Abstract
An association among the use of angiotensin converting enzyme (ACE) inhibitors and angiotensin‐receptor blockers (ARBs) with the clinical outcomes of coronavirus disease 2019 (COVID‐19) is unclear. PubMed, EMBASE, MedRxiv, and BioRxiv were searched for relevant studies that assessed the association between application of ACEI/ARB and risk of COVID‐19, inflammation level, severity COVID‐19 infection, and death in patients with COVID‐19. Ten studies were included with 13,944 patients. ACEI/ARB therapy might be associated with the reduced inflammatory factor (interleukin‐6) and elevated immune cells counts (CD3, CD8). Meta‐analysis showed no significant increase in the risk of COVID‐19 infection (odds ratio [OR]: 0.95, 95% CI: 0.89‐1.05) in patients receiving ACEI/ARB therapy, and ACEI/ARB therapy was associated with a decreased risk of severe COVID‐19 (OR: 0.75, 95% CI: 0.59‐0.96, p = 0.02) and mortality (OR: 0.57, 95% CI: 0.37‐0.87, p = 0.009). Subgroup analyses showed among the general population, ACEI/ARB therapy was not associated with reduced risks of severe COVID‐19 infection (OR: 0.85, 95% CI: 0.66‐1.08, p = 0.19) and all‐cause mortality (OR: 0.31, 95% CI: 0.13‐0.75), and COVID‐19 infection (OR: 0.97, 95% CI: 0.89‐1.05, p = 0.45) were not increased. Among patients with hypertension, the use of an ACEI/ARB was associated with a non‐significant lower severity of COVID‐19 (OR: 0.73, 95% CI: 0.51‐1.03, p = 0.07) and significant lower mortality (OR: 0.57, 95% CI: 0.37‐0.87, p = 0.009), without evidence of an increased risk of COVID‐19 infection (OR: 1.00, 95% CI: 0.90‐1.12, p = 1.00). On the basis of the available evidence, ACEI/ARB therapy should be continued in patients who are at risk for, or have COVID‐19, either in general population or hypertension patients. Our results need to be interpreted with caution considering the potential for residual confounders, and more well‐designed studies that control the clinical confounders are necessary to confirm our findings.
Keywords: ACEI/ARB, COVID‐19, hypertension, infectious disease, lung, pneumonia, SARS‐COV‐2
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic is becoming one of the most far‐reaching public health crises in recorded history. At right time of writing this review, on 29 April 2020, the number of infected persons worldwide has exceeded 3.01 million, with more than 200 000 reported deaths (http://2019ncov.chinacdc.cn/2019-nCoV/global.html). At present, there is no specifically targeted, effective treatment for patients with COVID‐19. There is an urgent need, therefore, to determine how to alleviate the severe clinical symptoms and reduce the morbidity and mortality due to this disease. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) shares the same cell entry receptor as SARS‐CoV, in which the viruses' spike proteins bind to the host cell surface. Angiotensin converting enzyme 2 (ACE2) receptors, which are an essential regulator of renin‐angiotensin‐aldosterone system (RAAS) activity. 1 , 2 The RAAS is a vital regulator of cardiovascular and renal function, including blood pressure, and which plays a vital role in regulating acute lung injury. 3 , 4 The ACE2 level is significantly reduced in patients following SARS‐CoV infection, resulting in RAAS system imbalance, and eventually cause severe acute lung injury. Angiotensin I converting enzyme inhibitors (ACEIs) and Angiotensin II receptor blockers (ARBs) are ACE2 receptor antagonists that can reduce the activity of the RAAS system and which have been widely used in the past several decades to treat cardiovascular diseases, such as hypertension and hypertrophic cardiomyopathy. 3 However, some animal experiments and clinical trials have shown that ACEIs potentially result in an increase in ACE2 receptors. 5 Some concern has therefore been expressed that the use of ACEIs/ARBs might increase the risk of COVID‐19 infection after exposure to SARS‐CoV‐2 and result in a poor prognosis. 6 On 12 April 2020, the European Society of Hypertension COVID‐19 Task Force issued the following statement: the current evidence does not support that RAAS inhibitors aggravate the condition of COVID‐19 patients. 5
Accumulating evidence revealed that inflammatory cytokine storm and dysfunction of immune system largely contribute to the severe COVID‐19, even cause death. 7 The latest evidence shows that peripheral blood interlekin‐6 (IL‐6) levels, a critical mediator of respiratory failure, shock, and multiorgan dysfunction, were significantly increased in COVID‐19 patients who had used a RAAS inhibitor. 8 , 9 Moreover, CD3 and CD8 cell counts were significantly reduced, suggesting that the level of inflammation in patients treated with ACEIs is relatively low. 8 Several retrospective studies have shown that the use of ACEIs/ARBs prior to COVID‐19 infection could reduce the severity of COVID‐19 and was associated with a better prognosis, especially in patients with both hypertension and COVID‐19. 10 , 11 , 12 However, other studies have shown that there is no significant correlation between the use of ACEIs/ARBs and the severity and mortality of patients with COVID‐19. 10 , 12 , 13 , 14 , 15 , 16 Because the affection of RAAS inhibitors on the clinical prognosis of COVID‐19 patients is unclear; in this article, we will review the current evidence and assess the clinical prognosis of COVID‐19 patients with or without hypertension treated with ACEs/ARBs.
