Abstract
As it has been shown that lopinavir (LPV) and hydroxychloroquine (HCQ) have in vitro activity against coronaviruses, they were used to treat COVID‐19 during the first wave of the epidemic in Lombardy, Italy. To compare the rate of clinical improvement between those who started LPV/ritonavir (LPV/r)+HCQ within 5 days of symptom onset (early treatment, ET) and those who started later (delayed treatment, DT). This was a retrospective intent‐to‐treat analysis of the hospitalized patients who started LPV/r + HCQ between 21 February and 20 March 2020. The association between the timing of treatment and the probability of 30‐day mortality was assessed using univariable and multivariable logistic models. The study involved 172 patients: 43 (25%) in the ET and 129 (75%) in the DT group. The rate of clinical improvement increased over time to 73.3% on day 30, without any significant difference between the two groups (Gray's test P = .213). After adjusting for potentially relevant clinical variables, there was no significant association between the timing of the start of treatment and the probability of 30‐day mortality (adjusted odds ratio [aOR] ET vs DT = 1.45, 95% confidence interval 0.50‐4.19). Eight percent of the patients discontinued the treatment becausebecause of severe gastrointestinal disorders attributable to LPV/r. The timing of the start of LPV/r + HCQ treatment does not seem to affect the clinical course of hospitalized patients with COVID‐19. Together with the severe adverse events attributable to LPV/r, this raises concerns about the benefit of using this combination to treat COVID‐19.
Keywords: antiviral treatment, COVID‐19, early, hydroxychloroquine, lopinavir, mortality
Highlights
Lopinavir/ritonavir (LPV/r) plus hydroxychloroquine (HCQ) have been repurposed to treat COVID‐19.
We studied the combination of LPV/r plus HCQ in 172 hospitalised patients with COVID‐19.
Early LPV/r plus HCQ treatment was not associated with a faster improvement in the patients clinical condition.
Early LPV/r plus HCQ treatment did not reduce 30‐day mortality.
Eight percent of the patients discontinued the treatment due to severe adverse events.
1. INTRODUCTION
The current coronavirus disease 2019 (COVID‐19) pandemic caused by severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) has seriously affected the public health systems of many countries worldwide (7 823 289 cases and 431 541 deaths as of 16 June 2020). 1
Although most SARS‐CoV‐2 infections are self‐limiting, about 15% of infected adults develop severe pneumonia requiring supplementary oxygen treatment, and 5% progress to critical illness requiring intensive care. 2 , 3 The pathogenetic mechanisms underlying COVID‐19 are still not fully understood, but increasing evidence indicates that the clinical deterioration observed during SARS‐CoV‐2 infection is attributable to direct viral damage followed by virus‐induced immune‐mediated injury. 4 The rapid spread and severity of COVID‐19 has prompted clinicians to identify possible therapeutic strategies on the basis of experimental data or clinical experiences with other coronaviruses such as severe acute respiratory syndrome (SARS) and Middle Eastern respiratory syndrome (MERS).
In late February 2020, Italy was the first Western country to be hit by the COVID‐19 epidemic, with the Lombardy region alone recording 91 917 cases and 16 457 deaths as of 16 June 2020. 5 During the first week of the epidemic, interim guidelines ("vademecum") were provided by the Lombardy section of the Italian Society of Infectious and Tropical Diseases (SIMIT), proposing the lopinavir/ritonavir (LPV/r) and hydroxychloroquine (HCQ) combination as a therapeutic protocol for hospitalized patients with the respiratory symptoms associated with COVID‐19. 6 , 7 This indication was based on the experimental studies showing that HCQ (an antimalarial drug that is also widely used to treat autoimmune disorders) has in vitro antiviral activity of against SARS‐CoV‐1, human coronavirus 229E (HCoV‐229E) and SARS‐CoV‐2, 8 , 9 , 10 and it has been postulated that it may benefit patients with COVID‐19 because of its modulatory effects on the production and release of tumor necrosis factor 1 (TNF‐1) and interleukin‐6 (IL‐6), both of which are thought to be involved in the inflammatory damage associated with late‐stage COVID‐19. 10 , 11 There were also data indicating that LPV, an HIV‐1 aspartate protease, has in vitro activity against SARS‐CoV‐1 and MERS coronavirus (MERS‐CoV), 12 , 13 and a clinical study conducted in Hong Kong in 2003 found that the addition of LPV co‐formulated with ritonavir (LPV/r) to a standard treatment protocol (ribavirin plus steroid therapy) was associated with improved clinical outcomes of patients affected by SARS‐CoV‐1. 14
However, very recent studies have questioned the clinical efficacy of LPV/r and HCQ against COVID‐19. In particular, one randomized controlled trial comparing the efficacy of LPV/r with that of standard of care in patients with severe COVID‐19 did not find any significant differences in mortality, clinical improvement or viral shedding, 15 and an observational study carried out in New York did not find any difference in mortality between severely ill patients with COVID‐19 who received HCQ and those who did not. 16 However, neither of these studies considered the possible effect of the timing of the start of treatment, although there is evidence that early treatment is crucial when assessing efficacy against acute respiratory infections. 17 , 18 , 19 , 20
The aim of this study was to analyze the combined effect of LPV/r and HCQ treatment on the course of COVID‐19 by examining the differences in clinical outcomes of patients who started treatment within 5 days of the onset of symptoms and those who started later.
