression adjustment and allow successful outcome as demonstrated in the current clinical case. Although the antiviral protection of the detected SARS-CoV-2-reactive T cells requires further evaluation, our data prove an ability mounting a strong SARS-CoV-2-reactive T cell response with functional capacity in immunosuppressed patients.
KEYWORDS: translational research, science, basic (laboratory) research, kidney transplantation, nephrology, immunosuppression, immune modulation, cellular biology, T cell biology, infection and infectious agents – viral, complication: infectious
Abbreviations: COVID-19, coronavirus disease 2019; GrzB, granzyme B; IFNγ, interferon gamma; MPA, mycophenolic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Tac, tacrolimus; TNFα, tumor necrosis factor alpha
1. CASE
The crucial role of cellular immunity for the antiviral protection is well known, and an association between T cell immunity and viral clearance has been shown previously by our and other groups.1 , 2 Because of immunosuppressive treatment required to prevent organ rejection, transplant patients are known to be at high risk of viral infections. In fact, enabling an immune response, capable of controlling viral infections under concomitant immunosuppression, is an important challenge in transplant medicine.
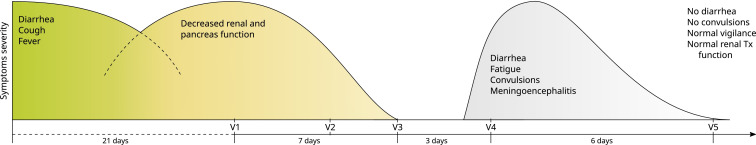
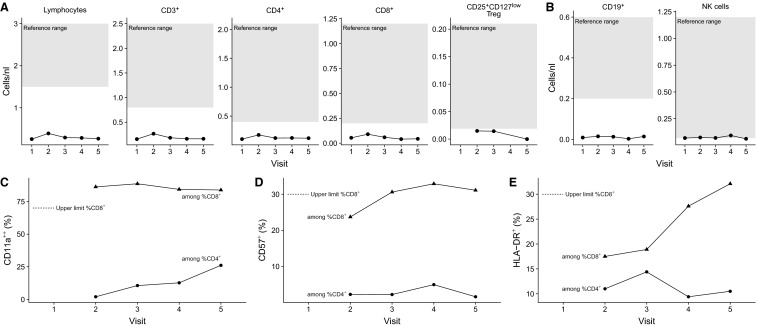
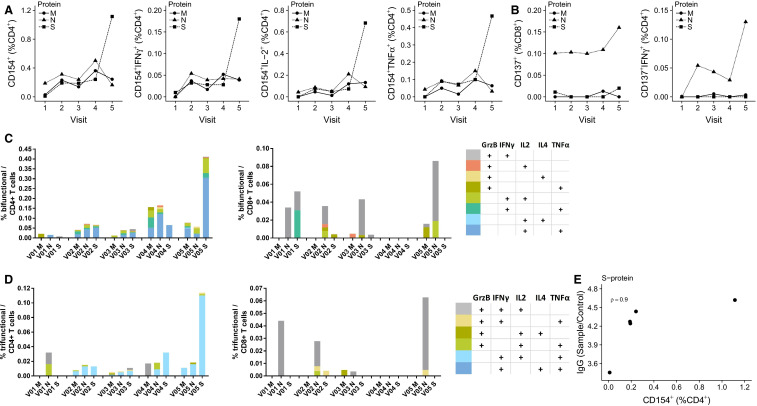
Here, we present in-depth monitoring of the general immune status and the analysis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reactive T cell and humoral immunity in clinical follow-up of a combined pancreas-kidney transplant patient accompanying successful outcome of severe coronavirus disease 2019 (COVID-19). We have very recently provided an in-depth description of his clinical course (Westhoff et al3). The maintenance triple immunosuppressive medication included tacrolimus (Tac) (trough level 4.6 mg/mL), mycophenolic acid (MPA), and prednisolone. Initially, the patient showed symptoms of COVID-19 pneumonia and gastroenteritis and was treated outside of our transplant center. The first positive SARS-CoV-2 polymerase chain reaction (PCR) was obtained 10 days after the first symptoms. Because of the very high level of tacrolimus (40 mg/mL) caused by severe diarrhea, tacrolimus medication was paused. Twenty-one days after the onset of the first symptoms, the patient suffered a gradual decrease of renal and pancreas graft function and was transferred to our transplant center. At this time point (V1, Figure 1) the maintenance immunosuppressive medication was replaced by intravenous hydrocortisone (200 mg/d) owing to prolonged convalescence and progressive COVID-19 pneumonia observed in computed tomography, and immune monitoring was initiated. Because of a modest increase in creatinine levels and proteinuria at V1, kidney allograft biopsy was