Abstract
We have provided an overview on the profound impact of COVID‐19 upon older people with Alzheimer's disease and other dementias and the challenges encountered in our management of dementia in different health‐care settings, including hospital, out‐patient, care homes, and the community during the COVID‐19 pandemic. We have also proposed a conceptual framework and practical suggestions for health‐care providers in tackling these challenges, which can also apply to the care of older people in general, with or without other neurological diseases, such as stroke or parkinsonism. We believe this review will provide strategic directions and set standards for health‐care leaders in dementia, including governmental bodies around the world in coordinating emergency response plans for protecting and caring for older people with dementia amid the COIVD‐19 outbreak, which is likely to continue at varying severity in different regions around the world in the medium term.
Keywords: Alzheimer's disease, COVID‐19, dementia, older people
1. EXECUTIVE SUMMARY
The coronavirus disease 2019 (COVID‐19) pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) poses a serious threat to older people with Alzheimer's disease (AD) and other dementias. Recent data suggest that apart from old age and medical comorbidities (eg, hypertension, diabetes), people with dementia are associated with an increased risk of having severe COVID‐19 and mortality associated with it. 1 , 2 , 3 , 4 , 5 In addition, the related public health interventions (eg, physical distancing or lockdown) have major adverse impacts upon the well‐being and the care of older people with dementia, and for those caring for them.
Recent simulation models suggested that outbreaks will recur after the initial wave of infections and that prolonged or intermittent physical distancing for more than a year may be required or until vaccination is available, which is expected to take 12 to 18 months or longer. 6 Although these simulation models may not be reliable, it will be prudent to take these predictions as the worst case scenario. Given this possibility that the threat of COVID‐19 may continue in the medium term, we aim to review the association between dementia and old age (the strongest risk factor for AD and other common dementias) with COVID‐19, including the association between apolipoprotein E (ApoE4) and COVID‐19 and the impact of COVID‐19 upon the brain and cognition. We also highlight the challenges encountered in the care and management of older people with dementia in different settings and propose strategies that health‐care providers (HCP) can take to tackle these challenges in regions with ongoing outbreaks and to enhance preparedness for recurrent outbreaks.
In this article, the term “people with dementia” in general encompasses those with any degree of severity, including mild cognitive impairment. Given that people with young onset dementia, including frontotemporal dementia, may have challenges different to that of older people with common dementias (eg, AD, vascular dementia [VD], dementia with Lewy bodies [DLB]), the present review will focus more on older people with dementia. The adverse impacts of COVID‐19 on clinical dementia research and related adaptive strategies were discussed in an earlier editorial of the Journal and will not be discussed here. 7
2. ASSOCIATIONS AMONG DEMENTIA, OLD AGE, AND COVID‐19
2.1. Increased risk of infection in people with dementia and older people
People with dementia are particularly vulnerable to being infected by and spreading SARS‐CoV‐2 because they may not adequately comprehend, execute, or recall any of the suggested public health measures (eg, physical distancing, use of face masks). Those with agitation, wandering, or disinhibition are probably at even higher risk of catching and spreading the infection. Physical distancing is not feasible for those who are dependent on others for performing their basic activities of daily living (ADL; eg, bathing), such as those with more severe dementia or VD/DLB with concurrent major physical disability. Many dementia or older patients are residing at care homes and such residents in congregant living situations are often living in close proximity with each other and share common areas (eg, dining and living rooms) and are therefore at high risk of infection. Moreover, because older people who are infected may present with non‐specific symptoms, for example, altered general activity, falls, or delirium without the typical COVID‐19 symptoms of fever, cough, and difficulty breathing, 8 their informal or professional caregivers may become infected as they have not been warned in time to take necessary precautions.
2.2. Increased risk of poor outcomes in older people and those with dementia
About 20% to 40% of COVID‐19 cases have been people older than 65 years. 9 , 10 , 11 Once infected with SARS‐CoV‐2, the risk of poor outcomes (eg, hospitalizations, severe pneumonia, need of ventilatory support, death) is high for older people with fatality rates ranging from 14.8% in China, to 25.5% in Korea, to as high as 41.8% for males (21.6% for females) in Italy among those 80 years or older. 1 , 12 , 13 In Europe, those older than 65 years accounted for more than 95% of COVID‐19 related deaths. 14 In the United Kingdom (UK), the OpenSAFELY platform showed that those 80 years or older were associated with a 12‐fold risk increase in mortality compared to those ages 50 to 59 years and males were associated with an almost two‐fold risk increase in mortality compared to females. 3 Reasons explaining the increased risk among older people (in particular among older men) may include inflamm‐aging and immune senescence. 15
RESEARCH IN CONTEXT
Systematic review: We reviewed the literature using PubMed on COVID‐19 and older people with dementia. While there are reviews on the neurological manifestations and involvement of COVID‐19, none had discussed the association between COVID‐19 and dementia. Moreover, an emergency response protocol with respect to the care for community dwelling dementia patients during lockdown does not exist in the literature.
Interpretation: Infected people with dementia and apolipoprotein E (ApoE4) carriers are at increased risk of severe disease and possibly of cognitive decline. We proposed a conceptual framework for home‐based care for older people with dementia and suggestions to optimize care at care homes and hospital settings.
Future directions: Studies are needed to evaluate the complete short‐ and long‐term cognitive impact of COVID‐19 upon the aging societies and the health outcomes of the proposed emergency response strategies, including cognitive telemedicine.