2. METHODS
This study was performed according to PRISMA guidelines (http://www.prisma-statement.org; Table S1). 17
2.1. Literature search
Two authors (L. X. and L. C‐Y.) independently searched the PubMed and Embase databases for published articles and the preprint platforms medRxiv (https://www.medrxiv.org/) and bioRxiv (https://www.biorxiv.org/) (since many studies are available on these websites prior to publication, which allows for collection of the latest data) without language restrictions. Furthermore, the research references were traced and cross‐checked. In the case of any discrepancy, it was resolved by consensus with the third author (H. K.). The databases were searched for articles from 1 February 2019 to 1 May 2020 using the following search terms: ACE inhibitor, angiotensin II receptor blocker, angiotensin‐converting enzyme inhibitor, 2019‐novel coronavirus, SARS‐CoV‐2, COVID‐19, and 2019‐nCoV.
2.2. Study selection
Studies were considered eligible for inclusion if they (a) were designed as a randomized controlled trial, case‐control study, or cohort study; and (b) assessed the relationship between ACEI/ARB use and the level of inflammation, disease severity, and mortality in patients with COVID‐19. If multiple studies used the same population, we selected the most recent publication. Certain publication types (eg, reviews, editorials, letters, conference abstracts, and animal studies) or studies with insufficient data were excluded from this analysis.
2.3. Data extraction and quality assessment
Two authors (L. X. and L. C‐Y.) independently extracted the study information and the basic characteristics of the articles using a standardized form, including the first author, year of publication, country, sample size, sex ratio, age, sample size, RAAS type, length of follow‐up, adjustments for confounders and adjusted odds ratio (OR) with 95% confidence intervals (CIs). Two authors independently used modified Jadad scores and Newcastle‐Ottawa Scale (NOS) scores to evaluate the quality of the randomized controlled trials (RCTs) and observational studies, respectively. A Jadad score >5 and an NOS score >7 were considered high quality scores. 19
2.4. Statistical analyses
The RevMan5.3 (Review Manager [RevMan], version 5.3, Cochrane Collaboration) software was used for statistical data processing, and the OR and 95% CIs were used to estimate the effect. Heterogeneity among the studies was analyzed using the I 2 test with the following interpretation: low heterogeneity, defined as I 2 < 50%; moderate heterogeneity, defined as I 2 = 50% to 75%; and high heterogeneity, defined as I 2 > 75%. 20 The meta‐analysis was performed using the random effects model when the heterogeneity was more than 25%, otherwise, the fixed effects model was applied. If there were ACEI and ARB subgroups in the study, the combined OR and the 95% CIs were pooled using the RevMan5.3 software. If the study did not report the OR directly, count data for outcomes were used to generate unadjusted ORs and 95% CIs. We also excluded reports with unadjusted ORs in the sensitivity analysis to assess the robustness of our results. A funnel plot was used to test for the presence of publication bias, and P value <.05 was considered statistically significant.
3. RESULTS
3.1. Study selection
The systematic search of the electronic databases identified 343 articles (PubMed = 54, EMBASE = 112, Medrxiv = 132, ArXiv = 45). After excluding duplicates and title/abstracts screened, 21 articles underwent a more detailed full‐text assessment, after which a total of 10 articles with 13,944 patients were included 8 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 21 , 22 (Figure 1).
Figure 1.
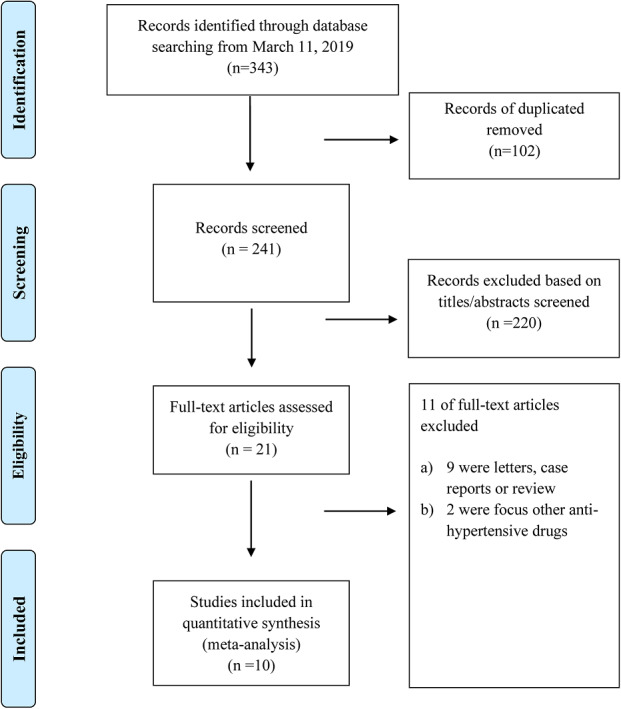
PRISMA flow diagram
3.2. Study characteristics and quality
Table 1 shows the basic characteristics of the included studies. Overall, the sample sizes included in the articles ranged from 42 to 6,272, and ages ranged from 55 to 67 years old. Among the 10 studies, 6 were published 8 , 10 , 14 , 15 , 16 , 22 and 4 were found on a preprint server 11 , 12 , 13 , 21 ; 2 studies were based on the general population of COVID‐19 patients 11 , 14 and 8 were based on patients with COVID‐19 and hypertension. 8 , 10 , 12 , 13 , 15 , 16 , 21 , 22 Four articles reported the effect of ACEIs/ARBs on the level of inflammation, 8 , 10 , 12 , 16 two studies assessed the risk of COVID‐19 inflection, 14 , 15 and all included studies evaluated the severity of disease or/and mortality. 8 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 21 , 22 All included studies were observational studies. The NOS score of all of the observational studies was >6, indicating that all of the studies were of high quality (Table S2).