2. MATERIALS AND METHODS
This retrospective cohort study involved patients with COVID‐19 pneumonia who were hospitalized at Luigi Sacco Hospital, Milan, Italy, between 21 February and 20 March 2020. COVID‐19 pneumonia was diagnosed on the basis of the detection of SARS‐CoV‐2 RNA on nasopharyngeal swab using a real‐time reverse‐transcriptase polymerase chain reaction (RT‐PCR) test processed using the automated ELITe InGenius® system and the GeneFinder™ COVID‐19 Plus RealAmp Kit assay (ELITechGroup, Puteaux, France) and a chest X‐ray with signs of pneumonia or ≤93% oxygen saturation (SpO2) while breathing room air. 21
In accordance with the SIMIT drug protocol, all patients with COVID‐19 pneumonia admitted to our hospital during the study period were offered off‐label treatment with LPV/r 400/100 mg (tablet or oral solution) twice daily plus HCQ 200 mg twice daily for a minimum of five and a maximum of 20 days depending on patients' clinical response. 6 , 7 The exclusion criteria were the presence of any condition that would not allow the treatment to be safely administered (including any known allergy or hypersensitivity to the drugs used in the protocol), severe liver or kidney disease, use of medications contraindicated with LPV/r that could not be replaced or discontinued, pregnancy or breast‐feeding, known HIV infection, a history of cardiomyopathy, arrhythmias or conduction disorders, as well as ocular macular disease or retinal damage.
The patients were included in the intention‐to‐treat analysis if they had received at least one dose of the scheduled treatment. Patients who died on the day of starting treatment were excluded from the analysis.
The study was approved by hospital's ethical committee (Comitato Etico Interaziendale Area 1), and all of the study patients gave their written informed consent to the administration of off‐label treatment (informed consent was waived in the case of those undergoing mechanical ventilation).
2.1. Data collection
The collected data included demographic data, the Charlson Comorbidity Index (CCI) unadjusted for age, date of onset of symptoms, signs and symptoms at the time of presentation, laboratory findings and disease severity at the time of starting the study treatment. In accordance with the China Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019‐nCoV) Infection, severity was classified as mild (only slight clinical symptoms and no imaging of pneumonia); moderate (with fever, respiratory symptoms and confirmed pneumonia); severe (with respiratory distress [>30 breaths per minute], or <93% resting oxygen saturation or PaO2/FiO2 < 300 mm Hg) or critically severe (with respiratory failure requiring mechanical ventilation, or shock, or any other organ failure needing intensive care). 22
The patients’ clinical status was monitored from the day of treatment initiation to day 30, and data concerning the requirement of oxygen support, laboratory values, serious adverse events, and discharge or death were recorded. The living status of the patients discharged before day 30 was assessed by means of telephone calls to the patients themselves.
2.2. Outcomes
The primary outcome was clinical improvement, defined as a decrease from baseline of at least two categories of the seven‐category ordinal scale recommended by the WHO R&D Blueprint Group, 23 which consists of 1 = not hospitalized, capable of resuming normal activities; 2 = not hospitalized, but unable to resume normal activities; 3 = hospitalized, but not requiring oxygen supplementation; 4 = hospitalized and requiring oxygen therapy; 5 = hospitalized and requiring high‐flow nasal oxygen therapy, noninvasive mechanical ventilation, or both; 6 = intensive care unit (ICU) hospitalization, requiring invasive mechanical ventilation or extra corporeal membrane oxygenation (ECMO), or both; 7 = deceased.