The OpenSAFELY platform also showed that mortality was associated with pre‐existing dementia/stroke (hazard ratio 1.79; independent of age). 3 Data from the UK Biobank suggested an association between pre‐existing dementia (odds ratio [OR] = 3.07) 5 and homozygous ApoE4 genotype (a known risk factor for AD; OR = 2.31) 16 with severe COVID‐19, as defined by having clinical signs (eg, pneumonia) necessitating hospitalization.
Possible reasons explaining the association between pre‐existing dementia and severe COVID‐19 include patients with dementia may be prone to having a high viral load because they are unable to comply with the health measures as discussed above, a majority of these dementia patients might come from care homes where there were high rates of infection, or a direct effect secondary to the underlying brain pathologies. Note further that if hospitals and intensive care units (ICU) are overwhelmed and ventilators or personal protective equipment (PPE) are in short supply, rationing of health care may be required, which could result in older people or those with dementia being denied intensive/ventilatory support, resulting in higher mortality. 17
The mechanisms explaining the potential association between homozygous ApoE4 genotype and severe COVID‐19 can be multiple. In the brain, ApoE4 is known to exacerbate microglia‐mediated neuroinflammation and subsequent neurodegeneration. 18 , 19 Peripherally, ApoE4 has also been shown to increase macrophage production of cytokine (eg, interleukin 6, tumor necrosis factor, compared to E2 or E3) in response to proinflammatory stimuli. 20 As cytokine storm is thought to account for COVID‐19 disease manifestations, ApoE genotype may thereby augment disease severity by impacting host immune response.
Other mechanisms by which ApoE4 interacts with viruses have been reported. For instance, herpes simplex virus‐1 infection involving the brain may be facilitated by ApoE4, 21 which could in part be related to the blood‐brain‐barrier (BBB) breakdown effect of ApoE4. 22 , 23 On the other hand, the ApoE genotype has been reported to influence human immunodeficiency virus cell entry, and interacts with hepatitis C virus non‐structural protein 5A. 21 It remains to be determined whether similar interactions may exist between ApoE and SARS‐CoV‐2 virus that may influence disease severity.
2.3. Adverse impacts of COVID‐19 upon the brain and cognition
The first report on the neurological manifestations of COVID‐19 showed that “impaired consciousness,” including reduced consciousness level or delirium occurred more frequently among severe COVID‐19 patients (13/88, 14.8%) compared to those with less severe disease (3/126, 2.4%). 24 While overt stroke also occurred more frequently (5/88, 5.7%, four ischemic stroke, and one hemorrhagic stroke) among those with severe disease compared to those with less severe disease (1/126, 0.8%). A single case of seizure was also reported among those with severe disease (1/88, 1.1%). Those with more severe disease tend to be older (mean age 58 years) than those with less severe disease (mean age 48.9 years). In a later study among COVID‐19 patients with acute respiratory distress syndrome (n = 58, median age 63 years) admitted to an ICU, agitation was reported in 69% (40/58) of patients, and two thirds of these agitated patients (26/40, 65%) were confused as well. 25 Among 13 encephalopathic patients who had brain magnetic resonance imaging (MRI), three (23%) showed evidence of acute/subacute ischemic strokes (two were silent strokes) on diffusion‐weighted imaging. Among 11 patients who had perfusion imaging, all revealed bilateral frontotemporal hypoperfusion. Cerebrospinal fluid was obtained from only seven patients; all showed no cells and reverse transcriptase polymerase chain reaction assays were negative for SARS‐CoV‐2 for all patients. 25 Other case reports showed that impaired consciousness in COVID‐19 could be due to direct viral invasion (ie, meningoencephalitis) 26 or to possible intracranial inflammation (ie, peripheral inflammatory cytokines triggering intracranial cytokine storm). 27 , 28 COVID‐19 related causes explaining impaired consciousness/delirium/agitation may be multiple, including hypoxia (as suggested by the bilateral frontotemporal hypoperfusion), 25 stroke (overt or subclinical), 24 , 25 postictal state, 24 inflammation, 27 , 28 and/or even direct viral invasion. 26 Overall, “impaired consciousness” was more common among those who died (22%) than those who recovered (1%). 29
It is important to recognize the brain and the vascular endothelium lining of the BBB expresses the angiotensin‐converting enzyme 2 (ACE2) receptor (ie, the SARS‐CoV‐2 receptor) that may facilitate SARS‐CoV‐2 entry into cells. 30 , 31 The virus may possibly cross the BBB either by directly infecting the vascular endothelial cells followed by spread to glial cells or by having infected peripheral leukocytes crossing the BBB (ie, Trojan horse mechanism). 31 , 32 An autopsy study showed that SARS‐CoV‐2 RNA could be detected in the brain tissues of 8 out of 21 patients (38%) and those with brain involvement were older (mean age 80 years) than those without (mean age 72 years). 33 One may speculate that in patients with increased BBB permeability associated with AD, cerebral small vessel disease, autonomic dysfunction associated with neurodegenerative conditions, longstanding cardiovascular comorbidities, or aging, there is an increased risk of SARS‐CoV‐2 being able to cross the BBB. The virus may also enter the brain via the olfactory nerve (ie, retrograde spreading via transsynaptic transfer) as anosmia is a common (as high as 80%) early symptom of COVID‐19. 31 , 34 , 35 A COVID‐19 case presenting with anosmia whose MRI revealed hyperintense signal at the olfactory bulb and the gyrus rectus (suggesting possible neuro‐invasion) was reported. 35 Some postulated the virus may spread from the olfactory nerve to the brainstem, possibly accounting for the respiratory failure of severe COVID‐19, which is typically characterized by lack of dyspnea, 36 while others have postulated that the SARS‐CoV‐2 may spread via transsynaptic transfer to the brainstem from the mechanoreceptors and chemoreceptors in the lung and lower respiratory airways. 37 Such postulates require confirmation from pathological study. Again here, the ApoE4 genotype may also play a pivotal role in facilitating viral entry into the brain as discussed above.
