Abstract
The full impact of coronavirus disease 2019 (COVID‐19) on pregnancy remains uncharacterized. Current literature suggests minimal maternal, fetal, and neonatal morbidity and mortality. COVID‐19 manifestations appear similar between pregnant and nonpregnant women. We present a case of placental severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus in a woman with mild COVID‐19 disease, then review the literature. Reverse transcriptase polymerase chain reaction was performed to detect SARS‐CoV‐2. Immunohistochemistry staining was performed with specific monoclonal antibodies to detect SARS‐CoV‐2 antigen or to identify trophoblasts. A 29‐year‐old multigravida presented at 40‐4/7 weeks for labor induction. With myalgias 2 days prior, she tested positive for SARS‐CoV‐2. We demonstrate maternal vascular malperfusion, with no fetal vascular malperfusion, as well as SARS‐CoV‐2 virus in chorionic villi endothelial cells, and also rarely in trophoblasts. To our knowledge, this is the first report of placental SARS‐CoV‐2 despite mild COVID‐19 disease (no symptoms of COVID‐19 aside from myalgias); patient had no fever, cough, or shortness of breath, but only myalgias and sick contacts. Despite her mild COVID‐19 disease in pregnancy, we demonstrate placental vasculopathy and presence of SARS‐CoV‐2 virus across the placenta. Evidence of placental COVID‐19 raises concern for placental vasculopathy (potentially leading to fetal growth restriction and other pregnancy complications) and possible vertical transmission—especially for pregnant women who may be exposed to COVID‐19 in early pregnancy. This raises important questions of whether future pregnancy guidance should include stricter pandemic precautions, such as screening for a wider array of COVID‐19 symptoms, increased antenatal surveillance, and possibly routine COVID‐19 testing throughout pregnancy.
Keywords: Coronavirus, public policy, SARS coronavirus
Highlights
We present a case of placental SARS‐CoV‐2 virus in a woman with mild COVID‐19 disease.
This is the first report of placental SARS‐CoV‐2 despite mild COVID‐19 disease (no symptoms of COVID‐19 aside from myalgias); patient had no fever, cough, or shortness of breath, but only myalgias and sick contacts.
Despite mild COVID‐19 disease in pregnancy, we demonstrate placental vasculopathy and presence of SARS‐CoV‐2 virus across the placenta.
This report raises important questions of whether future pregnancy guidance should include stricter pandemic precautions.
1. INTRODUCTION
As of 18 July 2020, the coronavirus disease 2019 (COVID‐19) pandemic has resulted in 3 630 587 cases and 138 782 deaths in the United States. 1 The full impact of COVID‐19 on pregnancy remains to be fully characterized, especially as women who were exposed to COVID‐19 early in their first trimester have yet to reach full term, as of the time of writing this manuscript. The current literature suggests minimal maternal, fetal, and neonatal morbidity and mortality for women with COVID‐19 in pregnancy. 2 COVID‐19 manifestations have appeared largely similar between pregnant and nonpregnant women, 3 until a recent publication from the US CDC Morbidity and Mortality Weekly Report (MMWR). 4
Before this study, we searched PubMed and Medline on 28 May 2020 (updated on 28 June 2020) for articles describing the impact of COVID‐19 in pregnancy, using the search terms “COVID‐19” or “SARS‐CoV‐2” and “pregnancy” or “placenta” with no time or language restrictions. We found only previously published research that describes finding severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus in the placentas of women with moderate‐to‐severe COVID‐19 disease. We found no published work about SARS‐CoV‐2 virus in the placentas of women with mild COVID‐19 disease. Two reports suggest that pregnant women with COVID‐19 may be more likely to be admitted to the intensive care unit (ICU) and more likely to be intubated, but all reports consistently state that severe COVID‐19 disease during pregnancy is uncommon. We found six manuscripts which describe SARS‐CoV‐2 virus in the placentas of women with moderate‐to‐severe COVID‐19 disease. We found no published work about SARS‐CoV‐2 virus immunohistochemistry (IHC) in the placentas of women with mild COVID‐19 disease.
