Abstract
In the absence of definitive therapy for coronavirus disease (COVID‐19), convalescent plasma therapy (CPT) may be a critical therapeutic option. This review was conducted to evaluate the impact of CPT in COVID‐19 patients based on the publications reported to date. A robust screening of electronic databases was conducted up to 10th July 2020. Randomized controlled trials (RCTs), cohort studies, and case series with a control group evaluating the effectiveness and safety of CPT in patients with COVID‐19 are included for the meta‐analyses. Our search retrieved seven studies, including two RCTs and five cohort studies, with a total of 5444 patients. In patients with COVID‐19, the use of CPT reduces mortality (odd's ratio [OR] 0.44; 95% CI, 0.25‐0.77), increases viral clearance (OR, 11.29; 95% CI, 4.9‐25.9) and improves clinically (OR, 2.06; 95% CI, 0.8 to 4.9). However, the evidence is of low quality (mortality reduction, and viral clearance), and very low quality (clinical improvement). CPT may be beneficial for reducing mortality, viral shedding and improving clinical conditions in COVID‐19 patients. However, further randomized control trials (RCT) are required to substantiate the safety margin, initiation, optimal dosage, titre and duration of CPT.
Keywords: coronavirus disease, convalescent plasma therapy, severe acute respiratory syndrome coronavirus‐2
1. INTRODUCTION
Convalescent Plasma Transfusion (CPT) has been traditionally tried during large‐scale epidemics in patients with viral infections whose critical condition is refractory to supportive care. 1 It is obtained from a recently recovered person from a viral illness and is expected to have the maximum levels of polyclonal antibodies directed against the virus. 2 Both passive immunity (reduction in viremia) 3 and active immunity (host immune response) 4 have been postulated for providing an immediate promising treatment option during the evaluation of existing drugs and developing new definitive therapies.The effectiveness of CPT has been tested ever since the Spanish Influenza pandemic in 1915‐1917, 5 severe acute respiratory syndrome (SARS) in 2003, 6 influenza A (H1N1) in 2009, 7 avian influenza A (H5N1), 8 and even in Ebola. 2
Recently, the US Food and Drug Administration has approved the use of CPT for patients with coronavirus disease (COVID‐19) under the emergency investigational new drug category and not for routine clinical use. 9
The absence of a definitive therapeutic modality for COVID‐19 has made CPT most relevant in the current grievous scenario. However, the clinical data for the studies involving COVID‐19, are still scarce. Thus, the aim of our study is to systematically analyze the current evidence on efficacy and safety of convalescent plasma therapy in COVID‐19 patients for decision‐making to prevent and control this pandemic. This study is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA‐P) guidelines.
2. METHODS
2.1. Search strategy
This systematic search was conducted with the major electronic databases (PubMed and Medline), Google Scholar (https://scholar.google.com), and preprint platforms MedRxiv (https://www.medrxiv.org) from 1st January2020 to 10th July 2020, independently by two researchers (SS and PK). The following terminologies: (“COVID‐19”) OR (“SARS‐CoV‐2”) AND (“plasma” OR “convalescent plasma”) were searched for.
2.2. Inclusion and exclusion criteria
We included randomized controlled trials (RCT), controlled clinical trials, prospective and retrospective comparative cohort studies, case‐control studies; cross‐sectional studies, and case series with a control group on steroid therapy for COVID‐19 patients. Our primary outcome of interest was mortality, and secondary outcomes included improvement in clinical conditions and clearance of viral shedding.We excluded articles written in languages other than English, absence of essential data, and without retrievable full text (PRISMA flow diagram). 10 , 11
2.3. Study selection
The available literature was screened independently after the removal of duplications by two researchers (SS and KDS). We screened all the abstracts primarily to exclude irrelevant articles. Finally, full‐texts of the potentially eligible studies were screened for inclusion. Disagreements involved consultation with a third researcher (PK).
2.4. Data extraction
Two researchers (SS and KDS) extracted the data independently from all included studies with the use of pre‐conceived data extraction sheet. The extracted information contained details of the intervention and control groups, mortality, clinical improvement, and viral clearance. The number of events along with the total number of patients per group was extracted for dichotomous data. Studies with missing or unusable data are reported in findings descriptively.
2.5. Risk of bias assessment
Two researchers (SS and PK) assessed the potential bias in each selected study independently. The third researcher (KDs) was consulted for resolving any difference of opinion.