The mechanisms underlying the increased risk of stroke in COVID‐19 may be related to a prothrombotic state, vascular endothelial dysfunction related to SARS‐CoV‐2 infection (ie, endotheliitis), or thrombocytopenia. 24 , 32 , 38 , 39 , 40 Such mechanisms may induce a large 38 or small vessel ischemic stroke (can be subclinical), 25 cerebral venous thrombosis, 41 or intracerebral/subarachnoid hemorrhage. 24 , 40
Taken together, people with dementia are at increased risk of infection. Once becoming infected, people with dementia, ApoE4 genotype carriers, and older people are associated with severe disease and increased mortality. Older people with more severe disease are at increased risk for reduced consciousness level, delirium, stroke, or possibly intracranial inflammation or viral neuro‐invasion. The pre‐existing brain vulnerability, including lack of brain resilience/cognitive reserve and increased BBB permeability, may make those with dementia/older patients at increased risk of various neurological complications, including decline in cognitive functions, which may be irreversible. Note that a third of the COVID‐19 patients discharged from the ICU were observed to have a dysexecutive syndrome. 25 Further studies are warranted to ascertain the complete short‐, medium‐, and long‐term impact of COVID‐19 upon the brain and cognition, especially among patients with dementia, ApoE4 carriers, or older people.
3. CHALLENGES IN THE CARE AND MANAGEMENT OF OLDER PEOPLE WITH DEMENTIA
3.1. Hospital settings
Apart from the likelihood of having a more severe disease (eg, pneumonia) and various neurological complications as discussed above, there are other challenges encountered with respect to the management of hospitalized older patients with dementia. The need to cohort patients or to isolate them in a single room for an extended period in an unfamiliar environment with lack of support from relatives because of visitation restrictions further increases the risk of delirium. 42 Agitation and wandering make compliance with infection control procedures extremely difficult: patients may remove masks, enter the bed spaces of other patients, come in contact with high‐touch surfaces or equipment, or reach out and touch clinical staff. Nasopharyngeal swabbing or blood sampling may not be possible.
Occult infection may be a particular issue in older patients in whom clinical presentations may be atypical as stated earlier. These patients are admitted initially to COVID‐19 “negative” areas and delayed diagnoses of occult or atypical cases may lead to spread of infection. Subsequent patient movement from “negative” to “positive” areas further increases likelihood of delirium and loss of continuity of care. Overall, the need for isolation and modification of some health‐care practices may increase the risk of other preventable harms related to dementia, such as falling, inadequate nutrition, and pressure sores.
Discharge planning is also more complex and delays to discharge are more likely. Care homes may be reluctant to take back patients for fear of infecting other residents. Patients who recovered from COVID‐19 or who tested negative may not be able to return to their usual care home residence owing to family and patient concerns that the particular care home has other residents with the disease. Furthermore, for patients with dementia living at home, professional caregivers may refuse to continue to care for a person with recent COVID‐19 infection.
3.2. Outpatient settings
During a lockdown, outpatient clinics for people with dementia are often suspended to attempt to reduce transmission or to redeploy staff to work at an infection ward. While usual medications will be continued in patients, changes or deterioration in clinical status requiring adjustment in medications, or other interventions, may be missed. Scheduled blood taking or neuroimaging appointments may be missed as well. Patients and caregivers may suffer as a consequence of losing the support that specialist clinics provide. Moreover, with postponement of appointments for new referrals, diagnosis and initiation of appropriate management may be delayed for individuals suffering from incipient dementia, adding to patient distress and caregiver burden. In an extreme case, some patients/caregivers may not return to picking up their usual medications (eg, anti‐thrombotics) and serious consequences (eg, stroke, myocardial infarction) may occur as a result of medication discontinuation.
3.3. Care home settings (eg, assisted living facilities, nursing homes)
As high as three quarters of COVID‐19 deaths were reported to occur in care homes in some regions because of reasons discussed above. 43 , 44 , 45 In addition, infection control training and PPE for staff may be inadequate in some of these care homes. Personal service workers may travel between different facilities providing part‐time care to residents in different facilities increasing the likelihood of spread to regular staff and residents. Taken together, both residents and staff are at high risk of infection. Because visits from relatives are usually banned and social or physical activities are cancelled, lack of social engagement, physical exercise, and emotional support may worsen the cognitive, behavioral, and physical condition of the residents.
3.4. Community dwelling people with dementia and informal caregivers
The need for people with dementia to be confined at home for extended periods is commonly associated with frustration or other behavioral symptoms. Preoccupation with negative news of the pandemic may also trigger negative emotions. In addition, many day‐to‐day activities that are beneficial to people with dementia, for example, outdoor exercise, social engagement, and/or recreational or rehabilitation programs that are conducted at older people's centers or day care centers are suspended or reduced, which may lead to worsening in cognitive, behavioral, and physical conditions. When acute medical problems occur (eg, chest pain), caregivers may be less inclined to bring patients to hospital for fear of infection. Similar concerns apply to visiting general practitioners in the community. Such delays in seeking medical help may increase risk of poor clinical outcomes, in such conditions as myocardial infarction, stroke, etc. The increased risk of cognitive decline or other clinical events associated with prolonged lockdown applies also to older people in general.