The secondary outcomes were 30‐day mortality and drug safety, including adverse events leading to premature treatment discontinuation. Adverse events were classified using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
2.3. Statistical analysis
The study population was divided into two groups: an early treatment (ET) group of patients who started LPV + HCQ treatment <5 days from the onset of symptoms; and a delayed treatment (DT) group of patients who started treatment ≥5 days from the onset of symptoms.
The baseline demographic and clinical characteristics of the two groups were compared using the χ 2 (or Fisher's exact test where necessary) for categorical variables, and Wilcoxon's rank‐sum test for continuous variables. The cumulative incidence of clinical improvement from day 1 (treatment start) to day 30 was estimated using death as a competing event and compared between groups using Gray's test. Uni‐ and multivariable logistic regression models were used to assess the influence of the timing of the start of treatment on the probability of 30‐day mortality. All of the factors judged to be clinically relevant to the study outcome were considered possible confounders in the multivariable model. The data were analyzed using SAS software, version 9.4, and a P < .05 was considered statistically significant.
3. RESULTS
Between 21 February and 20 March 2020, 172 patients with COVID‐19 pneumonia started LPV + HCQ treatment at our Hospital and received at least one dose: 43 (25%) in the ET group and 129 (75%) in the DT group. The median time from the onset of symptoms to starting the study treatment was 3 days (interquartile range [IQR], 2.5‐4) in the ET group and 8 days (IQR, 6‐10) in the DT group. The majority of the patients were males (72.1%) in their sixties presenting with moderate (53.4%) or severe disease (34.9%) associated with fever (72.7%).
Table 1 shows the baseline clinical and laboratory characteristics of the patients in the two groups. There were no significant between‐groups differences in terms of their demographic characteristics or disease severity, but the patients in the DT group had a higher burden of comorbidities (median CCI = 3; IQR 1‐5 vs 2, IQR 0‐3; P = .041), and more frequently presented with cough (58.9% vs 39.5%; P = .034) and fever (76.7% vs 60.4%; P = .045). They also had higher median white blood cell (P = .017) and neutrophil counts (P = .030), higher median C‐reactive protein levels (P = .045) and lower median PaO2 levels (P < .001).
Table 1.
Baseline characteristics of the study population at LPV/r + HCQ initiation
| Characteristic | Total (n = 172) | Early treatment (n = 43) | Delayed treatment (n = 129) | P |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 124 (72.1) | 29 (67.4) | 95 (73.6) | .556 |
| Female | 48 (27.7) | 14 (32.6) | 34 (26.4) | |
| Age, median (IQR) | 61.7 (50.9‐72.7) | 64.9 (55.0‐78.0) | 61.7 (50.2‐72.3) | .110 |
| BMI > 30, n (%) | 28 (16.3) | 7 (16.3) | 21 (16.3) | .999 |
| Charlson comorbidity indexa, median (IQR) | 0 (0‐1) | 0 (0‐1) | 0 (0‐1) | .077 |
| Symptoms, n (%) | ||||
| Cough | 93 (35.4) | 17 (39.5) | 76 (58.9) | .034 |
| Dyspnea | 61 (35.4) | 17 (39.5) | 44 (34.1) | .582 |
| Sore throat | 6 (3.5) | 0 (0.0) | 6 (4.6) | .338 |
| Arthralgia/myalgia | 6 (3.5) | 1 (2.3) | 5 (3.9) | .999 |