The RoB 2.0 tool, 12 was used for RCTs, which includes five domains: “randomization process”, “deviations from intended interventions”, “missing outcome data”, “measurement of the outcome”, and “selection of the reported result”. We used the Risk Of Bias In Non‐randomized Studies—of Interventions (ROBINS‐I) 13 tool for assessing the risk of bias in non‐randomized studies. It comprises seven domains: “bias due to confounding”, “selection of participants, classification of interventions”, “deviations from intended interventions”, “missing data”, “measurement of outcomes”, and “selection of the reported result”. Each domain is graded as “Low”, “Moderate”, “Serious”, and “Critical”.
2.6. Quality of the evidence
Two experienced researchers (PK and KDS) evaluated the quality of evidence by using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool. 14 , 15 It has five downgrading factors (study limitations, consistency of effect, imprecision, indirectness, and publication bias) and three upgrading factors (large magnitude of the effect, dose‐response relation, and plausible confounders or biases). The quality of evidence of each outcome is classified as “High”, “Moderate”, “Low” or “Very low”. 16 , 17 , 18 , 19 , 20 , 21 , 22
2.7. Data synthesis
Review manager version 5.4 was used for conducting the meta‐analysis. The Odd's ratio (OR) with 95% confidence intervals (CIs) was assessed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions. 23 Statistical heterogeneity was assessed with the I2 statistic, >50% indicating substantial heterogeneity. A funnel plot was used to assess publication bias.
The present study was not registered for rapid decision making in the context of the ongoing public health emergency.
3. RESULTS
3.1. Basic characteristics
We included 7 studies (2 RCTs and 5 cohort studies) out of 679 identified publications in this rapid review, after satisfying the inclusion criteria (Figure 1 and Table 1). The risk of bias was low in one of the included RCTs and another one had some concerns (Figure 2A). Out of the other five studies, four studies were associated with a moderate degree of bias (Figure 2B).
Figure 1.
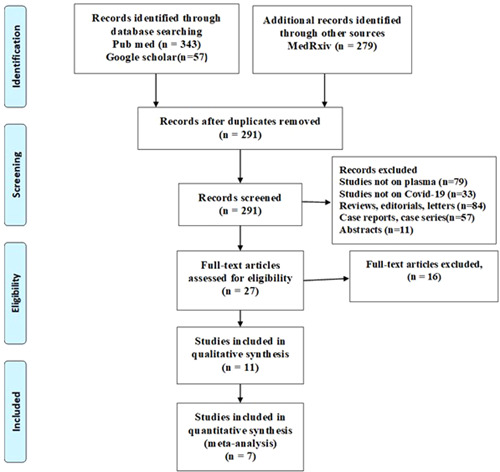
PRISMA‐2009 flow diagram
Table 1.
Characteristics of included studies
| SN | Studies (Year) | Type of study (centre) | No of patients | Patient condition | Time of administration | Dosage of CPT | Antibody titer | Concomitant therapy | Author's conclusion |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Chen et al 24 | Retrospective Observational, MC | 29 | Severely ill | 19 d (IQR, 14‐20) | 200‐500 mL (4‐5 mL/kg) | >1:160 | Not specified | Significant improvement in clinical outcomes in comparison to the untreated cases |
| 2 | Duan et al 25 | Pilot prospective cohort with a historical control group, SC | 20 | Severely ill | 16.5 d (IQR, 11‐19) | 200 mL | >1:640 | antiviral therapy, steroids and supportive care as appropriate | CPT shows a potential therapeutic effect and low risk in the treatment of severe COVID‐19 patients |
| 3 | Gharbharan et al 26 | Open‐label RCT, MC | 86 | Mild‐ moderately ill | 9 d (IQR, 7‐13) | 300 mL | 1:640 (IQR, 1:320‐1:1280) | Chloroquine, azithromycin, lopinavir/ritonavir, tocilizumab, anakinra as appropriate | No statistically significant differences in mortality (OR, 0.95, CI, 0.20‐4.67; P = .95) or improvement in the day‐15 disease severity (OR, 1.30; CI, 0.52‐3.32; P = .58) was observed when the study was suspended |
| 4 | Joyner et al 27 | Observational CT, MC | 5000 | Critically ill | Not specified | 200‐500 mL | Not specified | Not specified | Seven‐day mortality rate = 14.9% |
| 5 | Li et al 28 | Open label RCT, MC | 103 | Critically ill | 27 d (IQR, 22‐39) | 4‐13 mL/kg 200 mL (IQR, 200‐300) | >1:640 | antivirals, steroids, immunoglobulin, antibiotics and Chinese herbal medicines, as appropriate | In severe or life‐threatening COVID‐19 patients, in addition to standard treatment, CPT did not result in a statistically significant improvement in time to clinical improvement within 28 d. Interpretation is limited by early termination of the trial |
| 6 | Liu et al 29 | Case controlled study, SC | 185 | Moderate‐ critically ill | 4 d (IQR, 1‐7) | 2 units. Each unit of 250 mL | >1:320 | antivirals, anti‐biotics, steroid and immunoglobulins as appropriate | Plasma recipients also demonstrated improved survival, compared to control patients |
| 7 | Zeng et al 30 | Retrospective observational study, MC | 21 | Critically ill | 21.5 d (IQR, 17.8‐23) | 300 mL (IQR, 200‐600) | Not specified | antivirals, steroid and immunoglobulins as appropriate | CPT can discontinue the viral shedding and contribute longer survival duration in COVID‐19 patients with respiratory failure, although it cannot reduce the mortality in critically end‐stage patients |
Abbreviations: CPT, convalescent plasma transfusion; IQR, interquartile range; MC, multi‐center; OR, odds ratio; RCT, randomized controlled trial; SC, single center.