Home support for personal care or household chores may not be accessible during the outbreak. Caregivers may miss the respite during the daytime when their loved ones were being cared for at a day care center. Home visits from relatives and friends, and other social activities may also not be feasible. Caregivers may feel isolated, with no close family members nearby, all the local shops being closed, or being unable to travel or terrified of going out. Weeks to even months of being confined at home with an older patient may increase levels of stress, resulting in a feeling of helplessness and depression. Together, deterioration of psychological well‐being in both patients and caregivers triggers a vicious cycle that may result in worsening of overall health and quality of life for both parties.
4. COMMUNITY HOME‐BASED CARE STRATEGIES
Given that COVID‐19 pandemic is an unprecedented crisis faced by aging societies around the world, emergency response protocols with respect to care for community dwelling dementia patients during lockdown may not exist and are urgently needed (Figure 1). 46 The objectives of the strategies are to protect them from getting infected with COVID‐19 because of the potential serious complications associated with COVID‐19 and to mitigate the adverse impacts associated with the public health interventions as discussed above. 46
As hospitals may be overwhelmed during a major outbreak, community‐based HCP may need to assume a greater role or to be mobilized in supporting home‐based care for people with dementia during a lockdown. In most developed regions, community‐based services for dementia may include older people's centers, day care centers, groups that provide home personal care (eg, bathing, household chores), home nursing care (eg, wound care), or home rehabilitation (eg, physio‐ or occupational therapy), and care homes, and involvement of general practitioners/specialists, pharmacists, regional dementia support organizations, professional societies, or other volunteer groups. Ideally, community‐based HCP should be proactive in reaching out to needy families during the outbreak, for example, by setting up special emergency hotlines for vulnerable groups. Crises such as the COVID‐19 pandemic may create an opportunity for community‐based HCP in identifying needy families who are new to them and may benefit from their services.
In addition, in response to a crisis, the social support network of the patients/caregivers, which commonly consists of other family members (eg, children, grandchildren), friends, and/or neighbors, may evolve to provide care and support to families in need. This social network plays an even greater role in regions like India, Africa, or in rural regions where strong family‐based support networks already exist and institutional long‐term care services are less developed or needed.
On a macroscopic level, the health‐care leadership/governmental bodies should not only educate/update the community on public health measures, but also increase society's awareness of the challenges faced by vulnerable people groups (eg, older people with dementia/caregivers) 47 and initiate discussion/projects on how to provide tailor‐made care for patients/caregivers among various stakeholders (and to support these projects).
In situations in which one can identify people with dementia who are living alone without adequate support from their social network or community‐based services during the outbreak, social welfare service/patients’ guardians (if available) may probably need to discuss the option with the person of arranging urgent temporary placement to a care home as a last resort. Note however that because congregant living communities are at high risk of infection, such settings should follow governmental guidance for infection control. This category of people with dementia will not be further discussed here. Protocols for home care for patients with COVID‐19 presenting with mild symptoms are not the scope of this article. 48
FIGURE 1.
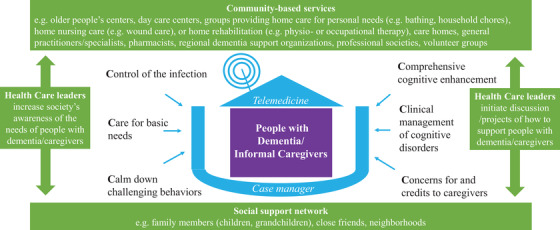
Conceptual framework of home‐based care strategies amid the COVID‐19 pandemic. It will be ideal if a case manager can help coordinate care across the continuum of community‐based services and partner with persons from the social support network, with special focus in managing the following key areas: implementing infection control measures at home, care for basic needs, tackling behavioral problems, maintaining brain‐healthy life‐style activities, managing medical/cognitive problems, showing concern for and appreciation to caregivers. Although user‐friendly telehealth technologies can facilitate better care delivery in many ways, making simple phone calls may sometime serve a similar purpose
During lockdown (ie, staying at home is required except for essential purposes, such as for fulfilling medical needs or grocery shopping), we propose that HCP can partner with persons from patients’ social support network in managing the following key areas that are relevant to dementia care (Table 1):
Control of the infection (ie, implementation of home‐based health measures)
Care for basic needs (ie, providing care for basic needs at home)
Calm down challenging behaviors (ie, tackling behavioral problems at home)
Comprehensive cognitive enhancement (ie, recommendations for home‐based lifestyle activities and rehabilitation programs)
Clinical management of cognitive disorders (ie, managing medical and cognitive problems at home)
Concerns for and give credits to caregivers
TABLE 1.
Suggestions for home‐based care strategies amid COVID‐19 pandemic
|
Control of the infection (ie, implementation of home‐based health measures)—for example,
|
|
Care for basic needs (ie, providing care for basic needs at home)—for example,
|
|
Calm down challenging behaviors (ie, tackling behavioral problems at home)—for example
|
|
Comprehensive cognitive enhancement (ie, recommendations for home‐based lifestyle activities and rehabilitation programs)—for example
|
|
Clinical management of cognitive disorders (ie, managing medical and cognitive problems at home)—for example,
|
|
Concerns for and credits to caregivers
|
Abbreviation: HCP, health care providers; PPE, personal protective equipment.