Table 1.
General characteristics of the included studies in the meta‐analysis
| References(first author, year, country/region) | Type of study | Study participants | Sample size | Hypertension% | Age (years), Male | Duration of follow‐up* | Outcomes reported | Adjustments for confounders |
|---|---|---|---|---|---|---|---|---|
| Bean, 2020, United Kingdom | RC | General population | 205 | 51.2% | 63.0,51.7% | Within 7 days | Severity of COVID‐19 | Age, sex, hypertension, diabetes mellitus, ischaemic heart disease, heart failure |
| Li,2020, China | RC | Hypertension | 1,178 | 100% | 55.5,46.3% | Median 19 days | Level of inflammatory cytokines Severity of COVID‐19Death | NA |
| Mancia, 2020, Italy | Case‐control | General population | 6,272 | Na | 68,63.3% | NA | Risk of COVID‐19Severity of COVID‐19 | CCB, diuretics, oral antidiabetic drugs, cardiovascular disease, respiratory diseases, kidney disease, cancer |
| Liu,2020, China | RC | Hypertension | 511 | 100% | 65.2,55.1% | NA | Severity of COVID‐19 | NA |
| Meng, 2020, China | RC | Hypertension | 42 | 100% | 64.5,57.1% | NA | Level of inflammatory cytokines Severity of COVID‐19 | NA |
| Yang, 2020, China | RC | Hypertension | 126 | 100% | 66.0,49.2% | Mean 30 day | Severity of COVID‐19Level of inflammatory cytokinesDeath | NA |
| Zeng, 2020, China | RC | Hypertension | 75 | 100% | 67.0,55% | 28‐days | Severity of COVID‐19Level of inflammatory cytokines | NA |
| Huang, 2020, China | RC | Hypertension | 50 | 100% | Na,54.0% | Mean 42.3 days | Death | NA |
| Zhang, 2020, China | RC | Hypertension | 1,128 | 100% | 63.4,53.2% | 28‐days | Death | Age, gender, fever, cough, dyspnea, diabetes, coronary heart disease, and chronic renal disease, CT‐diagnosed bilateral lung lesions, and incidence of increased CRP and creatinine, D‐dimer, procalcitonin, and unilateral lesion and antiviral drug and lipid lowering drug |
| Reynolds, 2020, United Status | RC | Hypertension | 4,357 | 100% | 64,50.8% | NA | Risk of COVID‐19Severity of COVID‐19 | Age; sex; race; ethnic group; body‐mass index; smoking history; history of hypertension, myocardial infarction, heart failure, diabetes, chronic kidney disease, and obstructive lung disease (e.g., asthma and obstructive pulmonary diseases); and other classes of medication |
Abbreviations: CCB: Calcium‐channel blockers, COVID‐19: Coronavirus disease 2019, NA: Not available, RC: Retrospective cohort. * the period of time for all‐cause mortality observed.
3.3. Inflammation level
There were four articles that evaluated the relationship between ACEI/ARB and level of inflammation in patients with COVID‐19, 8 , 13 , 17 , 22 but since the data were not reported in the same unit across studies, they were not pooled; therefore, we conducted a systematic review instead (Table 2 ). Meng et al. 8 first assessed the impact of RAAS inhibitors on the level of inflammation in patients with COVID‐19, and although they found no significant change in C‐reactive protein (CRP) levels from the peripheral blood in patients receiving ACEI/ARB treatment, IL‐6 level had a trend of decrease (no statically significance difference may limited by small sample size), and the immune cells (CD3, CD8 T cells) counts were significantly increased.
Table 2.