2. OBJECTIVES
In this case study, we present a case of placental SARS‐CoV‐2 virus in a woman with an uncomplicated pregnancy and mild COVID‐19 disease. Here, we review the literature on COVID‐19 disease and pregnancy, and subsequently discuss the key messages of this case.
3. STUDY DESIGN
A pregnant woman was evaluated at University of Missouri Women and Children's Hospital. Institutional review board approval was obtained; information was obtained from medical records. Reverse transcriptase‐polymerase chain reaction (RT‐PCR) was performed to detect SARS‐CoV‐2. A gynecological pathologist examined the placenta and performed histolopathology. Sections were formalin‐fixed and paraffin‐embedded; slides were cut and subjected to hematoxylin‐and‐eosin or IHC staining. IHC was performed with SARS‐CoV‐2 nucelocapsid‐specific or cytokeratin‐7 specific monoclonal antibody to detect SARS‐CoV‐2 antigen or to identify trophoblasts, respectively.
4. RESULTS/CASE PRESENTATION
In April 2020, a 29‐year‐old multigravida presented at 40‐4/7 weeks for labor induction. She had tested positive for SARS‐CoV‐2, with her only symptoms being myalgias 2 days prior. Her parents and siblings had been in self‐isolation for COVID‐19 positivity; her husband was asymptomatic and tested negative for COVID‐19, but he had been exposed to a workplace (meatpacking facility) outbreak.
Her prenatal course was uncomplicated, with no gestational hypertension or any other pregnancy complications. Her myalgias improved before admission. She was afebrile and asymptomatic with normal vital signs throughout hospitalization. Consistent with other reports of laboratory abnormalities associated with COVID‐19, her inpatient labwork showed some mild elevations in her liver function tests and C‐reactive protein, but were otherwise unremarkable (Table 1).
Table 1.
Significant maternal inpatient laboratory results
| Lab results | Normal values | 4/28/20 | 4/29/20 | 4/30/20 |
|---|---|---|---|---|
| C‐reactive protein, mg/dL | 0‐0.50 | 1.43 | ||
| D‐dimer, mcg/mL | 0‐0.50 | 0.96 | 1.43 | 1.21 |
| Procalcitonin, ng/mL | 0‐0.05 | 0.06 | ||
| Ferritin, ng/mL | 13.0‐150.0 | 35.9 | 48.3 | |
| Alkaline phosphatase, units/L | 35‐104 | 193 | 175 | 152 |
| AST‐SGOT, units/L | ≤32 | 65 | 57 | 43 |
| ALT‐SGPT, units/L | 10‐35 | 214 | 186 | 151 |
| LDH, units/L | 135‐214 | 215 | 226 | 197 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase.
A liveborn male infant was delivered vaginally. Newborn course was uneventful; with routine resuscitation and newborn APGARs of 8 and 9 at 1 and 5 minutes; points were taken off for color only. Birthweight was 3521 g (66th percentile), length was 48 cm (14th percentile) and head circumference was 35 cm (64th percentile), all appropriate for gestational age. His physical was unremarkable, with a small skin tag on his right cheek and dermal melanocytosis on buttock, appropriate for ethnicity. He passed hearing screen and screen for critical congenital heart defects (normal‐preductal 99%, postductal 99%). He was discharged home at 36 hours of life, with a plan for close follow‐up from his pediatrician. COVID‐19 RT‐PCR test was negative at 24 hours. At 1‐week follow‐up, newborn was breastfeeding well, with no fevers or respiratory distress. Two months later, mother and baby are doing well.
A gynecological pathologist examined the placenta and performed histolopathology. Sections were formalin‐fixed and paraffin‐embedded; slides were then cut and subjected to hematoxylin‐and‐eosin or IHC staining. Placenta had a marginally inserted three‐vessel umbilical cord; placental membranes were thin and transparent. Placental weight was 538 g, at the 60th percentile for gestational age. No gross lesions were seen on fetal or maternal placental surfaces; parenchymal sections were unremarkable. On histology, placental membranes showed decidua with scattered arterioles with thickened smooth muscle, consistent with hypertrophic arteriolopathy (Figure 1A) and subchorionic laminar necrosis (Figure 1B). Placental disc showed focal lympho‐histiocytic inflammation consistent with chronic villitis (Figure 1C) and scattered islands of extravillous trophoblasts (Figure 1D). No evidence of fetal thrombi (villous sclerosis or villous karyorrhexis) were seen. Overall histology is consistent with acute uterine hypoxia (subchorionic laminar necrosis) superimposed on chronic uterine hypoxia (extra‐villous trophoblasts and focal chronic villitis), of which hypertrophic arteriolopathy may be partly responsible.