It will be ideal if someone can serve as a “case manager,” 49 such as a nurse/social worker trained in dementia care or even a family member (eg, patient's son or daughter), who can coordinate the care across the continuum of community‐based services according to the needs of patients/caregivers. The proposed conceptual framework provides a reference for HCP to formulate detailed care plans that should be adjusted according to the needs of the patients/caregivers; types of available/affordable community‐based services/technologies; and assistance available from respective social support network, the health system, and the culture. Research is needed to evaluate the health outcomes, applicability, and resource implications of a home‐based care model in different regions.
We propose that health systems should quickly enable the use of telemedicine to facilitate better care for and communication with patients/caregivers. This may involve creating or adopting commercial simple user‐friendly technologies for secure videoconferencing with patients, advocating to reimburse telemedicine visits at the same rate as the equivalent care delivered in person, or providing compatible electronic health records. Ideally, governments should work to make high‐speed internet connections available to their whole populations during outbreaks. Lack of familiarity with the internet may be a barrier to accessing telemedicine for many older people, although telephone consultations, particularly for follow‐up, may be equally effective. The sound quality may be poor on telemedicine and difficult for those with hearing deficits. Also, the screen image may be quite small and many older people have visual impairments and may struggle to attend to such stimuli.
In addition, physicians/other HCP should be made aware of other telemedicine‐related issues on data privacy and security, and legal and insurance support for practice in telemedicine. 50 Physicians/other HCP may need to discuss with caregivers the potential limitations in providing advice without seeing the patients and consent may need to be obtained from patients/close relatives. Research on this and other barriers to cognitive telemedicine in different regions is urgently needed. Moreover, setting up an infrastructure for telemedicine will become useful for management of other patient groups, such as patients having great difficulty in traveling to hospitals because of a major disability and those living in remote areas during the usual times or other crises that may require lockdown of a region for an extended period.
5. STRATEGIES FOR CARE HOMES
Implementing high standards of infection prevention and control measures for care homes is of paramount importance because serious outbreaks that lead to high mortalities/overwhelm hospitals can happen in these institutions. 44 Details of infection prevention and control standards will differ according to regions/institutions and are beyond the scope of this article. 51 , 52 , 53 Regional or national regulatory agents need to monitor closely whether these institutions are compliant with the standards. Each institution should have emergency response protocols ready at any time with drilling (eg, what to do if a resident or staff gets infected with COVID‐19) to enhance preparedness for recurrent outbreaks. Care homes should ensure adequate stock of PPE for the staff. Ideally, all staff (and residents) should receive regular testing for COVID‐19.
All day‐to‐day routines and activities should be redesigned/adjusted so that physical distancing can be maintained, for example, staged meal times, smaller group activities, and keeping a minimum distance (eg, 1.5 meters) between the participants. Because outdoor activities for residents and visit from relatives are both restricted, where technologies are available, HCP should explore videoconferencing (or other creative means) between relatives and residents for social engagement and emotional support during the lockdown. Note, however, that such videoconferencing can also trigger negative reactions for some dementia patients. 44 Certain non‐pharmacological therapies can also be delivered online (eg, reminiscence, music therapy) for individuals with behavioral problems. Other therapies or advanced technologies for those with apathy or agitation that do not require close contact (eg, virtual reality, social robots) may be tried. 54 Regular or “as needed” medical/psychiatric consultation is best to be conducted via telemedicine during the outbreaks.
Moreover, in periods of lockdown during which family members may not have a full understanding on the needs of their loved ones residing at care homes, it is particularly important that they should maintain a close communication with an ombudsperson (or related agencies that advocate for the vulnerable residents) to ensure appropriate care is delivered to the residents.
6. IN‐HOSPITAL MANAGEMENT FOR OLDER PATIENTS WITH DEMENTIA
Emergency response protocols for major infectious disease outbreak at the hospital level are not the scope of this article. At the patient level, clinical management for older patients with dementia should be in line with the latest treatment guidelines for COVID‐19. 55 In addition, physicians or emergency room doctors should have high index of suspicion of COVID‐19 for older people presenting with non‐specific symptoms. Hospital management should facilitate physicians and/or nurses who have geriatric or related training including in delirium care to look after older dementia patients with COVID‐19. Specialist wards may be better able to facilitate online video platforms that allow frequent communication between patients and their family members, which may mitigate delirium and patient distress. Common reversible factors for delirium (eg, hypoxia, electrolyte disturbance, anemia, dehydration, pain, constipation, malnourishment, poor hygiene, polypharmacy, visual or hearing problems, immobilizations, sleep disturbance) should be monitored closely and corrected when possible. Because isolation measures and need of physical distancing may make it challenging to provide optimal first‐line management strategies for delirium, it is tempting to lower the threshold in prescribing antipsychotics. A short course of antipsychotic (eg, haloperidol, risperidone) may be considered where delirium presents a risk to the patient or to others including exposure of staff/other patients to infection, while benzodiazepines (and physical restraints) should be avoided if possible. At the same time, every effort should be made to identify the cause(s) of delirium and to correct it if possible. Note that corticosteroids, given empirically to some hospitalized patients with severe COVID‐19 experiencing cytokine storm 56 frequently worsen delirium and should be used with caution in dementia patients. Note however that in those with probable immune‐mediated severe encephalopathy associated with COVID‐19, corticosteroids may be helpful. 27 Yet again, risks and benefits of steroid use need to be carefully balanced for each patient. 55 In the ICU, the “ABCDEF safety bundles” (ie, A‐Assessment/treatment of pain; B‐Both Spontaneous Awakening Trial and Spontaneous Breathing Trial; C‐Choice of Sedation; D‐Delirium‐Regular monitoring and management; E‐Early mobility; F‐Family engagement) 57 provide a comprehensive framework for the prevention and management of delirium.