| Headache | 9 (5.2) | 5 (11.6) | 4 (3.1) | .044 |
| Asthenia | 21 (12.2) | 6 (13.9) | 15 (11.6) | .788 |
| Vomiting and/or diarrhea | 19 (11.0) | 3 (6.9) | 16 (12.4) | .410 |
| Fever > 37.3°C | 126 (72.7) | 26 (60.4) | 100 (76.7) | .045 |
| Disease severity,b n (%) | ||||
| Mild | 14 (8.1) | 7 (16.3) | 7 (5.42) | .125 |
| Moderate | 92 (53.4) | 19 (44.2) | 73 (56.6) | |
| Severe | 60 (34.9) | 16 (37.2) | 44 (38.1) | |
| Critical | 6 (3.5) | 1 (7.7) | 5 (3.9) | |
| Laboratory tests, median value (IQR) | ||||
| White blood cells, ×109/L | 5.73 (4.3‐7.7) | 4.7 (4.4‐7.2) | 5.8 (4.5‐7.9) | .017 |
| Lymphocytes, ×109/L | 0.97 (0.71‐1.22) | 0.92 (0.76‐1.22) | 0.98 (0.71‐1.23) | .505 |
| Neutrophils, ×109/L | 4.1 (2.9‐6.4) | 3.2 (2.5‐5.6) | 4.3 (3.1‐6.5) | .030 |
| Hemoglobin, g/dL | 13.8 (12.8‐14.8) | 13.7 (12.6‐14.4) | 13.9 (12.8‐15.0) | .104 |
| Platelets, ×109/L | 176 (137‐221) | 176 (135‐207) | 177 (141‐229) | .422 |
| D‐dimer, μg/L | 926 (585‐2054) | 929 (590‐2145) | 926 (577‐2037) | .978 |
| PaO2, mm Hg (n = 136) | 70 (61‐80) | 77 (69‐84) | 67 (59‐75) | <.001 |
| C‐reactive protein, mg/L | 51.6 (24.3‐122) | 35.6 (19.0‐95.3) | 58.8 (31.6‐140.8) | .045 |
| Creatinine, mg/dL | 0.96 (0.80‐1.14) | 0.90 (0.76‐1.10) | 0.99 (0.80‐1.14) | .234 |
| Lactate dehydrogenase, U/L | 350 (269‐452) | 321 (243‐448) | 358 (277‐450) | .160 |
| Creatine kinase, U/L | 111 (64‐249) | 109 (74‐184) | 113 (61‐273) | .255 |
| ALT, U/L | 32 (20‐55) | 32 (20‐57) | 32 (21‐55) | .717 |
| Bilirubin, mg/dL | 1.19 (1.05‐1.21) | 1.19 (0.94‐1.20) | 1.2 (1.10‐1.23) | .049 |
| Albumin, g/L | 29 (26‐32) | 29 (26‐32) | 29 (26‐32) | .718 |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; IQR, interquartile range; n, number.
Unadjusted for age.
Disease severity classification proposed by Wu et al. 19
The median duration of LPV/r + HCQ treatment was 6 days (IQR, 5‐8), with no significant difference between the groups.
A total of 40 patients (22.7%) discontinued the treatment before completing the minimum 5‐day course, with no significant difference between the ET and DT group (16.3% vs 25.5%; P = .296). The reasons for discontinuing were a switch to another treatment protocol (n = 18, 45%), adverse events (n = 14, 35%), early discharge (n = 5, 12.5%), death (n = 2, 5%) and possible interaction with other treatments (n = 1).
Sixty patients (34.9%: 19 [11.0%] who prematurely discontinued LPV/r + HCQ treatment and 41 [23.8%] who received it for >5 days) were administered other treatment/s during the study period, including remdesivir (n = 33, 19.2%), tocilizumab (n = 36, 20.9%) or both (n = 10, 5.8%). The proportion of patients who received other treatments was not significantly different between the two groups: remdesivir was given to 4 ET patients (9.1%) and 29 DT patients (22.5%) (P = .057), and tocilizumab was given to, respectively, 6 (13.6%) and 30 patients (23.5%) (P = .193).
3.1. Treatment outcomes
As shown in Figure 1, the cumulative incidence of clinical improvement increased over time from 36.6% on day 10 to 66.3% on day 20 and 73.3% on day 30, with no significant difference between the two groups (P = .213) (Figure 2).
Figure 1.
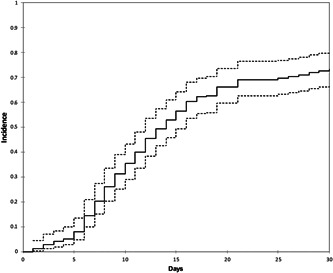
Cumulative incidence of improvement (solid line) and 95%Cis (dashed lines)
Figure 2.
