New forms of neurological complications of severe SARS‐CoV‐2 infection have been described, mainly including encephalopathy, agitation and confusion [1]. Only one publication reveals the emergence of de novo myoclonus in three patients [2], with most publications reporting the aggravation of pre‐existing abnormal movements disorders. Here, we identified and characterized in depth clinically a new type of delayed onset movement disorder in five patients who were admitted to the Assistance Publique – Hôpitaux de Paris intensive care unit (ICU) for severe SARS‐CoV‐2 infection. All patients underwent intubation and mechanical ventilation.
Abnormal movements developed 23 ± 7 days (mean ± SD) after ICU discharge. Upper limb postural and action tremor was observed in four patients; one of them (patient 2) also had irregular orthostatic tremor and one patient (patient 4) had bilateral upper limb jerky/myoclonic abnormal movements at rest and during posture and action (Table 1). Associated signs included a moderate proximal motor deficit attributed to a critical illness myopathy in four patients and a mild hemiparesis attributed to a critical illness neuropathy confirmed by electroneuromyography in one patient.
Table 1.
Clinical and radiological characteristics of five patients presenting with new onset movement disorder
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age, years | 51 | 67 | 34 | 66 | 48 |
| Sex | Male | Male | Male | Female | Male |
| Medical history | Disc herniation | Hypertension, poliomyelitis | Hepatitis B healed, typhoid | Hypertension, nephroangio‐sclerosis with severe renal insufficiency stage V, hepatitis B healed | Hypertension, obesity |
| Nasopharyngeal swabs for SARS‐CoV‐2 RNA | Positive | Positive | Positive | Positive | Positive |
| Delay between the onset of symptoms and the diagnosis of SARS‐CoV‐2, days | 9 | 8 | 5 | 11 | 7 |
| ICU | |||||
| Prone positioning | — | Yes | Yes | Yes | Yes |
| Extracorporeal membrane oxygenation procedure (ECMO), days | — | 14 | 9 | — | 18 |
| Tracheotomy, weeks | — | 3 | — | — | — |
| Average length of stay in ICU, days | 12 | 23 | 25 | 19 | 34 |
| Time to neurological presentation after extubation, days | 17 | 31 | 14 | 29 | 24 |
| Average weight loss, kg | 5 | 6 | 15 | 6 | 16 |
| Other complications of SARS‐CoV‐2 infection | Pneumopathy | Pneumopathy | Pneumopathy | Pneumopathy, worsening renal failure requiring dialysis, delirium after extubation requiring haloperidol medication that was rapidly discontinued | Pneumopathy |
| Neurological examination in rehabilitation unit | |||||
| Type of tremor | Action tremor predominant on the right hemibody | Postural and action tremor of upper and lower limbs + orthostatic tremor | Postural and action tremor of the upper limbs | Jerky tremor of the upper limbs | Postural and action tremor of the upper limb |
| Myoclonus | No | Cortical and subcortical | No | Cortical and subcortical | No |
| Pyramidal syndrome | No | No | No | No | No |
| Extrapyramidal syndrome | No | No | No | No | No |
| Motor deficit | Mild global proximal deficit predominating on the right side | Mild right hemiparesis | Mild global proximal deficit | Mild global proximal deficit | Mild proximal belt deficit |
| Critical illness neuropathy | Yes | No | No | No | No |
| Critical illness myopathy | Yes | No | Yes | Yes | Yes |
| Abnormal movement recording | — | Combination of cortical and subcortical myoclonus | — | Combination of cortical and subcortical myoclonus | — |
| MRI | |||||
| Standard MRI | Bilateral frontotemporal hypoperfusion | Corpus callosum microbleeds | Corpus callosum microbleeds | Deep and peripheral microbleeds | Corpus callosum microbleeds |
| Neuromelanin sequence | Loss of visibility of the nigrosomes | Normal | Normal | Normal | Normal |
| DaTScan | Normal | Normal | Normal | Normal | — |
DaTScan, 123I[A1] ‐FP‐CIT SPECT; ICU, intensive care unit; MRI, magnetic resonance imaging.
Electrophysiological exploration of the movement disorders was performed in patients 2 and 4 and recorded myoclonic jerks of short duration (40–60 ms) that were synchronous among the electromyography (EMG) traces, associated with post‐myoclonic inhibition period in one patient and with jerks of longer duration (50–100 ms) and increased long loop C‐reflex with latency of 50 ms recorded from thenar muscles in the second. Overall, the recordings supported a mixed cortical–subcortical pattern of myoclonic jerks (Fig. 1).
Figure 1.