In the case of overwhelming numbers with infection exceeding the supply of critical care beds and ventilators, health‐care systems will require triage protocols beyond the usual assessments for suitability for intubation and ventilation. Because the term dementia encompasses a wide range of severities, with many patients living in the community with a good quality of life, a dementia diagnosis should not be used as a blanket exclusion from critical care. Triage of patients with dementia should be based on objective predictions of short‐term mortality such as the Clinical Frailty Scale, 58 rather than unsubstantiated suppositions about quality of life. This has been advocated in countries such as the UK, but with the important caveat that its use was primarily based on the fact that it is a measure of physical health frailty, whereas many physically robust people with dementia would score poorly because of cognitive problems impacting on ADL. This should be taken into account to prevent people with dementia (and indeed other long‐term neurological and psychiatric conditions) being adversely discriminated against. If, despite their wishes, patients with dementia cannot be admitted to ICU, patients and family members deserve an explanation and good quality palliative care. 59
At the time of writing there are still no anti‐viral or immune‐based drugs that can reduce the mortality associated with COVID‐19. Preliminary results from Adaptive Covid‐19 Treatment Trial (ACTT‐1) showed that remdesivir could reduce time to recovery but not mortality. 60 Subgroup analysis showed that the benefit in shortening time to recovery was also observed in older subjects ≥65 years of age. It is important that ongoing clinical trials (eg, RECOVERY [Randomised Evaluation of COVID‐19 Therapy]) can incorporate cognition as an outcome measure. Note further that in many countries however, treatment for COVID‐19 beyond purely supportive care is unauthorized and specific therapies may only be given as part of a randomized clinical trial. Importantly, patients with COVID‐19 who have dementia should not be excluded from trials purely on the grounds of inability to give informed consent. Trial designs should allow for consultee consent, including from clinical professionals in the absence of any next of kin, for patients lacking capacity to avoid exclusion of patients with dementia impacting future generalizability of the trial findings.
7. CONCLUSION
Here we have provided an overview of the profound challenges faced by older people with dementia and their informal caregivers, as well as HCP during the COVID‐19 pandemic. We have put forward strategies for developing a comprehensive home‐based care for community dwelling people with dementia, as well as strategies for optimizing care and management of patients in care homes and hospital settings. Note that although we have focused mainly on dementia and its care, the mechanisms of how SARS‐CoV‐2 affects the brain and the proposed conceptual framework for caring for people with dementia may also apply to older people in general, with or without other neurological diseases (eg, stroke, parkinsonism). As the pandemic continues to evolve around the world, it is uncertain whether COVID‐19 may change the demographics or dementia incidence/prevalence of our societies.
We strongly believe the strategies proposed are of immediate relevance to regions that are still being hit hard by the infection at the time of writing. For regions that have contained the first wave of COVID‐19, we hope the strategies can guide health‐care leaders to formulate emergency response plans in case COVID‐19 outbreaks recur, so that we are better prepared to protect and promote the well‐being of older people with dementia.
CONFLICTS OF INTEREST
Philip B. Gorelick, MD, MPH, serves on a Data Monitoring Board for Novartis for a LCZ 696 trial which includes the study of cognitive outcomes in heart failure. Other authors have no conflicts of interest relevant to this article.
ACKNOWLEDGMENTS
We would like to pay tribute to all frontline health‐care providers (including doctors, nurses, therapists, psychologists, social workers, health care assistants, personal service workers, etc.) and informal caregivers, family members, neighbors, or volunteers, who have been providing the best care and support possible for older people with dementia around the globe during the COVID‐19 pandemic.
We would like to thank Dr Zaven Khachaturian and Dr Ara Khachaturian for suggesting the scope of this article and for enlightening how SARS‐CoV‐19 may impact the brain of older people. Our views in this article are consistent with the mission of The International Society of Vascular Behavioural and Cognitive Disorders (Vas‐Cog). Professor Philip M. Bath is Stroke Association Professor of Stroke Medicine and an Emeritus National Institute for Health Research Senior Investigator. We would also like to thank Mr Brain Yiu for his assistance in editing the article.