Effect of ACEI/ARB on level of inflammatory cytokines in patients with COVID‐19
| References (first author, year, country/region) | Study participants | Sample size | Effect on inflammatory cytokines | Values (median [IQR]) | ||
|---|---|---|---|---|---|---|
| ACEI/ARB | Non‐ACEI/ARB | P | ||||
| Li, 2020, China [16] | Hypertension | 1178 | Interleukin 6 ↓, pg/mL C‐reactive protein→, mg/dL |
7.5 (3.3‐22.2) 2.1 (0.3‐5.2) |
8.8 (4.1‐30.8) 2.6 (0.4‐6.0) |
.06 .99 |
| Meng, 2020, China [8] | Hypertension | 42 |
Interleukin 6 ↓ a C‐reactive protein → CD3 cell count↑ CD4 cell count → CD8 cell count↑ |
Na | Na | Na |
| Yang, 2020, China [12] | Hypertension | 126 |
Interleukin 6 → C‐reactive protein↓ |
14.3 (3.7‐121.1) 11.5 (4.0‐58.2) |
10.1 (5.0‐50.4) 33.9 (5.1‐119.2) |
.52 .049 |
| Zhang, 2020, China [10] | Hypertension | 1128 | C‐reactive protein b → | 74/124 (59.7) | 136/221 (61.5) | .73 |
Abbreviations: ACEI/ARB: angiotensin I converting enzyme inhibitors/angiotensin II receptor blockers; CT: computed tomography; Na: not available.
A trend of decrease, but no statically significance difference may limited by small sample size.
Values expressed as unit of increase> upper limit of normal, n/N (%); IQR: interquartile range; CD3.
In contrast, another study showed that there was no difference in IL‐6 levels, while high‐sensitivity CRP test was significantly decreased. 13 A study that included 342 COVID‐19 patients with hypertension who had taken ACEI/ARB drugs found that IL‐6 levels were significantly reduced, while CRP level shows no significant difference. 17 More recently, a multicenter study including 1128 patients with hypertension and COVID‐19 reported that the number of patients with high CRP values who had received ACEI/ARB therapy showed no significantly different than in the patients who had not received ACEI/ARB therapy. 10
3.4. Risk of COVID‐19 infection
Two 15 , 16 studies including a total of 10 629 patients reviewed the risk of COVID‐19 infection, including one general population‐based study and one study including patients with hypertension. There was no significant increase in the risk of COVID‐19 infection in patients receiving ACEI/ARB therapy (OR = 0.95, 95% CI: 0.89‐1.02; P = .14, I 2 = 0%), with no significant heterogeneity (Figure 2A). Subgroup analysis based on population showed that the results did not change in the general population (OR = 0.96, 95% CI: 0.89‐1.05; P = .45, I 2 = 0%) or in the hypertensive population (OR = 1.00, 95% CI: 0.90‐1.12; P = 1.00) (Figure 3A).
Figure 2.
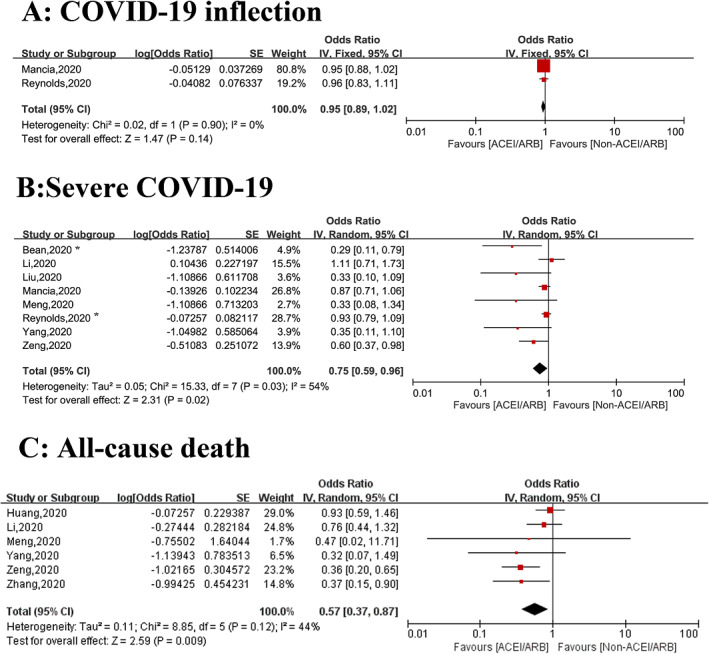
Summary of the associations between use of ACEI/ARB and clinical outcomes among patients with COVID‐19. A, Risk of COVID‐19 infection. B, Risk of severe COVID‐19 infection. C, All‐cause death. *severe COVID‐19 or death. ACEI, angiotensin I converting enzyme inhibitor; ARB, angiotensin II receptor blockers; COVID‐19, coronavirus disease 2019
Figure 3.