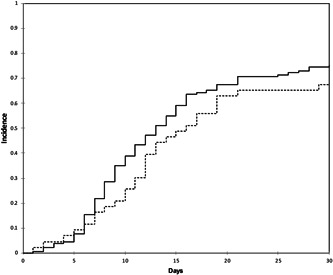
Cumulative incidence of improvement in the early treatment (ET) group (dashed line) vs delayed treatment (DT) group (solid line)
At the end of the study period, 23.2% of the patients in the ET group and 17% of those in the DT group had died. The univariable analysis did not reveal any significant association between the timing of the start of LPV/r + HCQ treatment and the probability of 30‐day mortality (odds ratio [OR] of <5 vs ≥5 days 1.58, 95% confidence interval [CI] 0.70‐3.56; P = .271). After adjusting for relevant clinical variables in the multivariable model, an earlier start of treatment was still not associated with a lower probability of 30‐day mortality (adjusted OR [aOR] of <5 vs ≥5 days 1.45; 95% CI, 0.50‐4.19) (Figure 3). Conversely, age per 10 years more (aOR 2.21; 95% CI, 1.38‐3.57), obesity (aOR 3.90; 95% CI, 1.19‐12.82), and undergoing invasive or noninvasive mechanical ventilation (aOR 4.75; 95% CI, 1.38‐16.34) were all independently associated with an increased probability of death (Figure 3).
Figure 3.
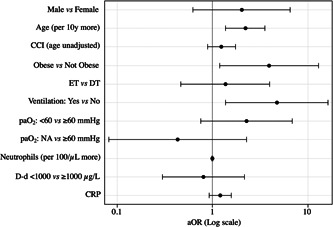
Multivariable model results (adjusted odds ratios). aOR: adjusted odds ratio; CCI, Charlson Comorbidity Index; CRP, C‐reactive protein; DT, delayed treatment group; D‐d, D‐dimer; ET, early treatment group; Log, logarithmic; NA, not assigned; paO2, partial oxygen pressure
3.2. Safety
The most frequent adverse events were an increase in hepatic enzymes to at least five times above the normal values (13 patients, 7.6%), and grade 2 to 3 nausea and/or diarrhea (14 patients, 8.1%). The treatment was discontinued in all of the 14 patients who developed grade 2 to 3 gastrointestinal disorders.
4. DISCUSSION
The spread of the COVID‐19 pandemic and the exponential increase in deaths worldwide has made the demand for clinical evidence concerning new and pre‐existing drugs increasingly pressing. Various molecules, including antivirals and immune modifiers, were rapidly evaluated in initial uncontrolled studies and are now being investigated in randomized controlled trials.
The search for an effective treatment of COVID‐19 also needs to consider the optimal time to start the use of effective drugs, taking advantage of the emerging data concerning the pathogenetic mechanisms underlying different stages of the disease. As it has been shown that the pathogenesis of COVID‐19 includes a viremic phase that peaks 5 to 6 days after infection, followed by an immune‐mediated phase characterized by an aggressive inflammatory response that is largely responsible for airway damage, 4 it is possible to hypothesize that the early use of effective antiviral drugs would reduce the progression and mortality of COVID‐19, as has been observed in the case of other acute viral respiratory illnesses. 17 , 18 , 19 , 20
However, our study assessing possible differences in the clinical outcomes of patients who received LPV/r + HCQ < 5 or >5 days after symptom onset did not reveal any difference in the time to clinical improvement or in the probability of 30‐day mortality between the two groups. This raises some doubts about the in vivo effect of LPV/r + HCQ treatment on SARS‐CoV‐2, which are also supported by emerging pharmacological considerations. It has been recently estimated that the protein‐adjusted 90% inhibitory concentrations (PA‐IC90) of LPV required to inhibit SARS‐CoV‐2 replication in plasma, epithelial lining fluid (ELF) and cerebrospinal fluid (CSF) are respectively 200‐fold, 20‐fold and 2000‐fold higher than those measured in vivo. 24 Moreover, a recently published mechanistic model has shown that, instead of the conventional lower dose of ≤400 mg/day, HCQ doses of >400 mg twice daily for ≥5 days would be required to obtain a rapid decrease in viral load, a reduction in the proportion of patients with detectable SARS‐CoV‐2 infection, and shorter treatment courses, 25 but it has been predicted that doses of >600 mg twice daily would prolong the QT interval and lead to a risk of arrhythmias, including torsade de pointes. 25
A total of 14 (8.1%) patients in our study were unable to complete the minimum 5‐day course of LPV/r +HCQ because of adverse events. The most frequent severe adverse events were gastrointestinal disorders (nausea and/or diarrhea) mainly attributed to LPV/r. Interestingly, a recent study has found that the trough plasma concentrations of LPV measured in COVID‐19 patientsare three times higher than those measured in patients who take the same dose to treat chronic HIV infection, which may explain why COVID‐19 patients poorly tolerate LPV. 26 Furthermore, Cao et al 15 found that nearly 14% of the patients who received LPV/r in their randomized trial could not complete the full course of 14 days mainly because of gastrointestinal intolerance and, as they did not find that LPV/r had a beneficial effect on the clinical course of COVID‐19, they suggest that its use may expose COVID‐19 patients to unnecessary toxicities.
