As the wave of COVID‐19 disease has spread across the globe, so medical publications have followed as part of scientific efforts to characterise, manage and hopefully prevent this disease. Papers pertaining to pathology have, by their very nature, lagged slightly behind those describing clinical and imaging cohorts, but we are now seeing increasing numbers of publications that give us insight into how human tissues are infected and damaged by the virus, three in this particular issue of Histopathology.
Menter et al. report a cohort of 21 patients dying of COVID‐19 infection. Acute lung injury was the main pathology at autopsy, primarily showing the exudative phase of diffuse alveolar damage (DAD), with a minority displaying the organising (or proliferative) phase. 1 In addition, there was frequent co‐existent vascular pathology, mainly thrombotic and more rarely vasculitic. In earlier small series and single cases, DAD was observed both with and without vascular damage. 2 , 3 , 4 Larger series provide a broader picture of the spectrum of COVID‐related lung pathology, 1 , 5 , 6 as well as confirming associations with male gender, hypertension, obesity and finding unexpected comorbidities such as senile cardiac amyloidosis. 1
The identification of vascular lung pathology in some, but not all, patients is also consistent with clinical and imaging data in relation to pro‐thrombotic states, 7 , 8 and we can conceptualise that this breadth of pathology findings represents several pathways through which patients, especially those who suffer severe disease, progress to a final common outcome of DAD. These include direct damage to the lungs by the virus and secondary bacterial infections, and systemic effects that manifest through immune dysregulation and increased levels of thrombosis (Figure 1). Indeed, it is likely that these pathways frequently interplay, and the next steps for the scientific community are unravelling these within individuals at the earliest stage possible to guide management.
Figure 1.
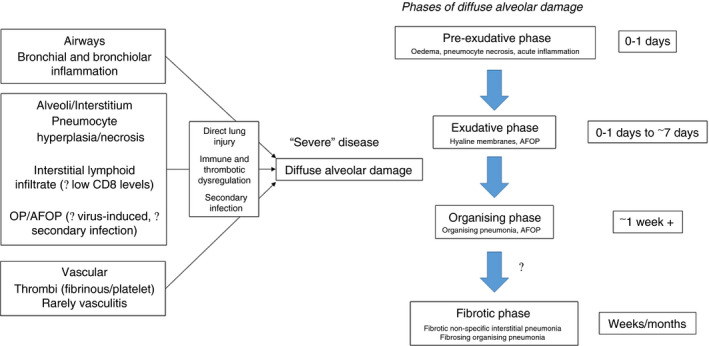
COVID‐19 virus‐related lung histopathological features. Histological features and patterns may vary and coexist dependent upon (A) the time‐point in disease evolution, (B) severity of disease and (C) the pathway(s) induced by viral infection.
In the second paper, Zeng et al. describe a patient operated on for a benign lung lesion before developing symptoms of COVID 19 infection. 9 Reported cases of pre‐mortem pathology are rare, and this case is a snapshot of what appears to be the disease in the earliest microscopically visible phase of DAD/acute lung injury. Although no hyaline membranes are seen there are oedematous exudates, one of the earliest features of DAD, identifiable on microscopy. 10 Typically, hyaline membranes take hours to develop. Similar features have been reported in lung cancer resections of patients later shown to have COVID‐19 infections. 11 , 12
The paper by Zeng et al. is also interesting for its description of a moderately intense lymphoid infiltrate within the interstitium, focally cuffing the pulmonary vasculature. This is also seen in other reports, 2 , 4 , 13 and is in keeping with some patients at autopsy reported by Menter et al., whereas a relative dearth of lymphoid infiltrate is reported in other cases. 1 Zeng et al. report a relative lack of CD8+ lymphocytes in the alveolar interstitium, also reported in the haematological literature. 14 Others report CD8+ lymphocytes outnumbering CD4+ lymphocytes. 2 Plasma cells and CD4+ lymphocytes were, however, present in the paper by Zeng et al., the latter again reported by others in bronchoalveolar lavage 15 and post‐mortem biopsy, respectively. 4 At present these are only very small series, but clearly this micro‐environment of interstitial inflammation and its relationship to the systemic, often lymphopaenic, 16 , 17 inflammatory state is a key subject for future investigation as we move beyond basic morphology in attempting to understand the pathogenesis of this disease and the interplay between pathways.