Figure 1.
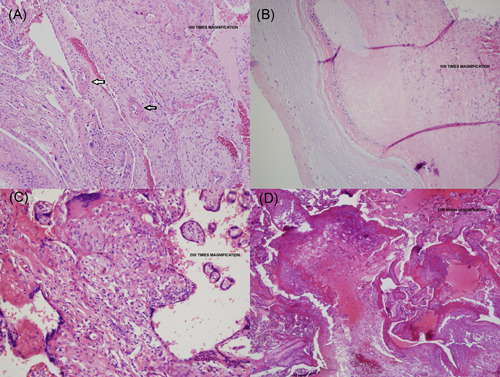
Placental vasculopathy in a pregnant woman with mild COVID‐19 disease. Placental membranes showed decidua with scattered arterioles with thickened smooth muscle, consistent with hypertrophic arteriolopathy (vasculopathy) (A, umbilical cord and placental membranes) and subchorionic laminar necrosis (B, placental parenchyma under the umbilical cord). Placental disc showed focal areas of lympho‐histiocytic inflammation consistent with chronic villitis (C, central placental parenchyma) and scattered islands of extravillous trophoblasts (D, peripheral placental parenchyma)
IHC was performed with SARS‐CoV‐2 nucelocapsid‐specific rabbit monoclonal antibody (Sino Biological, Wayne, PA) and goat anti‐rabbit IgG (Vector lab, Burlingame, CA). To identify trophoblasts, IHC was performed using rabbit recombinant anti‐cytokeratin 7 (CK7) monoclonal antibody (Abcam, Cambridge, MA) and goat anti‐rabbit IgG (Vector lab). IHC using SARS‐CoV‐2 nucleocapsid‐specific monoclonal antibody demonstrated SARS‐CoV‐2 antigens throughout the placenta under the umbilical cord (Figure 2B), and at the central (Figure 2C), and peripheral placenta disc (Figure 2D) in chorionic villi endothelial cells, and rarely in CK7‐expressing trophoblasts. Negative control placenta (November 2019 delivery; Figure 2A) and ferret nasal turbinate (not shown) were negative for SARS‐CoV‐2.
Figure 2.
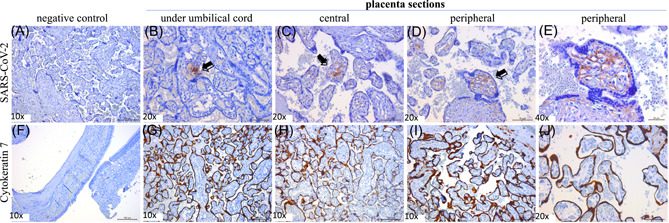
Presence of SARS‐CoV‐2 virus across the placenta in a patient with mild COVID‐19 disease. IHC staining of SARS‐CoV‐2 virus in a COVID‐19 negative patient, delivery before the COVID‐19 outbreak (A). IHC of SARS‐CoV‐2 from three placental sections ((B) under umbilical cord, (C) central placental disc, (D, E) peripheral placental disc at ×20 and ×40). IHC of CK‐7 marker in control ferret nasal turbinate tissue (F). IHC of CK‐7 from three placental sections ((G) under umbilical cord, (H) central placental disc, (I, J) peripheral placental disc at ×10 and ×20). Arrows or brown staining indicate immunoreactive antigens. Bars = 20/50/100 µm shown at the right bottom corner of each panel. IHC was performed with SARS‐CoV‐2 nucleocapsid‐specific rabbit monoclonal antibody (Sino Biological) and goat anti‐rabbit IgG (Vector lab). To identify trophoblasts, IHC was performed using rabbit recombinant anti‐CK7 monoclonal antibody (Abcam) and goat anti‐rabbit IgG (Vector lab). CK‐7, cytokeratin‐7; IHC, immunohistochemistry; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
5. DISCUSSION/REVIEW OF CURRENT LITERATURE
5.1. COVID‐19 in pregnancy
Thus far, there have been 12 case series in the literature, 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 which consistently observe that severe COVID‐19 disease during pregnancy is uncommon. Out of 431 patients reported in these 12 studies, 36 developed severe or critical disease, and 31 of the 36 severe cases progressed to critical disease and were admitted to the ICU. Overall, however, less than 10% of pregnant patients in these 13 studies are reported to have severe or critical disease. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Blitz et al 7 compared COVID‐19 ICU admissions between nonpregnant (n = 332; aged, 15‐49) and pregnant women (n = 82) at a large hospital system in New York State and found that pregnant women were not at an increased risk for ICU admission compared with nonpregnant women (P = .22); in this study, 15.1% of nonpregnant females were admitted to the ICU compared to 9.8% of pregnant patients. Similarly, Savasi et al 8 reported that out of 77 pregnant women with COVID‐19 across 12 hospitals in Italy, only six were admitted to the ICU and all patients eventually made a complete recovery. Reports of other nonrespiratory complications during pregnancy also appear to be rare. A study from Wuhan found that the rate of spontaneous abortion was 12.5% for patients in the first or second trimester (1 out of 8 COVID‐19 positive cases), while pre‐eclampsia occurred in 1.5% (1 out of 65 COVID‐19 positive cases). 16 Fortunately, none of these studies reported any maternal deaths.
Two other studies, 4 , 17 suggest that pregnant women with COVID‐19 have a higher risk of severe disease than nonpregnant women. A Swedish study showed pregnant Swedish women were five times more likely to be admitted to the ICU and four times more likely to receive mechanical ventilation than were nonpregnant women. 17
Also, a US CDC report reviewed 326 335 women of reproductive age (15‐44 years of age) with positive SARS‐CoV‐2 test results. 4 Data on pregnancy status was available for 28.0% (91 412 women), and 9% of that group (8207 women) were pregnant. 4 After adjusting for age, race/ethnicity, and underlying conditions, pregnant women were more likely to be admitted to intensive care (absolute risk reduction [aRR] = 1.5, 95% confidence interval [CI] = 1.2‐1.8) and to receive mechanical ventilation for breathing support (aRR = 1.7, CI, 1.2‐2.4), but with no significant differences in maternal mortality. 4 Based on absolute risks, the MMWR report noted that 1.5% of pregnant women with COVID‐19 required ICU admission, 4 so despite the finding of an increased risk of admission to critical care, pregnant women in this study had an even lower overall risk of ICU admission than the previous 12 case series. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16
All of these studies agree upon a low level of maternal mortality (16 deaths, or 0.2% according to MMWR) from COVID‐19. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 While this is reassuring overall, more data is needed, especially regarding pregnancies with exposure to COVID‐19 in the first trimester.
5.2. Possible vertical transmission in pregnancy
There are also several reports suggesting a potential low risk of in utero vertical transmission from mother to fetus. In an Italian series of 22 women affected by COVID‐19, Patanè et al 19 describe two cases in which the mothers, placentas, and neonates were all positive for SARS‐CoV‐2 by PCR testing. Penfield et al 20 describe an NYU study of 32 COVID‐19 positive pregnant patients, who had 11 placental or membrane swabs sent for analysis, three of which were positive for SARS‐CoV‐2; no infants tested positive for SARS‐CoV‐2 on days of life one through five, and none demonstrated COVID‐19 symptoms.
Baud et al 21 describe a second‐trimester miscarriage in which the mother (nasopharyngeal swab) and placental submembranes and placental cotyledons were positive for SARS‐CoV‐2 on RT‐PCR. Hosier et al 22 describe SARS‐CoV‐2 localization to syncytiotrophoblast cells at the maternal‐fetal interface of the placenta, with no evidence of vasculopathy. Chen et al 23 describe nine patients who had a caesarean section, with amniotic fluid, cord blood, neonatal throat swab, and breastmilk samples from six patients all testing negative for SARS‐CoV‐2.