Our study has a number of limitations. First, given the emergency context in which it was carried out, it was impossible to include a control group, and so we cannot exclude the possibility that the patients whose status improved after LPV/r + HCQ treatment would have improved regardless of any treatment. Second, a relatively large proportion of our patients received other experimental treatments during the study period, and this is clearly a confounding factor when analyzing the efficacy LPV/r + HCQ. However, as there was no between‐group difference in the proportion of patients who received other treatments, it is likely that this had no impact on our analysis of the effect that the time of starting treatment had on COVID‐19 outcomes.
Third, the treatment's virological efficacy (ie the reduction in viral load in nasopharyngeal secretions) could not be assessed because there was no regular monitoring of the presence of SARS‐CoV‐2 genome on nasopharyngeal swabs and the RT‐PCR available in our microbiology department only provides qualitative data.
Finally, the study was conducted in the ever‐changing scenario created by the dramatic escalation of the epidemic in Northern Italy. The Infectious Diseases Department of Luigi Sacco Hospital acts as a north Italian reference center for infectious diseases. Consequently, our findings concerning the potential use of LPV/r + HCQ relate to hospitalized patients in the early wave of the Italian pandemic and may not extend to outpatients with milder symptoms.
In conclusion we found that starting LPV/r + HCQ treatment within 5 days of symptom onset was not associated with a more rapid improvement in the clinical condition of patients hospitalized with COVID‐19 or a reduced probability of 30‐day mortality. Together with the relatively high rate of severe adverse event attributable to LPV/r, this raises some doubts about the benefit of combined LPV/r and HCQ treatment of COVID‐19. More rigorous controlled studies are needed to assess the real benefit‐to‐harm ratio of LPV/r and HCQ, and the use of the combination should be discouraged in other contexts.
CONFLICT OF INTERESTS
AG has received consultancy fees from Mylan and nonfinancial educational support from Gilead. GR has received grants and fees for speaker bureaux, advisory boards and CME activities from BMS, ViiV, MSD, AbbVie, Gilead, Janssen and Roche. SR has received grants, fees for speaker bureaux, advisory boards and CME activities from BMS, ViiV, MSD, AbbVie, Gilead and Janssen. CG has received grants and fees for speaker bureaux, advisory boards and CME activities from BMS, ViiV, MSD, AbbVie, Gilead, Janssen. DC has received grants and fees for speaker bureaux, advisory boards and CME activities from BMS, ViiV, MSD, Gilead, Janssen. SA has received support for research activities from Pfizer and Merck Sharp & Dome. MG has received grants and fees for speaker bureaux, advisory boards and CME activities from BMS, ViiV, MSD, AbbVie, Gilead, Janssen and Roche. GP, ALR, LO, FC, LP, LB, GC, SP, CB, VM, CC, EC and RC have nothing to declare.
AUTHOR CONTRIBUTIONS
AG and GP designed the study. LO, AG, and GP were responsible for the statistical analysis. All authors contributed in the patient's enrolment, data collection and interpretation. AG and GP had contributed to the preliminary draft of the manuscript. ALR and MG critically revised the manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank all the patients and their families, as well as the medical staff (paramedics, nurses and physicians) who are making every effort to ensure the best care for patients suffering from coronavirus disease.