As data amass on COVID‐19‐related lung pathology, it is worth taking a step back and reviewing the variety of patterns of interstitial lung disease that have been reported since the beginning of the pandemic, ranging from interstitial lymphoid infiltrates, oedematous exudates, acute fibrinous and organising pneumonia (AFOP) 18 , 19 and DAD in both the exudative and organising phases. Their ‘uniqueness’ has often been the thrust behind publication, but we believe that what one is seeing is the above pathways being ‘freeze‐framed’ and variably sampled at different time‐points in their evolution, additionally influenced by both extent of insult and individual patient susceptibility, be that genetic or acquired in the form of comorbidities (Figure 1). DAD is one of the histological patterns of interstitial pneumonia published in the ATS/ERS consensus document in 2002, 20 reviewed again in 2013. 21 This classification unified the clinical management of patients with interstitial lung disease across disciplines, a field that had hitherto been muddied by excessive terminology interpreted in different ways. AFOP (predominantly fibrinoid material forming intra‐alveolar buds) is also accepted as part of the classification system as a histological pattern that probably reflects a rate of response to lung injury between that of organising pneumonia (buds of granulation tissue) and exudative DAD [linear fibrinoid material (hyaline membranes) lining alveoli]: in essence, somewhere between subacute and acute response to lung injury. 21 First reported by Beasley et al. in 2002, 22 it is an accepted histological pattern of interstitial pneumonia, but is viewed neither as an idiopathic clinicopathological entity nor a disease‐specific histological pattern, as there are a variety of causes (drug, infections) and associations (connective tissue disorders, post‐transplantation). 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 It is also not infrequent to see areas of OP, AFOP and DAD in the same autopsy from a patient dying of acute lung injury, and computerised tomography data describe some patients with COVID‐19 who have disproportionate consolidation prior to, or alongside, classic DAD, 34 , 35 which may reflect a cohort with OP/AFOP as a sequelae of immune dysregulation, as seen in anti‐synthetase syndrome. 36 Therefore, the presence of AFOP may reflect the final common pathway of DAD, systemic virally induced immune dysregulation, 37 secondary infection or any combination (Figure 1).
Therefore, it is important that a balance is maintained between splitting COVID‐19 into a multitude of histological patterns for research into pathogenesis and merging them all together as ‘COVID‐lung’ for the purposes of management, as is the case with other interstitial lung diseases. 38 Unravelling this interplay between pathways may well be the middle‐ground for smarter splitting in terms of pathogenesis, and smarter merging in terms of targeting treatment to block specific pathways. Finally, this overlap with interstitial lung disease may well prove increasingly important as we start to see the chronic sequelae of COVID‐19 infection, given that the interstitial lung disease community has long been aware that fibrotic non‐specific interstitial pneumonia and organising pneumonia progressing to fibrosis are not infrequently seen in survivors of DAD/acute lung injury. As yet, we have little idea of the extent of chronic pathology in survivors, let alone those with milder infections not requiring hospitalisation.
The COVID‐19 pandemic also has seen many indirect consequences, as shown in the third paper by Binder et al., reporting an individual suffered erosive gastrointestinal injury following intentional repetitive sublethal ingestion of ethanol‐based hand cleaner. 39 Notwithstanding the musings of certain world leaders, this highlights the wide‐reaching effects of this disease, both physical and psychological. The risks to health workers should also not be underestimated, given that five individuals involved in treating the one patient later tested positive in the paper by Zeng et al. 9 It is impossible to say how extensive the ramifications of this chapter in medical history will be. As these three papers highlight, this is not just in terms of accrual of clinical data to combat the disease. We are seeing resultant and rapid changes in our work practices, the need for social distancing meaning that many support services have jumped to the front of the information technology queue. As an example, the paper from Menter et al. has wonderful direct links to whole slide images. Our approach to all forms of education will surely change. 40
However, the basics still apply. The autopsy remains a valuable source of information which, whether it be a full autopsy or minimally invasive, if coupled with thorough sampling and retention of tissue for research and taken with appropriate consent, is key to understanding diseases and helping in their prevention. 41 As with many lessons learned as a result of this pandemic, it is hoped that the value of autopsy, suddenly remembered, is not forgotten once more.