Ferraiolo et al 24 describe an asymptomatic woman with a positive nasopharyngeal swab for COVID‐19 in Italy, who had a Caesarean section and positive placental swabs for SARS‐CoV‐2 RNA. Dong et al 25 report a case of SARS‐CoV‐2 IgM in a neonate born to a COVID‐19 positive mother. Collectively, this literature suggests that there may be a low risk of vertical transmission of COVID‐19 from mother to fetus in utero.
In our report, SARS‐CoV‐2 was found throughout the placenta on IHC, and newborn was COVID‐19 negative. As this patient was exposed to COVID‐19 in her third trimester of pregnancy, it remains unclear whether women who are exposed to COVID‐19 earlier in pregnancy (or multiple times during pregnancy) may have a greater risk of in‐utero vertical transmission of COVID‐19 from mother to fetus. Further studies are needed, especially from pregnancies with exposure to COVID‐19 in the first trimester.
5.3. COVID‐19 in the placenta
COVID‐19 is known to interact with endothelial cells and cause a hypercoagulative state, which can lead to the formation of microthrombi. 26 This can potentially affect the placenta in two ways. First, effects on maternal perfusion of the placenta can manifest as accelerated villous maturation, infarction, intervillous thrombi, extravillous trophoblastic lesions, and subchorionic laminar necrosis in the membranes. Second, if the fetal circulation is affected, thrombosis of larger vessels, fibrous obliteration of vessels in the villi (villous sclerosis) and breakdown of the endothelial cells within the villous stroma (villous stromal vascular karyorrhexis) may be seen. In a study of placentas from COVID‐19 positive mothers in New York and New Jersey, Baergen et al 27 showed fetal vascular malperfusion in 8 of 20 placentas and maternal vascular malperfusion in 3 of 20 placentas. In a study at Northwestern University, Shanes et al 28 found that the most common findings in COVID‐positive placentas were decidual arteriopathy and intervillous thrombi. However, it is notable that signs of fetal and maternal malperfusion are nonspecific and can be seen in other conditions, such as hypercoagulable states, like lupus anticoagulant, and protein C or S deficiency, gestational hypertension, 29 pre‐eclampsia, as well as in patients with no specific medical history.
6. CASE DISCUSSION
The inexorable rise in COVID‐19 cases in the United States amid decreasing adherence to public health recommendations raises concern for the possibility of viral mutations. In light of the CDC's MMWR report, 4 the US CDC also issued updated (25 June 2020) recommendations 30 regarding pregnancy and COVID‐19, encouraging pregnant women to “limit interactions with other people as much as possible,” to “take precautions to prevent getting COVID‐19 when you do interact with others,” and to be aware that “some babies have tested positive for the virus shortly after birth. It is unknown if these babies got the virus before, during, or after birth.”
While two reports suggest that pregnant women with COVID‐19 may be more likely to be admitted to the ICU and more likely to be intubated than nonpregnant women, 4 , 17 all reports consistently state that severe COVID‐19 disease during pregnancy is uncommon. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 We found six manuscripts which describe SARS‐CoV‐2 virus in the placentas of women with moderate‐to‐severe COVID‐19 disease. To date, there is still no other published work about SARS‐CoV‐2 virus by IHC in the placentas of women with mild COVID‐19 disease.
To our knowledge, this is the first report of placental COVID‐19 despite mild COVID‐19 disease in pregnancy (with no symptoms of COVID‐19 aside from myalgias); specifically, this patient had no fever, cough, or shortness of breath, but only myalgias and sick contacts. Evidence of placental COVID‐19 raises concern for possible placental vasculopathy (potentially leading to fetal growth restriction, pre‐eclampsia, and other pregnancy complications) as well as for potential vertical transmission—especially for pregnant women who may be exposed to COVID‐19 in early pregnancy. Despite her having mild COVID‐19 disease in pregnancy, we demonstrate placental vasculopathy and presence of SARS‐CoV‐2 virus across the placenta.