performed to rule out acute allograft rejection. The histopathological findings ruled out acute renal allograft rejection, but moderate interstitial mononuclear cell infiltrate and tubular damage as well as SARS-CoV-2 RNA transcripts were detected by reverse transcription (RT)-PCR and in-situ hybridization (Westhoff et al3). The general immune status demonstrated a profound decrease of circulating immune cells including B, NK, and T cells ( Figure 2A, B). Furthermore, no general signs of acute immune activations were present as demonstrated by the frequencies of CD4+ or CD8+ T cells expressing activation molecules CD57 and/or HLA-DR (Figure 2C-E). Taking these immune monitoring data in consideration, pause of Tac medication was continued and hydrocortisone was continuously administered as immunosuppressive monotherapy (V2). During the next 7 days, we observed a continuous normalization of clinical symptoms and clinical laboratory parameters (V3). The monitoring of cellular immunity demonstrated a slight increase in the frequencies of certain activated differentiated effector T cell subsets (CD57 + CD8+; HLA-DR+CD8+) from V2 to V3; (Figure 2C-E). Considering the slight increase in the immune cell activation level, and sufficient number of SARS-CoV-2 reactive T cells (discussed later), Tac medication was reintroduced. Interestingly, at all analyzed time points, a stable or even rising magnitude of SARS-CoV-2 reactive T cells was observed, despite the observed T cell lymphopenia ( Figure 3A,B). In details, the analyzed magnitude of T cell responses against Spike (S), Nucleocapsid (N), and Membrane (M) proteins demonstrated a synchronous kinetics of SARS-CoV-2 S-, N-, and M-reactive T cells with slight differences in their magnitude and dynamics. Thus, N-protein activated CD4+T cells as well as activated CD4+T cells producing interferon gamma (IFNγ), interleukin-2 (IL-2), and/or tumor necrosis factor alpha (TNFα) demonstrated the highest magnitude for almost all analyzed time points, whereas the magnitude of S- and M-reactive T cells was lower and comparable to each other. Importantly, the majority of the detected SARS-CoV-2-reactive CD4 + T cells were of multifunctional Th1 phenotype simultaneously coproducing 2 or 3 proinflammatory cytokines (Figure 3C,D). For CD8+T cells, a clear immune dominance of N-protein was observed, because N-reactive CD8+CD137+ T cells producing effector cytokines or molecules were the mostly detectable protein reactivity (Figure 3C,D). We further observed increasing levels of antibodies against S-protein at each analyzed visit. Of note, a correlation between S-reactive T cells and S1-specific IgG antibodies was detected (Figure 3E) underscoring the specificity of the adaptive immunity findings. Furthermore, virus neutralization test of antibodies with ND50 titers ranging from 256 to 1494 demonstrated their antiviral protection.
FIGURE 1.
