To the Editor,
There is paucity of data on immune responses to COVID‐19 in severe asthmatics treated with biologics. Recent guidelines recommend continuing these agents (as clinically indicated) to maintain asthma control. Supporting this claim, Lommatzsch demonstrated that omalizumab could safely be continued during active COVID‐19 infection in a patient with allergic asthma. 1 There are other reports that treatment with benralizumab 2 and dupilumab 3 are not associated with significant negative impact. We had similar experience in a young patient with severe asthma who had previously been treated with dupilumab. However, her immunoglobulin responses to the virus were modest compared to two other patients who had not previously been exposed to dupilumab. We speculate if interruption of IL‐4 receptor signaling may prolong the development of antibody responses to SARS‐CoV‐2.
A 23‐year‐old female, who had predominant IL‐4/IL‐13‐driven disease (characterized by atopy, severe airway hyperresponsiveness with PC20 methacholine 0.08 mg/mL, mucus hypersecretion, sputum and blood eosinophilia, and FeNO of 74ppb), noticed significant improvement in asthma control (ACQ‐5:1.0, 26% improvement in FEV1) after receiving dupilumab (600 mg loading dose followed by 300 mg every 2 weeks, subcutaneously) from September‐December of 2019 (NCT03884842; clinicaltrials.gov). In mid‐March of 2020 (3 months after the last dose of dupilumab), she tested positive by PCR of a nasopharyngeal (NP) swab for SARS‐CoV‐2. Her asthma was uncontrolled (ACQ‐5:4.4) but her blood eosinophil (0.1 x 109/L) and lymphocyte (1.7x109.L) counts were normal. Sputum and spirometry were not examined. She remained on high doses of inhaled corticosteroids (800 µg mometasone) and formoterol, and her ACQ‐5 gradually improved over the following 8 weeks to 3.0. Blood eosinophils went up to 1.4 × 109/L, but sputum eosinophils were normal at 1%, FeNO was 15ppb and FEV1 was 2.2L (70% predicted). However, 9 weeks after her onset of symptoms, the virus was still detectable by PCR from her NP swab. There was insufficient viral load to attempt viral culture.
We examined some potential mechanisms (using archived sputum samples collected before and immediately after treatment with dupilumab) to investigate whether dupilumab may have contributed to the infection (by modulating ACE2 or TMPRSS2 expression) and the prolonged viral detection (by modulating anti‐SARS‐CoV‐2 antibody levels). ACE2 could not be detected in her sputum samples but TMPRSS2 expression had increased fourfold (by RT‐PCR normalized to GAPDH housekeeping gene, using validated primers from Bio‐Rad, CA, USA). It is plausible that the high dose of inhaled steroids decreased ACE2 expression 4 but probably did not affect TMPRSS2 expression that facilitated the infection. Inhaled steroids also probably prevented significant asthma deterioration and respiratory distress. Indeed, dexamethasone was demonstrated to improve COVID‐19 outcomes in hospitalized patients. 5 Additionally, serum antibodies (IgA, IgM, and IgG) to two SARS‐CoV‐2 antigens, the Spike‐protein (S) and receptor‐binding‐domain (RBD), were measured using an ELISA to purified recombinant proteins as previously described 6 in our index patient and two other control patients who developed the infection (an asthmatic on inhaled steroid only (fluticasone 500µg daily) and a steroid‐naïve nonasthmatic). In comparison with two control patients, the dupilumab‐treated patient had blunted IgG and IgM and absent IgA antibodies to two SARS‐CoV‐2 antigens, the Spike‐protein and receptor‐binding‐domain (Figure 1). It is unclear if previous treatment with dupilumab may have led to a less robust antibody response or reduced class switching, although this has not been observed with T‐cell–dependent and T‐cell–independent humoral immune responses to tetanus and meningococcal vaccines. 7 Lastly, our index patient had relative eosinopenia at the time of diagnosis, which has been associated with increased symptoms and a longer disease course, whereas high blood eosinophils are associated with COVID‐19 recovery. 8
FIGURE 1.