While our case shows features of maternal vascular malperfusion (extravillous trophoblastic lesions and subchorionic laminar necrosis), no evidence of fetal vascular malperfusion were seen. As this patient was exposed to COVID‐19 in her third trimester of pregnancy, it remains unclear whether women who are exposed to COVID‐19 earlier in pregnancy (or multiple times during pregnancy) may have a greater risk of fetal vascular malperfusion, which may result in pre‐eclampsia, fetal growth restriction, or other obstetric complications. Further studies are needed, especially from pregnancies with exposure to COVID‐19 in the first trimester.
This report also raises important concerns regarding the possibility of birth defects, miscarriages, and stillbirths with COVID‐19 infection, as well as the question about potential protective or detrimental effects of breastfeeding. Data responsive to these critical issues remain limited and preliminary. Of note, women who were exposed to COVID‐19 in their first trimester in the United States still have not had any full‐term deliveries, and remain in early stages of their pregnancies; thus, we are not able to comment on the possibilities of birth defects with COVID‐19 at the current time. Regarding miscarriage, one early meta‐analysis 2 noted four cases of miscarriage out of 324 pregnant women with COVID‐19; most related studies similarly lack a well‐characterized control group for appropriate comparative analyses. There are also isolated case reports suggesting that late miscarriage may be a presenting manifestation of COVID‐19. 21 , 31 Finally, an early report from the United Kingdom 32 demonstrated a concerning increase in stillbirth rates during the COVID‐19 pandemic; specifically, those authors report “3 stillbirths among 247 completed pregnancies” in women with confirmed COVID‐19, for a rate of 6.98 vs 1.19 per 1000 births (P = .01), with multifactorial causes likely.
Regarding breastfeeding, early data suggest that transmission of COVID‐19 through breastmilk is possible but rare. Cumulative data from six separate studies 23 , 25 , 33 , 34 , 35 , 36 found that out of 20 mothers with COVID‐19, only two had their breastmilk test positive with PCR. Of those two cases, one breastfed newborn tested positive for SARS‐CoV‐2 by PCR, and the other newborn tested negative for SARS‐CoV‐2; it is unclear whether that newborn breastfed or received expressed breastmilk. Ferrazi et al 36 reported that, of two women with COVID‐19 who breastfed without a mask, both had newborns who tested positive for COVID‐19 postpartum. In contrast, eight other COVID‐19 positive mothers in this study breastfed while mask‐wearing, and those eight newborns did not contract COVID‐19; there was no mention of whether or not the breastmilk was tested for SARS‐CoV‐2. 35 This suggests that close contact associated with breastfeeding may increase the risk of transmission of infected respiratory secretions, and that mask‐wearing may decrease this risk. Questions about birth defects, miscarriage, stillbirth, and breastfeeding with COVID‐19 remain critical areas for future research, but at the current time, data is limited, preliminary, and continually evolving.
7. CONCLUSION
To our knowledge, this is the first report of placental COVID‐19 despite mild COVID‐19 disease in pregnancy (with no symptoms of COVID‐19 aside from myalgias). All other reports have shown placental COVID‐19 in pregnancies with moderate‐to‐severe disease. This patient had no fever, cough, or shortness of breath, but only myalgias and sick contacts.
In this report, SARS‐CoV‐2 was found in the placenta, but the newborn was COVID‐19 negative. Our case shows maternal vascular malperfusion, with no features of fetal vascular malperfusion. Evidence of placental COVID‐19 raises concern for placental vasculopathy and potential vertical transmission. Our report raises the question of whether future pregnancy guidance should include even stricter pandemic precautions, such as prenatal screening for a wider array of COVID‐19 symptoms, increased antenatal surveillance recommendations, and possibly COVID‐19 testing on a regular basis throughout pregnancy.
AUTHOR CONTRIBUTIONS
ALH and X‐FW had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: ALH, EJ, AJS, NK, and X‐FW. Acquisition, analysis, or interpretation of data: ALH, MG, EJ, AJS, NK, BCT, and X‐FW. Drafting manuscript and critical revision of the manuscript for important intellectual content: all authors. Administrative, technical, or material support: ALH, EJ, and X‐FW. Supervision: ALH and X‐FW.