Clinical course of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-associated pneumonia, gastroenteritis, meningoencephalitis, kidney and pancreas graft impairment closely accompanied by in-depth immune monitoring. Symptoms, their relative severity and duration, together with accompanying sample monitoring at corresponding visits (V) are presented. The maintenance triple immunosuppressive medication in outpatient setting included tacrolimus (Tac) (trough level 4.6 mg/mL), mycophenolic acid (MPA), and prednisolone. Initially, the patient showed symptoms of coronavirus disease 2019 (COVID-19) pneumonia and gastroenteritis. Because of the very high level of tacrolimus (40 mg/mL) caused by severe diarrhea, tacrolimus medication was paused. Twenty-one days after the onset of the first symptoms, the patient suffered a gradual decrease of renal (creatinine rise from 1.2 to 2.2 mg/dL) and pancreas graft function (insulin-dependence) and was transferred to our transplant center. The symptoms occurred outside of our transplant centers are depicted left from V1 [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
Immune monitoring of circulating immune cells and their activation and differentiation status in clinical follow-up. A, Number of circulating lymphocytes, total CD3+, CD3+CD4+, CD3+CD8+, and CD25+CD127− regulatory T cells (Tregs). The shaded area corresponds to the normal range. B, Number of circulating CD19+ B cells and NK cells, defined by CD3−CD56+. The shaded area corresponds to the normal range. C, Frequency of CD11a++ lymphocytes among CD3+CD4+ and CD3+CD8+ T cells. D, Frequency of CD57+ lymphocytes among CD3+CD4+ and CD3+CD8+ T cells. E, Frequency of HLA-DR+ lymphocytes among CD3+CD4+ and CD3+CD8+ T cells
FIGURE 3.
Monitoring of adaptive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reactive immunity in clinical follow-up. A, Frequency of M-, N-, or S-protein specific CD4+ T cells, defined by expression of CD154+ (left panel) and subset by secretion of interferon gamma (IFNγ; panel middle left), interleukin-2 (IL-2; panel middle right), and tumor necrosis factor alpha (TNFα; right panel). B, Frequency of M-, N-, or S-protein specific CD8+ T cells, defined by expression of CD137+ (left panel) and subset by secretion of IFNγ (right panel). C, Frequency of CD154+ bifunctional T cells among CD4+ T cells. Bifunctional is defined at expression of any pairwise combination of the cytokines granzyme B (GrzB), IFNγ, IL-2, IL-4, and TNFα. D, Frequency of CD154+ trifunctional T cells among CD4+ T cells. Bifunctional is defined at expression of any triple combination of the cytokines GrzB, IFNγ, IL-2, IL-4, and TNFα. E, Association between the frequency S-protein specific CD4+ T cells, defined by expression of CD154+, and S1 specific IgG expressed as the ratio of sample to calibrator [Color figure can be viewed at wileyonlinelibrary.com]
Acute deterioration of the vigilance with convulsive seizure and diarrhea occurred within the next days (V4). Laboratory findings showed the constellation of microangiopathic hemolysis and thrombopenia indicating thrombotic microangiopathy. Magnetic resonance tomography indicated meningoencephalitis and RT-PCR of the cerebrospinal fluid revealed a positive finding of SARS-CoV-2-RNA suggesting progression of SARS-CoV-2 infection. Tac was withdrawn again. The positive detection of SARS-CoV-2 was not owing to the blood contamination, because SARS-CoV-2 PCR performed in peripheral blood was negative. The accompanied monitoring of SARS-CoV-2 reactive CD4 + T cells demonstrated a strong antiviral response with slightly increased frequencies compared to the previous visits V1-V3 (Figure 3A,B). Supported by antiviral immune surveillance data, the immunosuppressive regimen remained unchanged and hydrocortisone infusion in combination with hydroxychloroquine medication (duration of hydroxychloroquine therapy was 4.5 days) was continued. The following 5 days were characterized by clinical improvement of neurological symptoms and diarrhea, accompanied by a stable level of SARS-CoV-2 reactive T cells and low, but stable levels of unspecific bulk T cell subsets comparable to the initial findings (V5). Because of the significance improvement of neurological symptoms, we refrained from the repeated puncture for SARS-CoV-2-PCR in follow-up. In total, the detection of SARS-CoV-2 by PCR was performed 6 times from nasopharyngeal swabs during the treatment in our center. The first 2 swabs were positive, and all remaining swabs were negative. PCR testing of peripheral blood samples was performed every 3-5 days in follow-up showing negative results. Complete resolution of SARS-CoV-2-associated symptoms (cessation of diarrhea, normalization of renal graft function, normalization of vigilance) was observed at day 18 and patient´s discharge could be prepared. Finally, supported by immune monitoring data demonstrating a strong SARS-CoV-2 reactive polyfunctional T cell response and neutralizing capacity of Spike protein-specific antibodies, his initial Tac/MPA-based immunosuppressive therapy could be reintroduced at discharge.