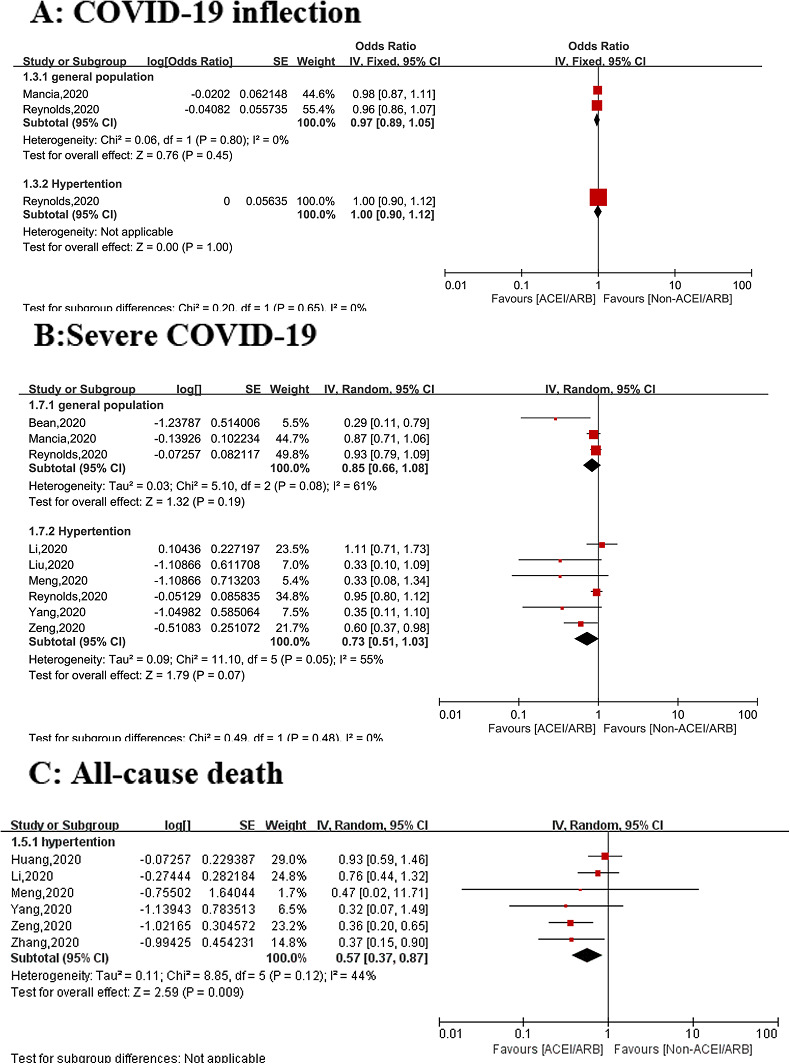
Subgroup analysis of the associations between use of ACEI/ARB and clinical outcomes among patients with COVID‐19 stratified by general population and hypertensive population: A, Risk of COVID‐19 infection. B, Risk of severe COVID‐19 infection. C, All‐cause death. *severe COVID‐19 or death. ACEI, angiotensin I converting enzyme inhibitor; ARB, angiotensin II receptor blockers; COVID‐19, coronavirus disease 2019
3.5. Risk of severe COVID‐19
Eight studies involving 12,766 patients assessed the relationship between the use of ACEI/ARB therapy and severe COVID‐19, 8 , 11 , 12 , 13 , 14 , 15 , 16 , 21 with two reports in the general population, 11 , 14 and six reports in patients with hypertension. 8 , 12 , 13 , 15 , 16 , 21 Compared with the non‐ACEI/ARB group, the risk of severe COVID‐19 infection decreased by 35% (OR = 0.75, 95% CI: 0.59‐0.96; P = .02) in patients treated with an ACEI/ARB, with moderate heterogeneity (I 2 = 54%) (Figure 2B). However, in the sensitivity analysis, the results were not statistically significant when the unadjusted studies 8 , 12 , 13 , 16 , 21 were excluded (OR = 0.85, 95% CI: 0.66‐1.08; P = .19).
Subgroup analysis showed that there was no statistical significant association between the risk of severe COVID‐19 infection and ACEI/ARB use either in the general population (OR = 0.85, 95% CI: 0.66‐1.08; P = .19, I 2 = 61%) or in hypertensive patients with (OR = 0.73, 95% CI: 0.51‐1.03; P = .07, I 2 = 55%) (Figure 3B).
3.6. All‐cause mortality
Six studies reported all‐cause mortality in 2,599 patients with COVID‐19, 8 , 10 , 12 , 16 , 21 , 22 and ACEI/ARB therapy was associated with a decreased risk of all‐cause mortality (OR: 0.57, 95% CI: 0.37‐0.87; P = 0.009) with modest of heterogeneity (I 2 = 44%) (Figure 2C). There is no evidence of heterogeneity (I 2 = 3%) when one 22 study was excluded, with no change of the conclusion (OR: 0.49, 95% CI: 0.34‐0.71; P = 0.0001). In the sensitivity analysis, the results were statistically significant when the unadjusted studies 8 , 12 , 16 , 21 , 22 were excluded (OR = 0.37, 95% CI: 0.15‐0.90; P = 0.03).
Subgroup analysis of hypertensive patients 8 , 10 , 12 , 16 , 21 , 22 (N = 2,599) showed the use of an ACEI/ARB is associated with a decreased risk of all‐cause mortality (OR: 0.57, 95% CI: 0.37‐0.87; P = 0.009, I 2 = 44%) (Figure 3C).