ACKNOWLEDGMENTS
Authors thank Danny Schust, MD; Taylor Nelson, DO; Jane McElroy, PhD; Dima Dandachi, MD; and Holly Ford, MD. This study was supported by the Department of Obstetrics and Gynecology, University of Missouri School of Medicine.
Hsu AL, Guan M, Johannesen E, et al. Placental SARS‐CoV‐2 in a pregnant woman with mild COVID‐19 disease. J Med Virol. 2021;93:1038–1044. 10.1002/jmv.26386
Contributor Information
Albert L. Hsu, Email: hsual@health.missouri.edu.
Xiu‐Feng Wan, Email: wanx@missouri.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. COVID‐19 cases in the US. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed July 18, 2020.
- 2. Juan J, Gil MM, Rong Z, Zhang Y, Yang H, Poon LC. Effects of coronavirus disease 2019 (COVID‐19) on maternal, perinatal and neonatal outcomes: a systematic review. Ultrasound Obstet Gynecol. 2020;56:15‐27. 10.1002/uog.22088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muhidin S, Behboodi Moghadam Z, Vizheh M. Analysis of maternal coronavirus infections and neonates born to mothers with 2019‐nCOV: a systematic review. Arch Acad Emerg Med. 2020;8(1):e49. [PMC free article] [PubMed] [Google Scholar]
- 4. Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status—United States, January 22‐June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):769‐775. 10.15585/mmwr.mm6925a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breslin N, Baptiste C, Gyamfi‐Bannerman C, et al. COVID‐19 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2(2):100118. 10.1016/j.ajogmf.2020.100118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breslin N, Baptiste C, Miller R, et al. Coronavirus disease 2019 in pregnancy: early lessons. Am J Obstet Gynecol MFM. 2020;2(2):100111. 10.1016/j.ajogmf.2020.100111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blitz M, Grünebaum A, Tekbali A, et al. Intensive care unit admissions for pregnant and non‐pregnant women with COVID‐19. Am J Obstet Gynecol. 2020. 10.1016/j.ajog.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Savasi, Parisi F, Patanè L, et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID‐19). Obstet Gynecol. 2020;136(2):252–258. 10.1097/aog.0000000000003979 [DOI] [PubMed] [Google Scholar]
- 9. Ferrazzi E, Frigerio L, Savasi V, et al. Vaginal delivery in SARS‐CoV‐2‐infected pregnant women in Northern Italy: a retrospective analysis. Int J Obstet Gynaecol. 2020;127(9):1116‐1121. 10.1111/1471-0528.16278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Govind, Essien S, Karthikeyan A, et al. Novel coronavirus COVID‐19 in late pregnancy: outcomes of first nine cases in an inner city London hospital. Eur J Obstet Gynecol Reprod Biol. 2020;251:272–274. 10.1016/j.ejogrb.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ceulemans D, Thijs I, Schreurs A, et al. Screening for COVID‐19 at childbirth: does it deliver? Ultrasound Obstet Gynecol. 2020;56:113‐114. 10.1002/uog.22099 [DOI] [PubMed] [Google Scholar]
- 12. Lokken E, Walker C, Delaney S, et al. Clinical characteristics of 46 pregnant women with a SARS‐CoV‐2 infection in Washington State. Am J Obstet Gynecol. 2020;S0002‐9378(20):30558–30565. 10.1016/j.ajog.2020.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu Y, Liu C, Dong L, et al. Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. Int J Obstet Gynaecol. 2020;127(9):1109–1115. 10.1111/1471-0528.16276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buonsenso D, Costa S, Sanguinetti M, et al. Neonatal late onset infection with severe acute respiratory syndrome coronavirus 2. Am J Perinatol. 2020;37(8):869–872. 10.1055/s-0040-1710541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qiancheng X, Jian S, Lingling P, et al. Coronavirus disease 2019 in pregnancy. Int J Infect Dis. 2020;95:376‐383. 10.1016/j.ijid.2020.04.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 (COVID‐19) in pregnant women: A report based on 116 cases. Am J Obstet Gynecol. 2020;223(1):111.e1–111.e14. 10.1016/j.ajog.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collin J, Byström E, Carnahan A, Ahrne M. Public health agency of Sweden's brief report: pregnant and postpartum women with SARS‐CoV‐2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99:819‐822. 10.1111/aogs.13901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Habib H. Has Sweden's controversial COVID‐19 strategy been successful? BMJ. 2020;369:m2376. 10.1136/bmj.m2376 [DOI] [PubMed] [Google Scholar]