Conflicts of interest
None.
Author contributions
All authors contributed to the writing and editing of the manuscript.
Nicholson A G, Osborn M, Devaraj A & Wells A U. (2020) Histopathology 77, 169–172. 10.1111/his.14131 CD34 positive tubular basement membrane in testicular germ cell tumours: a potential staging pitfall
References
- 1. Menter T, Haslbauer JD, Nienhold R et al Post‐mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID‐19 autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020; 153; 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu Z, Shi L, Wang Y et al Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020; 8; 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yao XH, Li TY, He ZC et al [A pathological report of three COVID‐19 cases by minimally invasive autopsies]. Zhonghua Bing Li Xue Za Zhi 2020; 49; E009. [DOI] [PubMed] [Google Scholar]
- 5. Carsana L, Sonzogni A, Nasr A et al Pulmonary post‐mortem findings in a large series of COVID‐19 cases from Northern Italy: A Two‐ Centre Study. Lancet Infect Dis. 2020; S1473‐3099(20)30434‐5. 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wichmann D, Sperhake JP, Lutgehetmann M et al Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann. Intern. Med. 2020; M20‐2003. 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Helms J, Tacquard C, Severac F et al High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intens. Care Med. 202046 6; 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dolhnikoff M, Duarte‐Neto AN, de Almeida Monteiro RA et al Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19. J. Thromb. Haemost. 2020; 18; 1517–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeng Z, Xu L, Xie XY et al Pulmonary pathology of early phase COVID‐19 pneumonia in a patient with a benign lung lesion. Histopathology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corrin B, Nicholson AG. Acute alveolar injury and repair In Pathology of the lungs. 3rd edn Edinburgh: Elsevier, 2011; 135–154. [Google Scholar]
- 11. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early‐phase 2019 novel coronavirus (COVID‐19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020; 15; 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pernazza A, Mancini M, Rullo E et al Early histologic findings of pulmonary SARS‐CoV‐2 infection detected in a surgical specimen. Virchows Arch. 2020; 1–6. 10.1007/s00428-020-02829-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tian S, Xiong Y, Liu H et al Pathological study of the 2019 novel coronavirus disease (COVID‐19) through postmortem core biopsies. Mod. Pathol. 2020; 33; 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang M, Guo Y, Luo Q et al T cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of COVID‐19. J. Infect. Dis. 2020. jiaa252. 10.1093/infdis/jiaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giani M, Seminati D, Lucchini A, Foti G, Pagni F. Exuberant plasmocytosis in bronchoalveolar lavage specimen of the first patient requiring extracorporeal membrane oxygenation for SARS‐CoV‐2 in Europe. J. Thorac. Oncol. 2020; 15; e65–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang F, Nie J, Wang H et al Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J. Infect. Dis. 2020; 221; 1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang F, Hou H, Luo Y et al The laboratory tests and host immunity of COVID‐19 patients with different severity of illness. JCI Insight 2020221 111; 1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Copin MC, Parmentier E, Duburcq T et al Time to consider histologic pattern of lung injury to treat critically ill patients with COVID‐19 infection. Intens. Care Med. 2020; 46; 1124–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang H, Zhou P, Wei Y et al Histopathologic changes and SARS‐CoV‐2 immunostaining in the lung of a patient with COVID‐19. Ann. Intern. Med. 2020; 172; 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Thoracic Society/European Respiratory Society . International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2002; 165; 277–304. [DOI] [PubMed] [Google Scholar]
- 21. Travis WD, Costabel U, Hansell DM et al An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. J. Respir. Crit. Care Med. 2013; 188; 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch. Pathol. Lab. Med. 2002; 126; 1064–1070. [DOI] [PubMed] [Google Scholar]