3.7. Publication bias
Publication bias was not performed this study, as the publication bias could not be ascertained as number of studies included for each item is <10. 24
4. DISCUSSION
This study was the first systemic assessment of ACEI/ARB therapy and the clinical prognosis of patients infected with COVID‐19. We did not find an association between ACEI/ARB therapy and the increased risk of COVID‐19 infection either in general population or in hypertensive patients. Moreover, we found that ACEI/ARB therapy could reduce the risk of severe COVID‐19; in a subgroup analysis, RAAS inhibitors reduced the risk of severe COVID‐19 by 15% (OR = 0.85, 95% CI: 0.66‐1.08; P = 0.19) and 27% (OR = 0.73, 95% CI: 0.51‐1.03; P = 0.07) in the general population and the hypertensive population, respectively, although this benefit did not achieve statistical significance. Finally, ACEI/ARB therapy reduced the rate of all‐cause death in patients with COVID‐19 by 43% (OR: 0.57, 95% CI: 0.37‐0.87; P = 0.009) in the hypertensive population.
Studies have shown that, similar to SARS‐CoV, SARS‐CoV‐2 directly binds to the host cell ACE2 receptor which is highly expressed in human lung tissue, gastrointestinal tract, vascular endothelial cells, and arterial smooth muscle cells in vivo. 1 , 24 Animal tests have shown that the expression of ACE2 receptors is significantly increased during ACEI/ARB therapy, 5 leading some scholars to worry that the use of RAAS system inhibitors may contribute to the spread of COVID‐19 in the population. 6 On 13 April 2020, the European Society of Hypertension COVID‐19 Task Force updated their recommendations on the use of RAAS inhibitors in patients with COVID‐19 and stated that the available evidence does not support a deleterious effect of RAAS blockers in COVID‐19 infections. 5 Our research also determined that there is no significantly increased risk of infection with SARS‐CoV‐2 in patients receiving ACE/ARB therapy. There are several possible explanations for these findings. First, although some clinical studies have reported that RAAS inhibitors might increase the expression of ACE2 receptors, these results are inconsistent 5 and most are small studies with small sample sizes. 25 , 26 , 27 Furthermore, these studies detected circulating ACE2 or soluble ACE2 receptor levels in the urine, and the level of soluble ACE2 receptors may not accurately reflect the true level of ACE2 receptors in the organs and tissues. 25 , 26 , 27 One study assessed the expression of ACE2 receptors in the duodenal and ileal tissues of 21 patients with RAAS inhibition; the researchers found that the expression of ACE2 receptor mRNA and protein increased significantly in the brush border of the small intestinal epithelial cells. 28 However, we know that SARS‐CoV‐2 is mainly transmitted through the respiratory tract ACE2 receptors, 1 , 29 and to date, there have been no reports published on the expression of ACE2 receptors in lung tissue after ACEI/ARB treatment, which is an important direction for future study.
Our study found that RAAS inhibitors reduced the risk of severe COVID‐19 infection by 25% (OR = 0.75, 95% CI: 0.59‐0.96; P = 0.02), which might be related to the previously confirmed anti‐inflammatory effects of RAAS inhibitors. 4 ACEIs and AT1R (Angiotensin type 1 receptor) inhibitors are commonly used RAAS system inhibitors that have been widely used in the treatment of hypertension, diabetic nephropathy, and congestive heart failure. 3 Lot of studies have shown that RAAS system is an important target for the treatment of acute lung injury. 30 For example, a retrospective analysis showed that ACEIs and AT1R inhibitors can also reduce the incidence of radiation pneumonitis. 31 In patients with severe COVID‐19, the levels of many pro‐inflammatory factors (eg, IL‐6, IL‐2, and tumor necrosis factor‐alpha) were significantly elevated, and levels of regulatory T cells decreased significantly. 7 A nonrandomized controlled clinical trial showed that the IL‐6 inhibitor tocilizumab could significantly reduce oxygen consumption, imaging abnormalities, and clinical prognosis in patients with COVID‐19. 32 Moreover, in patients receiving ACEI/ARB therapy, IL‐6 and CRP levels were significantly decreased, and the level of CD3 andCD8 increased significantly. 8 Overall, this evidence suggests that ACEI/ARB therapy might reduce lung injury and infection severity by downregulating inflammation levels in patients with COVID‐19.
Although we found use of ACEI/ARB was associated with the decreased risk of severe COVID‐19 in the overall analysis, this benefit did not reach statistical significance in the sensitive analysis (with excluding unadjusted studies).Furthermore, subgroup analysis showed the benefit was also not statistically significant either in the general population or in patients with hypertension. The possible reason for this result may be related to the limited sample size and existence of many confounding factors in the baseline characteristics, such as cardiovascular disease, and the use of drugs may affect these results. Age has been proven to be a strong risk factor for both infection with COVID‐19 and a higher severity of COVID‐19 infection. 12 Additionally, population‐based studies have estimated that the prevalence of hypertension ranges from 9.5% to 31.2% in COVID‐19 patients, and many studies have shown that hypertension can also significantly increase the severity of COVID‐19. 33 Therefore, more studies with large sample sizes with adjusted for these potential confounders are needed to clarify the impact of ACEI/ARB use on the severity of COVID‐19 in both the general population and in hypertensive patients. Recently, one multicenter study that included 1128 adult patients with hypertension also showed that ACEI/ARB use reduced the risk of septic shock by 62% without reducing the risk of other serious complications, such as acute respiratory distress syndrome and disseminated intravascular coagulation after matching baseline factors, suggesting that ACEI/ARB therapy led to a different type of severity of COVID‐19. 10 Therefore, the effect of RAAS inhibitors on the severity of COVID‐19 remains controversial and requires further study.