Giacomelli A, Pagani G, Ridolfo AL, et al. Early administration of lopinavir/ritonavir plus hydroxychloroquine does not alter the clinical course of SARS‐CoV‐2 infection: A retrospective cohort study. J Med Virol. 2021;93:1421–1427. 10.1002/jmv.26407
Andrea Giacomelli, Gabriele Pagani, and Anna L. Ridolfo contributed equally to the study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐19) Situation Report– 111. Data as received by WHO from national authorities by 10:00 CEST, 15 June 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200615-covid-19-sitrep-147.pdf?sfvrsn=2497a605_4
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giacomelli A, Ridolfo AL, Milazzo L, et al. 30‐day mortality in patients hospitalized with COVID‐19 during the first wave of the Italian epidemic: A prospective cohort study [published online ahead of print, May 22, 2020]. Pharmacol Res. 2020;158:104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:1‐12. 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Covid‐19 Regione Lombardia, 16 June 2020. https://experience.arcgis.com/experience/0a5dfcc103d0468bbb6b14e713ec1e30/
- 6. Società Italiana di Malattie Infettive e Tropicali . Sezione regione Lombardia. Linee guida sulla gestione terapeutica e di supporto per pazienti con infezione da coronavirus COVID‐19. Edizione marzo 2020. http://www.simit.org/medias/1555-covid19-linee-guida-trattamento-01mar.pdf
- 7. Lombardy Section Italian Society Infectious And Tropical Diseases . Vademecum for the treatment of people with COVID‐19 Infez Med. 2020;28:143–152. [PubMed] [Google Scholar]
- 8. Keyaerts E, Vijgen L, Maes P, Neyts J, Ranst MV. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blau DM, Holmes KV. Human coronavirus HCoV‐229E enters susceptible cells via the endocytic pathway. In: Lavi E, Weiss SR, Hingley ST, eds. The Nidoviruses. Advances in Experimental Medicine and Biology. 494. Boston, MA: Springer; 2001. [DOI] [PubMed] [Google Scholar]
- 10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Conti P, Ronconi G, Caraffa A, et al. Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by Coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): anti‐inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327‐331. [DOI] [PubMed] [Google Scholar]
- 12. Chen F, Chan KH, Jiang Y, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31:69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan JF, Chan K‐H, Kao RY, et al. Broad‐spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013;67:606‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan KS, Lai ST, Chu CM, et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9:399‐406. [PubMed] [Google Scholar]
- 15. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe covid‐19. N Engl J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid‐19. N Engl J Med. 2020;382:2411‐2418. 10.1056/NEJMoa2012410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta‐analysis of randomised controlled trials. Lancet. 2015;385:1729‐1737. [DOI] [PubMed] [Google Scholar]
- 18. Kim SJ, Kim K, Park SB, Hong DJ, Jhun BW. Outcomes of early administration of cidofovir in non‐immunocompromised patients with severe adenovirus pneumonia. PLOS One. 2015;10:e0122642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oboho IK, Reed C, Gargiullo P, et al. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory‐confirmed influenza. J Infect Dis. 2016;214:507‐515. [DOI] [PubMed] [Google Scholar]
- 20. Ko J‐H, Lim JU, Choi JY, et al. Early cidofovir administration might be associated with a lower probability of respiratory failure in treating human adenovirus pneumonia: a retrospective cohort study. Clin Microbiol Infect. 2019. pii: S1198‐743X(19)30545‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. WHO. Clinical management of severe acute respiratory infection when COVID‐19 is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected
- 22. Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID‐19) in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020;71:706‐712. 10.1093/cid/ciaa199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. WHO R&D Blueprint and COVID‐19 . https://www.who.int/teams/blueprint/covid-19 [Google Scholar]
- 24. Cattaneo D, Corbellino M, Clementi E, Galli M, Riva A, Gervasoni C. Does lopinavir really inhibit SARS‐CoV‐2? Pharmacol Res. 2020;158:104898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia‐Cremades M, Solans BP, Hughes E, et al. Optimizing hydroxychloroquine dosing for patients with COVID‐19: an integrative modeling approach for effective drug repurposing. Clin Pharmacol Ther. 2020;108:253‐263. 10.1002/cpt.1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baldelli S, Corbellino M, Clementi E, Cattaneo D, Gervasoni C. Lopinavir/ritonavir in COVID‐19 patients: maybe yes, but at what dose? Journal of Antimicrobial Chemotherapy. 2020. 10.1093/jac/dkaa190 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