Figure 2.
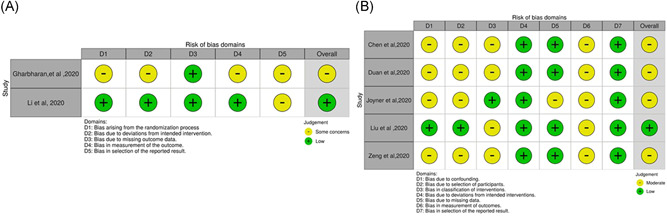
A, ROB2 tool assessment for the included RCTs. B, ROBINS‐I assessment for the included non‐randomized cohort studies
3.2. Meta‐analysis
Mortality was assessed in seven articles (two RCTs and five cohort studies) with a total of 5444 patients. The use of CPT reduced the risk of mortality almost by half in COVID‐19 (OR, 0.44; 95% CI, 0.25 to 0.77; I 2 = 0), which is statistically significant (Figure 3).
Figure 3.
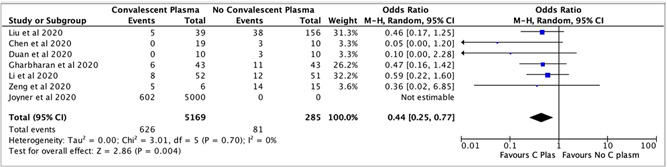
The efficacy of convalescent plasma therapy on mortality in COVID‐19 patients
Five studies with a total of 259 patients assessed the clinical improvement in COVID‐19. The majority of the COVID‐19 patients who received CPT showed clinical improvement than that in patients who received no CPT (OR, 2.06; 95% CI, 0.8 to 4.9; I 2 = 44%) (Figure 4A). However, the finding is not statistically significant.
Figure 4.
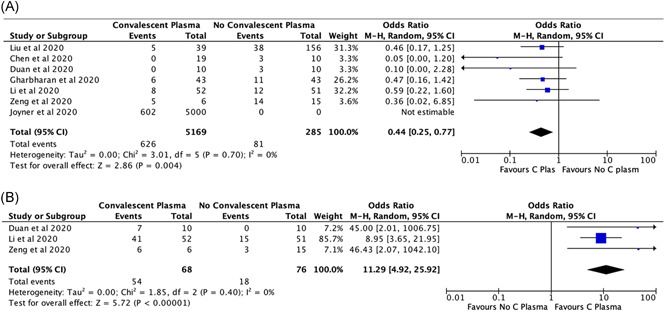
A, The impact of convalescent plasma therapy on clinical improvement in COVID‐19 patients. B, The effect of convalescent plasma therapy on viral clearance in COVID‐19 patients
The incidence of viral clearance was assessed in two studies with a total of 144 patients. It is found that the use of CPT helps in viral clearance (OR, 11.29; 95% CI, 4.9 to 25.9; I 2 = 0%) significantly (Figures 4B).
Apart from mild heterogeneity among studies on assessing clinical improvement (I 2 = 44), the overall findings are homogenous. In view of the high homogeneity, the overall effect seems to be conclusive.
3.3. Quality of evidence
The quality of evidence on the impact of CPT on mortality and viral clearance in COVID‐19 is of low quality, and that of clinical improvement is of very low quality (Table 2).
Table 2.