Mok VCT, Pendlebury S, Wong A, et al. Tackling challenges in care of Alzheimer's disease and other dementias amid the COVID‐19 pandemic, now and in the future. Alzheimer's Dement. 2020;16:1571–1581. 10.1002/alz.12143
REFERENCES
- 1. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infect Dis. 2020;20(6):669‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williamson E, Walker AJ, Bhaskaran KJ, et al. OpenSAFELY: factors associated with COVID‐19‐related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv. 2020. 2020.05.06.20092999. [Google Scholar]
- 4. Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID‐19): a pooled analysis of published literature. Int J Stroke. 2020;15(4):385‐389. 1747493020921664. [DOI] [PubMed] [Google Scholar]
- 5. Atkins JL, Masoli JA, Delgado J, et al. Preexisting comorbidities predicting severe COVID‐19 in older adults in the UK biobank community cohort. medRxiv. 2020. 2020.05.06.20092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS‐CoV‐2 through the postpandemic period. Science. 2020;368(6493):860‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alzheimer's disease research enterprise in the era of COVID‐19/SARS‐CoV‐2. Alzheimers Dement. 2020;16:587‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nguyen S, Major K, Cochet C, et al. [COVID‐19 infection in the elderly in French‐speaking Switzerland: an inventory of beliefs, convictions and certainties]. Rev Med Suisse. 2020;16:835‐838. [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Etard J‐F, Vanhems P, Atlani‐Duault L, Ecochard R. Potential lethal outbreak of coronavirus disease (COVID‐19) among the elderly in retirement homes and long‐term facilities, France, March 2020. Euro Surveill. 2020;25(15):2000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. COVID C, Team R . Severe outcomes among patients with coronavirus disease 2019 (COVID‐19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrari R, Maggioni AP, Tavazzi L, Rapezzi C. The battle against COVID‐19: mortality in Italy. Eur Heart J. 2020;41(22):2050‐2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. A marzo mortalità aumentata del 49,4%, ma il virus lascia un'Italia spaccata: bergamo +568%, Roma ‐9,4%. Chronicle; 2020. May 4, 2020. [Google Scholar]
- 14. Kluge H. Statement–Older People Are at Highest Risk From Covid‐19, But All Must Act To Prevent Community Spread. Copenhagen, Denmark: World Health Organization; 2020. [Google Scholar]
- 15. Bonafè M, Prattichizzo F, Giuliani A, Storci G, Sabbatinelli J, Olivieri F. Inflamm‐aging: why older men are the most susceptible to SARS‐CoV‐2 complicated outcomes. Cytokine Growth Factor Rev. 2020;53:33‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuo C‐L, Pilling LC, Atkins JL, et al. APOE e4 genotype predicts severe COVID‐19 in the UK biobank community cohort. J Gerontol A Biol Sci Med Sci. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cesari M, Proietti M. COVID‐19 in Italy: ageism and decision making in a pandemic. J Am Med Dir Assoc. 2020;21:576‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Konttinen H, Cabral‐da‐Silva MEC, Ohtonen S, et al. PSEN1ΔE9, APPswe, and APOE4 confer disparate phenotypes in human iPSC‐derived microglia. Stem Cell Reports. 2019;13:669‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi Y, Yamada K, Liddelow SA, et al. ApoE4 markedly exacerbates tau‐mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549:523‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang H, Wu L‐M, Wu J. Cross‐talk between apolipoprotein E and cytokines. Mediators Inflamm. 2011;2011:949072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tudorache IF, Trusca VG, Gafencu AV. Apolipoprotein E ‐ a multifunctional protein with implications in various pathologies as a result of its structural features. Comput Struct Biotechnol J. 2017;15:359‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med. 2020;382(23):2268‐2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. Int J Infect Dis. 2020;94:55‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pilotto A, Odolini S, Masciocchi S, et al. Steroid‐responsive encephalitis in Covid‐19 disease. medRxiv. 2020. 2020.04.12.20062646. [Google Scholar]
- 28. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID‐19‐associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020:201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS‐CoV‐2. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID‐19) and anosmia. JAMA Neurol. 2020. [DOI] [PubMed] [Google Scholar]
- 36. Román GC, Spencer PS, Reis J, et al. The neurology of COVID‐19 revisited: a proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J Neurol Sci. 2020;414:116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92(6):552‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oxley TJ, Mocco J, Majidi S, et al. Large‐vessel stroke as a presenting feature of Covid‐19 in the young. N Engl J Med. 2020;382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zulfiqar A‐A, Lorenzo‐Villalba N, Hassler P, Andrès E. Immune thrombocytopenic purpura in a patient with Covid‐19. N Engl J Med. 2020;382:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hemasian H, Ansari B. First case of Covid‐19 presented with cerebral venous thrombosis: a rare and dreaded case. Rev Neurol (Paris). 2020;176:521‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. LaHue SC, James TC, Newman JC, Esmaili AM, Ormseth CH, Ely EW. Collaborative delirium prevention in the age of COVID‐19. J Am Geriatr Soc. 2020;68(5):947‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rada AG. Covid‐19: the precarious position of Spain's nursing homes. BMJ. 2020;369:m1554. [DOI] [PubMed] [Google Scholar]
- 44. Trabucchi M, De Leo D. Nursing homes or besieged castles: cOVID‐19 in northern Italy. Lancet Psychiatry. 2020;7:387‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Comas‐Herrera A, Zalakain J, Litwin C, Hsu AT, Lane N, Fernandex JL. Mortality associated with COVID‐19 outbreaks in care homes: early international evidence. 21 May 2020.
- 46. Chan EYY, Gobat N, Kim JH, et al. Informal home care providers: the forgotten health‐care workers during the COVID‐19 pandemic. Lancet. 2020;395(10242):1957‐1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sutcliffe C, Giebel C, Bleijlevens M, et al. Caring for a person with dementia on the margins of long‐term care: a perspective on burden from 8 European countries. J Am Med Dir Assoc. 2017;18:967‐973 e1. [DOI] [PubMed] [Google Scholar]
- 48. World Health Organization . Home Care For Patients With Covid‐19 Presenting With Mild Symptoms and Management of Their Contacts: Interim Guidance, 17 March 2020. [Google Scholar]
- 49. Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295:2148‐2157. [DOI] [PubMed] [Google Scholar]
- 50. Bloem BR, Dorsey ER, Okun MS. The coronavirus disease 2019 crisis as catalyst for telemedicine for chronic neurological disorders. JAMA Neurol. 2020. [DOI] [PubMed] [Google Scholar]
- 51. World Health Organization . Infection Prevention and Control Guidance For Long‐Term Care Facilities In The Context of Covid‐19: Interim Guidance, 21 March 2020. [Google Scholar]
- 52. Centers for Disease Control and Prevention . Preparing for COVID‐19 in Nursing Homes. 25 June 2020.