2. DISCUSSION
COVID-19 is a challenging problem for health care worldwide. Patients suffering from hypertension, atherosclerotic diseases, diabetes, and malignancy are usually at a higher risk for developing a severe disease.4 For allograft transplant patients, not only the high rate of these comorbidities and frequent contact with medical care, but also—and especially—immunosuppressive therapy can contribute to disease severity. Thus, a crucial role of T cell immunity for the viral clearance and vaccination response has been shown in previous studies.5, 6, 7 Because of immunosuppression, the quantity and the functionality of antiviral immunity can be impaired, whereas the impact of different immunosuppressive drugs can vary.6 The role of SARS-CoV-2-reactive cellular immunity for COVID-19 evolution is not understood. Based on the data of the closely related Middle East respiratory syndrome coronavirus8 and the general knowledge of the most acute viral infections,1 T cell response should be crucial for the antiviral control in SARS-CoV-2 infection, too. Series of studies on transplant patients demonstrated more severe presentations of COVID-19 in transplant populations compared to nonimmunosuppressed patients, which might reflect a negative impact of immunosuppression on disease progression. In a report from the outbreak in New York City the authors describe a mortality of 28% compared to < 5% in the general population.9 Furthermore, compared to hospitalized cohort of nontransplant patients, Pereira et al demonstrated a higher rate of severe diseases and mortality in transplant patients.10 In order to enable a more efficient antiviral immunity, immunosuppressive treatment was discontinued or significantly reduced in the majority of the published case reports.11 , 12 On the other hand, opposite data suggesting immune dysregulation as underlying cause of severe COVID-19 manifestations have been reported.13 Such observations can implicate a positive effect of immunosuppression for the clinical course of SARS-CoV-2 infection in transplant patients. In line with this, Seminari et al demonstrated mild course of COVID-19 in transplant patients.4 Moreover, Johnson et al reported in their review on 3 patients who remained on triple calcineurin inhibitor (CNI)-based immunosuppressive therapy.4 , 11 , 14 All 3 patients showed uncomplicated clinical course with stable graft function during the infection and COVID-19 convalescence within a short period of time.11
Taken together, a variety of clinical case reports demonstrated different approaches for the adjustment of immunosuppression leading to a different outcome. Despite the differences in details, the majority of patients received corticosteroid monotherapy, and in only a few cases the maintenance immunosuppression was not changed.11 However, outside of supportive care, the optimal management of immunosuppression in transplant recipients with COVID-19 remains largely uncertain. The main concern of the most physicians taking care of transplant patients is the ability of immunosuppressed patients to generate and mount the sufficient antiviral immunity for antiviral protection. Even for immunocompetent populations, limited data are available about adaptive SARS-CoV-2 immunity or proteins recognized by T cells. It is currently not clear whether a CD4+T helper cell response facilitating the generation of SARS-CoV-2 specific antibodies or a CD8+cytotoxic T cell response that leads to the killing of infected cells would be preferable for a most effective virus elimination. In addition, the role of cytotoxic CD4+T cells for the SARS-CoV-2 clearance as shown by our and other groups for other viruses is also not clear.15 Few data on SARS-CoV-2 reactive T cells have been published so far regarding nonimmunosuppressed patient cohorts.16, 17, 18, 19, 20 Data on SARS-CoV-2 reactive cellular immunity in immunosuppressive patients are lacking.