In addition, we found that ACEI/ARB therapy reduced the risk of mortality by 43%, and in the subgroup analysis, this mortality benefit remained significant for hypertensive patients. These results remained consistent in the sensitivity analysis, which verified the robustness of our study. Finally, studies have shown that COVID‐19 patients with hypertension receiving ACEI/ARB therapy have lower mortality compared with those nonhypertensive individuals with COVID‐19 34 , 35
4.1. Study limitations
Our research has several limitations. First, all of the included articles were observational and therefore cannot confirm the cause‐and‐effect relationship between ACEI/ARB therapy and the clinical prognosis of patients with COVID‐19; a large‐scale RCT is needed to confirm our results. Second, coexisting conditions, such as hypertension, have been shown a key prognostic determinant (eg, severity and mortality) in patients with COVID‐19. The guidelines recommend that patients with hypertension and COVID‐19 continue their ACEI/ARB therapy, and our study reinforced this recommendation and further showed that the use of ACEI/ARB therapy might be associated with better clinical outcomes in hypertensive patients with COVID‐19. However, the benefit of RAAS inhibitors in nonhypertensive patients might differ from those with hypertensive patients. Due to data limitations, we cannot analyze the severity and clinical prognosis of ACEI/ARB therapy in patients with COVID‐19 without hypertension. Third, studies have shown that ACEIs and ARBs may play different roles in patients with COVID‐19, due to limited data, we were unable to perform subgroup analyses of ACEIs and ARBs. Fourth, some characteristic clinical values (eg, drug variables) were missing. For example, the specific details of RAAS inhibitors were lacking in all studies, which might have impact on our results. Fifth, considering that all of the included studies were retrospective, the introduction of recall bias was inevitable and may affect the reliability of the conclusions. Sixth, although we did not register the protocol of this meta‐analysis in the PROSPERO database, no relevant protocols of this topic were found in this database at the writing of this review.
5. CONCLUSIONS
On the basis of the available evidence, we found that the use of ACEI/ARB therapy was not associated with an increased risk of COVID‐19 and that there was a decreased trend between ACEI/ARB use and severe COVID‐19 infection in both the general population and in patients with hypertension, although this association was not statistically significant. The risk of all‐cause death was decreased with ACEI/ARB therapy in patients with hypertension. Overall, RAAS inhibitors should be continued use in patients who are at risk for, are being evaluated for, or have COVID‐19. Our results need to be interpreted with caution considering the potential for residual confounders, and more well‐designed studies that control clinical confounders are necessary to confirm our foundlings.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
Kui Hong was responsible for the entire project and revised the draft. L. X and L. C‐Y. performed the systematic literature review and drafted the first version of the manuscript. H. K, X. Q‐M, S. Y‐H, C. Ch, and M. J‐Y reviewed, interpreted, and checked data. All authors took part in the interpretation of the results and prepared the final version of the manuscript.
Supporting information
Supplemental Table S1 PRISMA checklist
Supplemental Table S2. Quality assessment of included studies
Liu X, Long C, Xiong Q, et al. Association of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with risk of COVID‐19, inflammation level, severity, and death in patients with COVID‐19: A rapid systematic review and meta‐analysis. Clin Cardiol. 2020;47:1–10. 10.1002/clc.23421
Dr Xiao Liu, and Dr Chuyan Long are co‐first authors.
Funding information National Natural Science Foundation of China, Grant/Award Number: 81530013;81600243; Postgraduate Innovation Foundation of Jiangxi Province, Grant/Award Number: CX‐2017198; Scientific and Technical Innovation Group of Jiangxi Province, Grant/Award Number: 20181BCB24012
[Correction added on 18 October 2024, after first online publication: Two references have been removed from the reference list and three references (18, 34, and 35) have been added. Changes have been made to the Abstract, Introduction, Methods, Results, and Discussion. Figures 1, 2, and 3 as well as Tables 1 and 2 have been amended to reflect the new list of articles included in the systematic review and meta‐analysis. These changes have been made due to the retraction of an article originally included in the systematic review and meta‐analysis.]