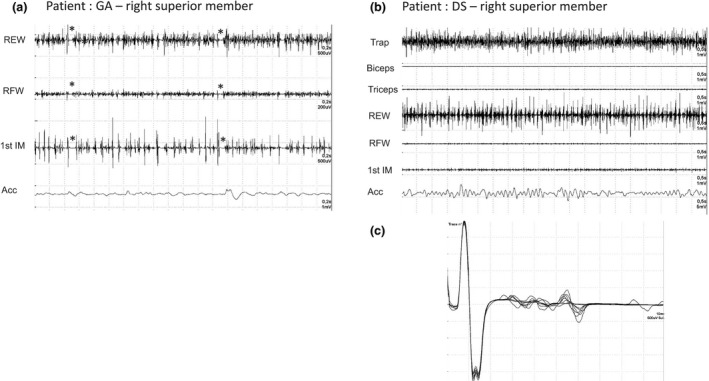
Myoclonus electrophysiology (patients 2 and 4). REW, radial extensor of wrist; RFW, radial flexor of wrist; 1st MI, first interossal muscle; Acc, accelorometer; Trap, trapezius muscle. (a) Surface EMG of right superior member, patient 2: REW, myoclonic bursts of 40–44 ms with 36–44 ms of post‐myoclonic inhibition period (indicated with *); RFW, myoclonic bursts of 24 ms with 66 ms of post‐myoclonic inhibition period (indicated with *); 1st MI, myoclonic bursts of 36 ms with 86 ms of post‐myoclonic inhibition period (indicated with *). (b) Surface EMG of right superior member, patient 4: irregular myoclonic activity on the EMG of trapezius and REW with 70–94 ms of duration of myoclonic bursts. (c) Long loop C reflex with latency of 50 ms recorded from the thenar muscles of patient 4.
Magnetic resonance imaging (MRI) (3 T) performed in all patients showed in four patients microbleeds which are non‐specific injuries associated with the resuscitation setting [3] and a bilateral frontotemporal hypoperfusion in one patient. Neuromelanin‐sensitive MRI showed that the dorsal nigral hyperintensity sign was bilaterally present in four patients but was asymmetrical and only present in one hemisphere in patient 1. Single photon emission computed tomography (SPECT) with 123I‐FP‐CIT (DaTScan) performed in four patients showed no significant decrease of striatal uptake in any of them.
Several pathophysiological mechanisms may be hypothesized here: (i) direct central nervous system damage by SARS‐CoV‐2 or of post infectious/immune‐mediated origin, (ii) metabolic (renal failure) and post‐hypoxic myoclonus [4, 5] no hypothesis is exclusive of the others.
For the first hypothesis, SARS‐CoV‐2 is known to enter the brain [5], where it can bind to the enzyme angiotensin‐converting enzyme 2 and cause neuronal death. In line with that, we observed MRI abnormalities such as alterations of nigrosomes (substantia nigra) and frontotemporal hypoperfusion on MRI perfusion sequence that could be directly or indirectly related to SARS‐CoV‐2 [4]. The delayed onset (3 weeks after ICU discharge) of these movement disorders can also be in favor of SARS‐COV‐2 related immune implication. Alternatively, cortical and subcortical myoclonus may be related to post‐infectious myoclonus, although this hypothesis is less likely in the absence of opsoclonus or ataxia. Finally, electrophysiological exploration documented cortical (abnormal long loop C‐reflex) and subcortical (long duration bursts) myoclonus. We thus cannot exclude a combination of chronic post‐hypoxic myoclonus with action and intention myoclonus of subcortical and cortical origin with possible additional metabolic origin in patient 4 with renal failure syndrome.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest.
References
- 1. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med 2020; 15: 2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rábano‐Suárez P, Bermejo‐Guerrero L, Méndez‐Guerrero A, et al. Generalized myoclonus in COVID‐19. Neurology 2020; 21: 10. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fanou EM, Coutinho JM, Shannon P, et al. Critical illness‐associated cerebral microbleeds. Stroke 2017; 48: 1085–1087. [DOI] [PubMed] [Google Scholar]
- 4. Sutter R, Ristic A, Rüegg S, Fuhr P. Myoclonus in the critically ill: diagnosis, management, and clinical impact. Clin Neurophysiol 2016; 127: 67–80. [DOI] [PubMed] [Google Scholar]
- 5. Gupta HV, Caviness JN. Post‐hypoxic myoclonus: current concepts, neurophysiology, and treatment. Tremor Other Hyperkinet Mov (N Y) 2016; 6: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgements
The authors thank the Cohort COVID‐19 Neurosciences (CoCo Neurosciences) study sponsored by APHP and funded by the support of the FIA Foundation and donors of Paris Brain Institute – ICM.