Here, we present data on in-depth monitoring of cellular and humoral immunity in a kidney-pancreas transplant patient. In this severe course of disease with multiorgan SARS-CoV-2 involvement, we demonstrated a strong antiviral immunity. The detected T cells were reactive to all 3 SARS-CoV-2 proteins with N-protein inducing the highest magnitude of CD4+ and CD8+ T cell responses at almost all analyzed time points. To our knowledge, this is a first report demonstrating a higher magnitude of N-protein-reactive T cells in SARS-CoV-2 infection. Confirming immunogenicity of N-protein, generation of B cell responses against N-protein has been demonstrated for the previous SARS-CoV infection.21
Analyzing the kinetics of SARS-CoV-2 reactive T cells we observed an increased magnitude of cellular immunity in clinical follow-up. Confirming the specificity of our finding, antibody titers measured during the disease evolution were also increasing and a clear correlation between the frequencies of S-reactive T cells and S-reactive antibodies was observed. Of note, our analyses were initiated at the time point of irregular CNI administration with the trough CNI level of 3.0 ng/mL. Therefore, a conclusion on the capability of transplant patients to generate SARS-CoV-2 reactive T cells under full blown triple immunosuppression cannot be finally drawn. However, our data indicate that at least for the low-level immunosuppressive therapy, a strong antiviral response can be generated and mounted. Furthermore, viral clearance and resolution of severe COVID-19 manifestation was accompanied by increasing magnitude of adaptive immunity. Importantly, the analysis of functional activity of SARS-CoV2-reactive T cells showed that the majority of T cells were bi- or trifunctional producing Th1 proinflammatory cytokines TNFα, IFNγ, IL-2, or granzyme B. Because such polyfunctional cells are commonly considered as indicators of protective immunity,23 , 24 the detection of polyfunctionality within SARS-CoV2-reactive T cells points to their protective antiviral capacity.
In conclusion, we report a clinical case on successful outcome of SARS-CoV-2-associated pneumonia, meningoencephalitis, pancreas, and renal graft function impairment accompanied by in-depth immune monitoring. A strong SARS-CoV-2 reactive T cell and antibody response could be observed despite the preexisting immunosuppression. Although analysis of circulating T cell subsets with activated memory phenotype provided information on general immune activation level, analysis of SARS-CoV-2 reactive cellular and humoral immunity enabled specific information on antiviral response. Combined monitoring of both arms of immunity facilitated clinical decision-making, allowing risk-adjusted guidance of immunosuppressive regimen and successful outcome of severe life-threating COVID-19 complications.
3. MATERIALS AND METHODS
Peripheral blood mononuclear cells (PBMCs) were prepared from whole blood by gradient centrifugation and frozen as recently described.19 Defrosted PBMCs were stimulated with 1 µg/mL SARS-CoV-2 overlapping peptide pools (V1-V4, or JPT V5, Miltenyi Biotec, Bergisch Gladbach, Germany) containing 15mer peptides from S, N, and M protein, respectively, for 16h. Brefeldin A (1µg/ml, Sigma Aldrich, St. Louis, MO) was added after 2h. Immune phenotyping was performed using EDTA-treated whole blood. All samples were stained with antibodies directed against the indicated molecules and immediately acquired on a CytoFlex flow cytometer (Beckman Coulter, Brea, CA). SARS-CoV-2 IgG titers were analyzed using a commercially available kit (EUROIMMUN, Lübeck, Germany) per manufacturer’s instructions. Flow cytometry data were analyzed using FlowJo version 10.6.2 (BD Biosciences, San Jose, CA), R version 3.6.2, and Prism version 8 (GraphPad Software, LaJolla, CA). For the virus neutralization assay, a propagation-incompetent VSV*ΔG(FLuc) pseudovirus system bearing the SARS-CoV-2 spike protein in the envelope was incubated with serial dilutions of immune sera prior to infections of Vero E6 cells, as described before.24 At 18 hours post infection, firefly luciferase (FLuc) reporter activity was determined and the reciprocal antibody dilution causing 50% inhibition of the luciferase reporter calculated (PVN50). The virus neutralization assay has been kindly provided by Dr. Gert Zimmer.24 The spike protein was kindly provided by Prof. Pöhlmann.
ACKNOWLEDGMENT
Open access funding enabled and organized by Projekt DEAL.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Funding information Stiftung Mercator; Bundesministerium für Bildung und Forschung, Grant/Award Number: 01ZX1612A
Footnotes
Ulrik Stervbo and Timm H. Westhoff share senior authorship and contributed equally.
[Correction added on September 22, 2020, after first online publication: Projekt Deal funding statement has been added.]