REFERENCES
- 1. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romero CA, Orias M, Weir MR. Novel RAAS agonists and antagonists: clinical applications and controversies. Nat Rev Endocrinol. 2015;11(4):242‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin‐angiotensin systems. Physiol Rev. 2006;86(3):747‐803. [DOI] [PubMed] [Google Scholar]
- 5. Kreutz R, Algharably EAE, Azizi M, et al. Hypertension, the renin‐angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID‐19. Cardiovasc Res. 2020. [published online ahead of print. 10.1093/cvr/cvaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garami AR. Preventing a covid‐19 pandemic–is there a magic bullet to save COVID‐19 patients? We can give it a try. BMJ. 2020;368:m810. [DOI] [PubMed] [Google Scholar]
- 7. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020. [published online ahead of print]. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meng J, Xiao G, Zhang J, et al. Renin‐angiotensin system inhibitors improve the clinical outcomes of COVID‐19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coomes EA, Haghbayan H. Interleukin‐6 in COVID‐19: a systematic review and meta‐analysis. MedRxiv. 2020. 03.30.20048058; 10.1101/2020.03.30.20048058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang P, Zhu L, Cai J, et al. Association of Inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126(12):1671‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bean DM, Kraljevic Z, Searle T, et al. Angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID‐19 infection in a multi‐site UK acute hospital trust. European Journal of Heart Failure. 2020. [published online ahead of print]. 10.1002/ejhf.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang G, Tan Z, Zhou L, et al. Angiotensin II Receptor Blockers and Angiotensin‐Converting Enzyme Inhibitors Usage Is Associated with Improved Inflammatory Status and Clinical Outcomes in COVID‐19 Patients with Hypertension. MedRxiv. 2020. 03.31.20038935; 10.1101/2020.03.31.20038935 [DOI] [Google Scholar]
- 13. Liu Y, Huang F, Xu J, et al. Anti‐Hypertensive Angiotensin II Receptor Blockers Associated to Mitigation of Disease Severity in Elderly COVID‐19 Patients. MedRxiv. 2020.03.20.20039586; 10.1101/2020.03.20.20039586 [DOI] [Google Scholar]
- 14. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin‐angiotensin‐aldosterone system blockers and the risk of Covid‐19. N Engl J Med. 2020;382:2431‐2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin‐angiotensin‐aldosterone system inhibitors and risk of Covid‐19. N Engl J Med. 2020;382:2441‐2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J, Wang X, Chen J, Zhang H, Deng A. Association of Renin‐Angiotensin System Inhibitors with Severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Open Med. 2009;3(3):e123‐e130. [PMC free article] [PubMed] [Google Scholar]
- 18. Mcpheeters ML. Newcastle‐Ottawa Quality Assessment Scale. 2012.
- 19. Liu X, Ma J, Huang L, et al. Fluoroquinolones increase the risk of serious arrhythmias: a systematic review and meta‐analysis. Medicine (Baltimore). 2017;96(44):e8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng Z, Sha T, Zhang Y, et al. Hypertension in Patients Hospitalized with COVID‐19 in Wuhan, China: a Single‐Center Retrospective Observational Study. MedRxiv. 2020. 04.06.20054825. 10.1101/2020.04.06.20054825 [DOI] [Google Scholar]
- 22. Huang Z, Cao J, Yao Y, et al. The effect of RAS blockers on the clinical characteristics of COVID‐19 patients with hypertension. Ann Transl Med. 2020;8(7):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]The Cochrane Collaboration, 2011. https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm. 2011.
- 24. Lan J, Ge J, Yu J, et al. Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215‐220. [DOI] [PubMed] [Google Scholar]
- 25. Abe M, Oikawa O, Okada K, Soma M. Urinary angiotensin‐converting enzyme 2 increases in diabetic nephropathy by angiotensin II type 1 receptor blocker olmesartan. J Renin Angiotensin Aldosterone Syst. 2015;16(1):159‐164. [DOI] [PubMed] [Google Scholar]
- 26. Furuhashi M, Moniwa N, Mita T, et al. Urinary angiotensin‐converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28(1):15‐21. [DOI] [PubMed] [Google Scholar]
- 27. Mariana CP, Ramona PA, Ioana BC, et al. Urinary angiotensin converting enzyme 2 is strongly related to urinary nephrin in type 2 diabetes patients. Int Urol Nephrol. 2016;48(9):1491‐1497. [DOI] [PubMed] [Google Scholar]
- 28. Vuille‐dit‐Bille RN, Camargo SM, Emmenegger L, et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE‐inhibitors. Amino Acids. 2015;47(4):693‐705. [DOI] [PubMed] [Google Scholar]
- 29. Guo Y‐R, Cao Q‐D, Hong Z‐S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak–an update on the status. Mil Med Res. 2020;7(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pinter M, Kwanten WJ, Jain RK. Renin–angiotensin system inhibitors to mitigate cancer treatment–related adverse events. Clin Cancer Res. 2018;24(16):3803‐3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117(20):10970‐10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng Y‐Y, Ma Y‐T, Zhang J‐Y, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng X, Cai G, Wen X, et al. Clinical characteristics and fatal outcomes of hypertension in patients with severe COVID‐19. Aging (Albany NY). 2020;12(23):23436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baral R, White M, Vassiliou VS. Effect of renin‐angiotensin‐aldosterone system inhibitors in patients with COVID‐19: a systematic review and meta‐analysis of 28,872 patients. Current atherosclerosis reports. 2020;22(10):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1 PRISMA checklist
Supplemental Table S2. Quality assessment of included studies