GRADE evidence profile of COVID‐19 studies
| Out come | No. of participants | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Quality of evidence (Grade) | Relative effect | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total no. | Intervention | Control | ||||||||
| Mortality | 5444 | 5169 | 285 | Yes | No | No | No | None | Low ⊕⊕⊝⊝ | OR 0.44 (95% CI, 0.25 to 0.77) |
| Clinical improvement | 259 | 130 | 129 | Yes | No | No | Yes | None | Very low ⊕⊝⊝⊝ | OR 2.06 (95% CI, 0.8 to 4.9) |
| Viral Clearance | 144 | 68 | 76 | Yes | No | No | No | None | Low ⊕⊕⊝⊝ | OR 11.29 (95% CI, 4.9 to 25.9) |
Abbreviations: CI, confidence interval; COVID‐19, coronavirus disease 2019; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MD, mean difference; OR, odds ratio.
3.4. Publication bias
We assessed publication bias for the studies on COVID‐19 mortality. The funnel plot indicates a publication bias is likely in view of smaller studies with a large effect (Figure S1).
4. DISCUSSION
We have identified low‐quality evidence with variability that the convalescent plasma therapy is associated with around 44% reduction in the mortality in COVID‐19 patients.
A similar systematic review and meta‐analysis on severe acute respiratory syndrome (SARS), reported that the CPT is beneficial for reducing the mortality (OR, 0.25; 95% CI, 0.14 to 0.45; I 2 = 0%) in comparison to placebo or no therapy. 31
Another recent systematic review on CPT in COVID‐19 patients reported about a potential reduction in mortality but was unable to provide any opinion regarding the efficacy of CPT in COVID‐19 due to paucity in quantitative synthesis. 32
The present study has identified that very low‐quality evidence regarding improvement in clinical conditions and low‐quality evidence for viral clearance are associated with CPT.
A recent systematic review on the efficacy of CPT for the management of COVID‐19 also reported a significant decrease in viral loads and improvement in clinical symptoms within 3 to 26 days post‐transfusion. 33 Rajendran et al 32 also reported similar findings in their systematic review.
Another meta‐analysis on the efficacy and safety of convalescent plasma have found uninformative results regarding complete recovery (OR, 1.04; 95% CI, 0.69 to 1.64), length of stay (mean difference, 1.62; 95% CI, –3.82 to 0.58) and reduction in viral load on day 3 (RR, 1.07; 95% CI, 0.58 to 1.8), and day 7 (RR, 1.32; 95% CI, 0.97 to 1.81). However, the quality of evidence was very low due to the presence of high level of indirectness. 34
Salazar et al 35 reported out of 25 critically ill patients, who received CPT on the 7th post‐transfusion day, 9 patients improved, while 13 remained static, and 3 deteriorated, and on the 14th post‐transfusion day 19 patients had a better clinical status, as per 6 points WHO ordinal scale.
The studies have shown significant variation regarding the timing of initiation, dosage and neutralizing antibody titer, and concomitant therapy.
However, a dilemma exists on finding a concrete conclusion about the favorable outcome being due to CP therapy alone based on the given evidence and not due to natural disease progression or concomitant therapies.
4.1. Adverse events
The overall incidence of serious adverse events was very low. None of the patients, who received CPT in two studies, Gharbharan et al 26 (n = 43) and Zeng et al 30 (n = 6) showed any adverse event. Joyner et al 27 reported the incidence of serious adverse events after CPT was low (<1%) in 5,000 patients. They reported about transfusion‐associated circulatory overload (TACO) (n = 7), transfusion‐related lung injury (TRALI) (n = 11) and severe allergic reactions (n = 3). Dua et al 25 reported about rashes in one patient out of 10 patients, who received CPT. Another study reported about TRALI in one patient and rashes in one patient out of 52 patients. 28
4.2. Strengths and limitations
Our study is one of the first comprehensive and systematic reviews of the effectiveness and safety of convalescent plasma therapy for patients with COVID‐19 using data from COVID‐19 studies and may be considered at the moment as the best evidence for decision‐making.
Although in the current scenario, CPT is an effective therapeutic option in addition to current antiviral, antimicrobial agents, a wide range of variation regarding selection of the donor, clinical stage of the recipient, initiation time, antibody titer, volume, dose and duration of CPT is noted across the available studies so far. We could not conduct subgroup analyses due to lack of data. We also acknowledge the procedure is yet to be standardized, and information in this regard is still evolving.
5. CONCLUSION
CPT may be an effective therapeutic option, until the availability of therapeutic and/or prophylactic agents for COVID‐19, with some early promising evidence on safety, viral clearance, and reduction in mortality. However, large multi‐center clinical trials are the need of the hour for establishing a stronger quality of evidence along with the optimal doses, titer, and initiation time point for CPT for effective use.
5.1. Summary statement
Impact of convalescent plasma therapy in COVID‐19 management:
↓ Mortality (OR, 0.44; 95% CI, 0.25 to 0.77).
↑ Viral clearance (OR, 11.29; 95% CI, 4.9 to 25.9).