REFERENCES
- 1.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33(4):451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babel N, Volk H-D, Reinke P. BK polyomavirus infection and nephropathy: the virus–immune system interplay. Nat Rev Nephrol. 2011;7(7):399–406. doi: 10.1038/nrneph.2011.59. [DOI] [PubMed] [Google Scholar]
- 3.Westhoff TH, Seibert FS, Bauer F, et al. Allograft infiltration and meningoencephalitis by SARS-CoV-2 in a pancreas-kidney transplant recipient [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.16223. [DOI] [PMC free article] [PubMed]
- 4.Seminari E, Colaneri M, Sambo M, et al. SARS Cov-2 infection in a renal-transplanted patient: A case report. Am J. Transplant. 2020;20:1882–1884. doi: 10.1111/ajt.15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stervbo U, Nienen M, Weist BJD, et al. BKV clearance time correlates with exhaustion state and T-cell receptor repertoire shape of BKV-specific T-cells in renal transplant patients. Front Immunol. 2019;10(767) doi: 10.3389/fimmu.2019.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weist BJD, Wehler P, El Ahmad L, et al. A revised strategy for monitoring BKV-specific cellular immunity in kidney transplant patients. Kidney Int. 2015;88(6):1293–1303. doi: 10.1038/ki.2015.215. [DOI] [PubMed] [Google Scholar]
- 7.Dziubianau M, Hecht J, Kuchenbecker L, et al. TCR repertoire analysis by next generation sequencing allows complex differential diagnosis of T cell-related pathology. Am J Transplant. 2013;13(11):2842–2854. doi: 10.1111/ajt.12431. [DOI] [PubMed] [Google Scholar]
- 8.Shin H-S, Kim Y, Kim G, et al. Immune responses to middle east respiratory syndrome coronavirus during the acute and convalescent phases of human infection. Clin Infect Dis. 2019;68(6):984–992. doi: 10.1093/cid/ciy595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson KM, Belfer JJ, Peterson GR, Boelkins MR, Dumkow LE. Managing COVID-19 in renal transplant recipients: a review of recent literature and case supporting corticosteroid-sparing immunosuppression. Pharmacother: J Human Pharmacol Drug Ther. 2020;40(6):517–524. doi: 10.1002/phar.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Chen Y, Yuan Q, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020;77(6):742–747. doi: 10.1016/j.eururo.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weist BJD, Schmueck M, Fuehrer H, Sattler A, Reinke P, Babel N. The role of CD4+ T cells in BKV-specific T cell immunity. Med Microbiol Immunol. 2014;203(6):395–408. doi: 10.1007/s00430-014-0348-z. [DOI] [PubMed] [Google Scholar]
- 16.Weiskopf D, Schmitz KS, Raadsen MP, et al. Phenotype of SARS-CoV-2-specific T-cells in COVID-19 patients with acute respiratory distress syndrome. medRxiv 2020. 2020.2004.2011.20062349. [DOI] [PMC free article] [PubMed]
- 17.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun J, Loyal L, Frentsch M, et al. Presence of SARS-CoV-2 reactive T cells in COVID-19 patients and healthy donors. medRxiv 2020. 2020.2004.2017.20061440.
- 19.Anft M, Paniskaki K, Blazquez-Navarro A, et al. A possible role of immunopathogenesis in COVID-19 progression. medRxiv 2020. 2020.2004.2028.20083089.
- 20.Thieme CJ, Weist BJD, Mueskes A, et al. The TreaT-assay: a novel urine-derived donor kidney cell-based assay for prediction of kidney transplantation outcome. Sci Rep. 2019;9(1):19037. doi: 10.1038/s41598-019-55442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan Y-J, Goh P-Y, Fielding BC, et al. Profiles of antibody responses against severe acute respiratory syndrome coronavirus recombinant proteins and their potential use as diagnostic markers. Clin Diagn Lab Immunol. 2004;11(2):362–371. doi: 10.1128/CDLI.11.2.362-371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duvall MG, Precopio ML, Ambrozak DA, et al. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol. 2008;38(2):350–363. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J, Zhao J, Mangalam Ashutosh K, et al. Airway memory CD4+ T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44(6):1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zettl F, Meister TL, Vollmer T, et al. Rapid Quantification of SARS-CoV-2-Neutralizing Antibodies Using Propagation-Defective Vesicular Stomatitis Virus Pseudotypes. Vaccines. 2020;8(3):386. doi: 10.3390/vaccines8030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.