- 19. Patanè L, Morotti D, Giunta MR, et al. Vertical transmission of COVID‐19: SARS‐CoV‐2 RNA on the fetal side of the placenta in pregnancies with COVID‐19 positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020:1001452(3):100145. 10.1016/j.ajogmf.2020.100145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Penfield CA, Brubaker SG, Limaye MA, et al. Detection of SARS‐COV‐2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM. 2020:1001332(3):100133. 10.1016/j.ajogmf.2020.100133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baud D, Greub G, Favre G, et al. Second‐trimester miscarriage in a pregnant woman with SARS‐CoV‐2 infection. JAMA. 2020;323(21):2198‐2200. 10.1001/jama.2020.7233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hosier H, Farhadian SF, Morotti RA, et al. SARS‐CoV‐2 infection of the placenta. J Clin Invest. 2020:139569 130(9):4947–4953. 10.1172/JCI139569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809‐815. 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferraiolo A, Barra F, Kratochwila C, et al. Report of positive placental swabs for SARS‐CoV‐2 in an asymptomatic pregnant woman with COVID‐19. Medicina. 2020;56:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dong L, Tian J, He S, et al. Possible vertical transmission of SARS‐CoV‐2 from an infected mother to her newborn. JAMA. 2020;323(18):1846‐1848. 10.1001/jama.2020.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Connors J, Levy J. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baergen RN, Heller DS. Placental pathology in COVID‐19 positive mothers: preliminary findings. Pediatr Dev Pathol. 2020;23(3):177‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shanes ED, Mithal CB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID‐19. Am J Clin Pathol. 2020;154:23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heider A. Fetal vascular malperfusion. Arch Pathol Lab Med. 2017;141:1484‐1489. [DOI] [PubMed] [Google Scholar]
- 30. CDC recommendations on Coronavirus Disease 2019 (COVID‐19) . If you are pregnant, breastfeeding, or caring for young children. https://www.cdc.gov/coronavirus/2019-ncov/need-extra&hyphen-qj18;-precautions/pregnancy-breastfeeding.html. Accessed June 26, 2020.
- 31. Hachem R, Markou GA, Veluppillai C, Poncelet C. Late miscarriage as a presenting manifestation of COVID‐19 [published online ahead of print, 2020 Jul 13]. Eur J Obstet Gynecol Reprod Biol. 2020;S0301‐2115(20):30466‐30468. 10.1016/j.ejogrb.2020.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O'Brien P, Magee L. Change in the Incidence of stillbirth and preterm delivery during the COVID‐19 pandemic. JAMA. 2020;324(7):705–706. 10.1001/jama.2020.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan C, Lei D, Fang C, et al. Perinatal transmission of COVID‐19 associated SARS‐CoV‐2: should we worry? Clin Infect Dis. 2020;ciaa226. 10.1093/cid/ciaa226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grob R, Conzelmann C, Müller J, et al. Detection of SARS‐CoV‐2 in human breastmilk. Lancet. 2020;395(10239):1757–1758. 10.1016/s0140-6736(20)31181-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu Y, Liu C, Dong L, et al. Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. Int J Obstet Gynaecol. 2020;127(9):1109–1115. 10.1111/1471-0528.16276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferrazzi E, Frigerio L, Savasi V, et al. Vaginal delivery in SARS‐CoV‐2‐infected pregnant women in Northern Italy: a retrospective analysis [published online ahead of print April 27, 2020]. Int J Obstet Gynaecol. 2020. 10.1111/1471-0528.16278 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