↑ Clinical‐improvement (OR, 2.06; 95% CI, 0.8 to 4.9).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
SS: conceptualization search strategy study selection, data extraction, risk of bias assessment, and drafted the manuscript; KDS: study selection, data extraction, risk of bias assessment, quality of the evidence assessment, data synthesis, and editing; PK: conceptualization, search strategy, study selection, risk of bias assessment, quality of the evidence assessment, and editing.
Supporting information
Supplementary information
Supplementary information
Sarkar S, Soni KD, Khanna P. Convalescent plasma is a clutch at straws in COVID‐19 management! A systematic review and meta‐analysis. J Med Virol. 2021;93:1111–1118. 10.1002/jmv.26408
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Casadevall A, Pirofski L‐A. The convalescent sera option for containing COVID‐19. J Clin Invest. 2020;130(4):1545‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Use of Convalescent Whole Blood or Plasma Collected from Patients Recovered from Ebola Virus Disease for Transfusion, as an Empirical Treatment During Outbreaks. Geneva: World Health Organization; 2014. www.who.int/csr/resources/publications/ebola/convalescent-treatment/en [Google Scholar]
- 3. Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schoofs T, Klein F, Braunschweig M, et al. HIV‐1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV‐1. Science. 2016;352:997‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta‐analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599‐609. [DOI] [PubMed] [Google Scholar]
- 6. Soo YO, Cheng Y, Wong R, et al. Retrospective comparison of convalescent plasma with continuing high‐dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004l;10(7):676‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357(14):1450‐1451. [DOI] [PubMed] [Google Scholar]
- 9. Investigational COVID‐19 Convalescent Plasma‐Emergency INDs . U.S. Food and Drug Administration. 2020 [cited July 16, 2020]. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma G. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 13. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomized studies of interventions. BMJ. 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Norris SL, Meerpohl JJ, Akl EA, et al. The skills and experience of GRADE methodologists can be assessed with a simple tool. J Clin Epidemiol. 2016;79:150‐158. [DOI] [PubMed] [Google Scholar]
- 15. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383‐394. [DOI] [PubMed] [Google Scholar]
- 16. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401‐406. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 17. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64:407‐415. [DOI] [PubMed] [Google Scholar]
- 18. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64:1277‐1282. [DOI] [PubMed] [Google Scholar]
- 19. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283‐1293. [DOI] [PubMed] [Google Scholar]
- 20. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294‐1302. [DOI] [PubMed] [Google Scholar]
- 21. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64:1303‐1310. [DOI] [PubMed] [Google Scholar]
- 22. Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311‐1316. [DOI] [PubMed] [Google Scholar]
- 23. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen B, Xia R. Early experience with convalescent plasma as immunotherapy for COVID‐19 in China: Knowns and unknowns. Vox Sang. 2020. 10.1111/vox.12968 [DOI] [PMC free article] [PubMed]
- 25. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490‐9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gharbharan A, Jordans CCE, GeurtsvanKessel C, et al. Convalescent Plasma for COVID‐19. A randomized clinical trial. medRxiv. 2020. 10.1101/2020.07.01.20139857 [DOI]
- 27. Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID‐19 convalescent plasma in 5,000 patients [published online ahead of print June 11, 2020]. J Clin Invest. 2020. 10.1172/JCI140200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: a randomized clinical trial [published online ahead of print June 3, 2020]. JAMA. 2020;324:460. 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu STH, Lin H‐M, Baine I, et al. Convalescent plasma treatment of severe COVID‐19: A matched control study. medRxiv. 2020. 10.1101/2020.05.20.20102236 [DOI] [PubMed]
- 30. Zeng QL, Yu ZJ, Gou JJ, et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis. 2020;222(1):38‐43. 10.1093/infdis/jiaa228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211(1):80‐90. 10.1093/infdis/jiu396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, et al. Convalescent plasma transfusion for the treatment of COVID‐19: systematic review. J Med Virol. 2020:1‐9. 10.1002/jmv.25961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khadka S, Saleem M, Shrestha D, Budhathoki P. Safety and efficacy of convalescent plasma therapy for the management of COVID‐19: A systematic review. 2020.
- 34. Devasenapathy N, Ye Z, Loeb M, et al. Efficacy and safety of convalescent plasma for severe COVID‐19 based on evidence in other severe respiratory viral infections: a systematic review and meta‐analysis. CMAJ. 2020;192(27):E745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salazar E, Perez KK, Ashraf M, et al. Treatment of COVID‐19 patients with convalescent plasma in houston. Texas. medRxiv. 2020. 10.1101/2020.05.08.20095471 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