- 53. England PH. Infection Prevention and Control: An Outbreak Information Pack for Care Homes ‐ The "Care Home Pack". London, UK: Public Health England; 2017. [Google Scholar]
- 54. Ferguson C, Shade MY, Blaskewicz Boron J, Lyden E, Manley NA. Virtual reality for therapeutic recreation in dementia hospice care: a feasibility study. Am J Hosp Palliat Care. 2020. 1049909120901525. [DOI] [PubMed] [Google Scholar]
- 55. World Health Organization . Clinical Management of COVID‐19: Interim Guidance, 27 May 2020. [Google Scholar]
- 56. Yang SS, Lipes J. Corticosteroids for critically ill COVID‐19 patients with cytokine release syndrome: a limited case series. Can J Anaesth. 2020:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hayhurst CJ, Pandharipande PP, Hughes CG. Intensive care unit delirium: a review of diagnosis, prevention, and treatment. Anesthesiology. 2016;125:1229‐1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smith EE, Couillard P, Fisk JD, et al. Allocating scare resources to persons with dementia during a pandemic. Can Geriatr J. 2020. in press. [Google Scholar]
- 60. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19 — preliminary report. N Engl J Med. 2020. [DOI] [PubMed] [Google Scholar]
- 61. Gauthier S, Cummings J, Ballard C, et al. Management of behavioral problems in Alzheimer's disease. Int Psychogeriatr. 2010;22:346‐372. [DOI] [PubMed] [Google Scholar]
- 62. Nissen RM, Serwe KM. Occupational therapy telehealth applications for the dementia‐caregiver dyad: a scoping review. Phys Occup Ther Geriatr. 2018;36:366‐379. [Google Scholar]
- 63. Shaw CA, Williams KN, Perkhounkova Y, Hein M, Coleman CK. Effects of a video‐based intervention on caregiver confidence for managing dementia care challenges: findings from the FamTechCare clinical trial. Clin Gerontol. 2020:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cotelli M, Manenti R, Brambilla M, et al. Cognitive telerehabilitation in mild cognitive impairment, Alzheimer's disease and frontotemporal dementia: a systematic review. J Telemed Telecare. 2019;25:67‐79. [DOI] [PubMed] [Google Scholar]
- 65. Ceravolo MG, De Sire A, Andrenelli E, Negrini F, Negrini S. Systematic rapid "living" review on rehabilitation needs due to covid‐19: update to march 31st 2020. Eur J Phys Rehabil Med. 2020. [DOI] [PubMed] [Google Scholar]
- 66. Chen Y, Abel KT, Janecek JT, Chen Y, Zheng K, Cramer SC. Home‐based technologies for stroke rehabilitation: a systematic review. Int J Med Inform. 2019;123:11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Costanzo MC, Arcidiacono C, Rodolico A, Panebianco M, Aguglia E, Signorelli MS. Diagnostic and interventional implications of telemedicine in Alzheimer's disease and mild cognitive impairment: a literature review. Int J Geriatr Psychiatry. 2020;35:12‐28. [DOI] [PubMed] [Google Scholar]
- 68. Poon P, Hui E, Dai D, Kwok T, Woo J. Cognitive intervention for community‐dwelling older persons with memory problems: telemedicine versus face‐to‐face treatment. Int J Geriatr Psychiatry. 2005;20:285‐286. [DOI] [PubMed] [Google Scholar]
- 69. Phillips NA, Chertkow H, Pichora‐Fuller MK, Wittich W. Special issues on using the Montreal cognitive assessment for telemedicine assessment during COVID‐19. J Am Geriatr Soc. 2020;68(5):942‐944. [DOI] [PubMed] [Google Scholar]
- 70. Kim H, Jhoo JH, Jang JW. The effect of telemedicine on cognitive decline in patients with dementia. J Telemed Telecare. 2017;23:149‐154. [DOI] [PubMed] [Google Scholar]
- 71. Wong A, Nyenhuis D, Black SE, et al. Montreal cognitive assessment 5‐minute protocol is a brief, valid, reliable, and feasible cognitive screen for telephone administration. Stroke. 2015;46:1059‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pendlebury ST, Welch SJ, Cuthbertson FC, Mariz J, Mehta Z, Rothwell PM. Telephone assessment of cognition after transient ischemic attack and stroke: modified telephone interview of cognitive status and telephone Montreal cognitive assessment versus face‐to‐face Montreal cognitive assessment and neuropsychological battery. Stroke. 2013;44:227‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wong A, Fong CH, Mok VC, Leung KT, Tong RK. Computerized cognitive screen (CoCoSc): a self‐administered computerized test for screening for cognitive impairment in community social centers. J Alzheimers Dis. 2017;59:1299‐1306. [DOI] [PubMed] [Google Scholar]
- 74. Charalambous AP, Pye A, Yeung WK, et al. Tools for App‐ and Web‐based self‐testing of cognitive impairment: systematic search and evaluation. J Med Internet Res. 2020;22:e14551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Arya A, Buchman S, Gagnon B, Downar J. Pandemic palliative care: beyond ventilators and saving lives. Can Med Assoc J. 2020;192:E400‐E404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bossen AL, Kim H, Williams KN, Steinhoff AE, Strieker M. Emerging roles for telemedicine and smart technologies in dementia care. Smart Homecare Technol Telehealth. 2015;3:49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Khazaeili M, Zargham Hajebi M, Mohamadkhani P, Mirzahoseini H. The effectiveness of mindfulness‐based intervention on anxiety, depression and burden of caregivers of multiple sclerosis patients through web conferencing. Pract Clin Psychol. 2019;7:21‐32. [Google Scholar]