- 23. Kim JY, Doo KW, Jang HJ. Acute fibrinous and organizing pneumonia: imaging features, pathologic correlation, and brief literature review. Radiol. Case Rep. 2018; 13; 867–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feinstein MB, DeSouza SA, Moreira AL et al A comparison of the pathological, clinical and radiographical, features of cryptogenic organising pneumonia, acute fibrinous and organising pneumonia and granulomatous organising pneumonia. J. Clin. Pathol. 2015; 68; 441–447. [DOI] [PubMed] [Google Scholar]
- 25. Hariri LP, Unizony S, Stone J et al Acute fibrinous and organizing pneumonia in systemic lupus erythematosus: a case report and review of the literature. Pathol. Int. 2010; 60; 755–759. [DOI] [PubMed] [Google Scholar]
- 26. Prahalad S, Bohnsack JF, Maloney CG, Leslie KO. Fatal acute fibrinous and organizing pneumonia in a child with juvenile dermatomyositis. J. Pediatr. 2005; 146; 289–292. [DOI] [PubMed] [Google Scholar]
- 27. Perry R, Christidis D, Nicholson AG, Schomberg L, Cheent K. A case report of adult‐onset Still's disease presenting with acute fibrinous and organising pneumonia. J. R. Soc. Med. Open 2020; 11; 0954406220913584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishiwata T, Ebata T, Iwasawa S et al Nivolumab‐induced acute fibrinous and organizing pneumonia (AFOP). Intern. Med. 2017; 56; 2311–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupta A, Sen S, Naina H. Acute fibrinous and organising pneumonia: a rare histopathological variant of chemotherapy‐induced lung injury. BMJ Case Rep. 2016. bcr2016214721. 10.1136/bcr-2016-214721.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gomes R, Padrao E, Dabo H et al Acute fibrinous and organizing pneumonia: a report of 13 cases in a tertiary university hospital. Medicine 2016; 95; e4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jamous F, Ayaz SZ, Choate J. Acute fibrinous organising pneumonia: a manifestation of trimethoprim–sulfamethoxazole pulmonary toxicity. BMJ Case Rep. 2014; 2014; bcr2014205017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Otto C, Huzly D, Kemna L et al Acute fibrinous and organizing pneumonia associated with influenza A/H1N1 pneumonia after lung transplantation. BMC Pulm. Med. 2013; 13; 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ribera A, Llatjos R, Casanova A, Santin M. Chlamydia pneumoniae infection associated to acute fibrinous and organizing pneumonia. Enferm. Infecc. Microbiol. Clin. 2011; 29; 632–634. [DOI] [PubMed] [Google Scholar]
- 34. Revel MP, Parkar AP, Prosch H et al COVID‐19 patients and the radiology department – advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur. Radiol. 2020; 1–7. 10.1007/s00330-020-06865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu J, Pan J, Teng D et al Interpretation of CT Signs of 2019 novel coronavirus (COVID‐19) pneumonia. Eur. Radiol. 2020; 1–8. 10.1007/s00330-020-06915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Debray MP, Borie R, Revel MP et al Interstitial lung disease in anti‐synthetase syndrome: initial and follow‐up CT findings. Eur. J. Radiol. 2015; 84; 516–523. [DOI] [PubMed] [Google Scholar]
- 37. Hussein HM, Rahal EA. The role of viral infections in the development of autoimmune diseases. Crit. Rev. Microbiol. 2019; 45; 394–412. [DOI] [PubMed] [Google Scholar]
- 38. Wells AU, Brown KK, Flaherty KR, Kolb M, Thannickal VJ; IPFCW consensus Working Group . What's in a name? That which we call IPF, by any other name would act the same. Eur. Respir. J. 2018; 51; 1800692. [DOI] [PubMed] [Google Scholar]
- 39. Binder L, Hogenauer C, Langner C. Gastrointestinal effects of an attempt to ‘disinfect’ from COVID‐19. Histopathology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mukhopadhyay S, Booth AL, Calkins SM et al Leveraging technology for remote learning in the era of COVID‐19 and social distancing: tips and resources for pathology educators and trainees. Arch. Pathol. Lab. Med. 2020. 10.5858/arpa.2020-0201-ED. [DOI] [PubMed] [Google Scholar]
- 41. Ledford H. Autopsy slowdown hinders quest to determine how coronavirus kills. Nature 2020. 10.1038/d41586-020-01355-z. [DOI] [PubMed] [Google Scholar]