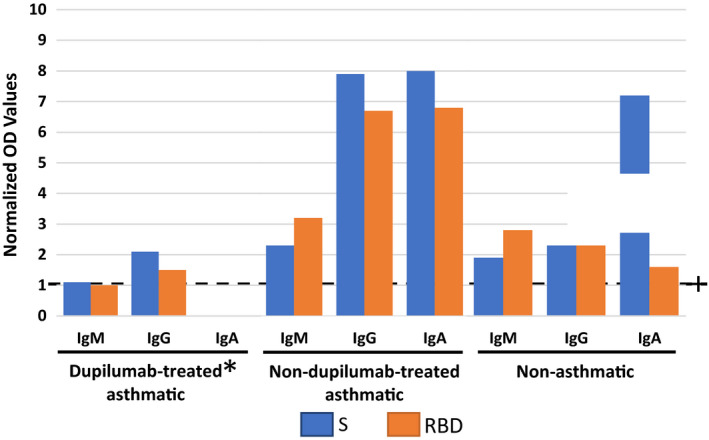
Serum antibodies against SARS‐CoV‐2 in dupilumab‐treated asthmatic and in two nondupilumab‐treated patients (one of whom was on inhaled steroid and the other steroid‐naïve nonasthmatic). The level of detection was determined using the optical density (OD) of pre‐COVID sera. Levels of antibody are determined for each antibody/antigen combination by determining a cutoff value (optical density + 2 standard deviations for pre‐COVID plasma), and optical density values are divided by this cutoff value. Levels above one are considered positive (dashed line). Dupilumab‐treated patient remained PCR positive for 9 wk, while the nondupilumab‐treated patients tested negative between 4 and 5 wk
In conclusion, we suggest that while dupilumab, when used along with glucocorticosteroids for asthma may be safe during SARS‐CoV‐2 pandemic, further research is necessary to understand its effect on the sero‐immune responses to the virus, and whether it may prolong the time to recover from the infection.
CONFLICT OF INTEREST
Dr Bhalla is supported by the Frederick E. Hargreave fellowship in Airway Diseases. Ms Radford, Ms Kjarsgaard, and Dr Nazy do not have any disclosures. Dr Mukherjee is supported by investigator award from Canadian Institutes of Health Research and Canadian Allergy, Asthma, and Immunology Foundation. She has received grants from Methapharm Specialty Pharmaceuticals and honorarium from AZ. Dr Bowdish is supported by a Canada Research Chair in Aging & Immunity. She has received honoraria from Pfizer, Astra Zeneca, and Abbvie. Dr Nair is supported by the Frederick E. Hargreave Teva Innovation Chair in Airway Diseases. He has received honoraria from AZ, Sanofi, Teva, Merck, Novartis, and Equillium, and his university has received research grants from AZ, Teva, Sanofi, Novartis, BI, and Methapharm.
REFERENCES
- 1. Lommatzsch M, Stoll P, Virchow JC. COVID‐19 in a patient with severe asthma treated with Omalizumab. Allergy. 2020;75:2705‐2708. 10.1111/all.14456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. García‐Moguel I, Díaz Campos R, Alonso Charterina S, Fernández Rodríguez C, Fernández CJ. COVID‐19, severe asthma, and biologics. Ann Allergy Asthma Immunol. 2020. 10.1016/j.anai.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Förster‐Ruhrmann U, Szczepek AJ, Bachert C, Olze H. COVID‐19 in a patient with severe chronic rhinosinusitis with nasal polyps during therapy with dupilumab. J Allergy Clin Immunol. 2020;146(1):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peters MC, Sajuthi S, Deford P, et al. COVID‐19 related genes in sputum cells in asthma: relationship to demographic features and corticosteroids. Am J Respir Crit Care Med 2020;202(1):83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID‐19. Preliminary report. medRxiv. 2020. 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 6. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS‐CoV‐2 seroconversion in humans. Nat Med 2020;26(7):1033‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blauvelt A, Simpson EL, Tyring SK, et al. Dupilumab does not affect correlates of vaccine‐induced immunity: a randomized, placebo‐controlled trial in adults with moderate‐to‐severe atopic dermatitis. J Am Acad Dermatol. 2019;80(1):158. [DOI] [PubMed] [Google Scholar]
- 8. Liu F, Xu A, Zhang Y, et al. Patients of COVID‐19 may benefit from sustained Lopinavir‐combined regimen and the increase of Eosinophil may predict the outcome of COVID‐19 progression. Int J Infect Dis. 2020;95:183‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]


