Abstract
Various comorbidities represent risk factors for severe coronavirus disease 2019 (COVID‐19). The impact of smoking on COVID‐19 severity has been previously reported in several meta‐analyses limited by small sample sizes and poor methodology. We aimed to rigorously and definitively quantify the effects of smoking on COVID‐19 severity. MEDLINE, Embase, CENTRAL, and Web of Science were searched between 1 December 2019 and 2 June 2020. Studies reporting smoking status of hospitalized patients with different severities of disease and/or at least one clinical endpoint of interest (disease progression, intensive care unit admission, need for mechanical ventilation, and mortality) were included. Data were pooled using a random‐effects model. This study was registered on PROSPERO: CRD42020180920. We analyzed 47 eligible studies reporting on 32 849 hospitalized COVID‐19 patients, with 8417 (25.6%) reporting a smoking history, comprising 1501 current smokers, 5676 former smokers, and 1240 unspecified smokers. Current smokers had an increased risk of severe COVID‐19 (risk ratios [RR]: 1.80; 95% confidence interval [CI]: 1.14‐2.85; P = .012), and severe or critical COVID‐19 (RR: 1.98; CI: 1.16‐3.38; P = .012). Patients with a smoking history had a significantly increased risk of severe COVID‐19 (RR: 1.31; CI: 1.12‐1.54; P = .001), severe or critical COVID‐19 (RR: 1.35; CI: 1.19‐1.53; P < .0001), in‐hospital mortality (RR: 1.26; CI: 1.20‐1.32; P < .0001), disease progression (RR: 2.18; CI: 1.06‐4.49; P = .035), and need for mechanical ventilation (RR: 1.20; CI: 1.01‐1.42; P = .043). Patients with any smoking history are vulnerable to severe COVID‐19 and worse in‐hospital outcomes. In the absence of current targeted therapies, preventative, and supportive strategies to reduce morbidity and mortality in current and former smokers are crucial.
Keywords: coronavirus, epidemiology, pandemics, pathogenesis, respiratory tract, virus classification, zoonoses
Highlights
The first high‐quality systematic review and meta‐analysis assessing the impact of smoking on COVID‐19 severity.
To date, the largest meta‐analysis among peer‐reviewed literature assessing the impact of smoking on COVID‐19 severity, including 32,849 hospitalised patients with COVID‐19.
Patients who were current smokers had an increased risk of severe COVID‐19 and severe or critical COVID‐19.
Patients with a smoking history had an increased risk of severe COVID‐19, severe or critical COVID‐19, in‐hospital mortality, disease progression and need for mechanical ventilation.
1. INTRODUCTION
As of 28 July 2020, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has infected 16 341 920 patients, with 650 805 deaths across 188 countries. 1 , 2 Risk factors for poor outcome in patients with coronavirus disease 2019 (COVID‐19) include older age, male sex, hypertension, diabetes, cardiovascular disease, and respiratory disease. 3 , 4 , 5 Remarkably, current peer‐reviewed data surrounding the effect of smoking tobacco on the clinical severity of COVID‐19 has thus far been controversial, and there is an urgent need for definitive answers. 6
An early systematic review without meta‐analysis concluded that smoking is most likely associated with negative progression and outcomes in COVID‐19, 7 however, a preliminary meta‐analysis showed that active smoking is not significantly associated with increased risk of severe disease. 8 Four subsequent meta‐analyses have shown an increased risk of severe COVID‐19 associated with smoking. 9 , 10 , 11 , 12 A summary of the six previously published systematic reviews 7 , 8 , 9 , 10 , 11 , 12 alongside assessment of their methodological quality using A Measurement Tool to Assess systematic Reviews 2 13 (AMSTAR 2) is provided in the Appendix (Appendix pp2‐3). The articles ranged from critically poor to moderate quality, indicating that significant methodological flaws in critical domains exist with all six currently published reviews assessing the impact of smoking on COVID‐19 severity. It is therefore likely that the true effect of smoking on COVID‐19 severity reported in these analyses is clouded by considerable bias.
Furthermore, as a result of several nonpeer reviewed preprint articles falsely equating the prevalence of smoking in COVID‐19 study populations with population estimates for smoking prevalence, there has been widespread attention paid to recent mass media reports that smoking may exert a protective effect against COVID‐19 infection. 14 This led to the World Health Organization releasing a statement on 11 May urging caution with regards to these claims, and emphasizing the lack of evidence confirming a link between smoking or nicotine in the prevention or treatment of COVID‐19. 15 Consequently, there remains a distinct lack of clarity and high‐quality evidence regarding the relationship between smoking and the severity of COVID‐19. Therefore, to address this important clinical question, this systematic review and meta‐analysis aimed to evaluate the effect of smoking status, including current smoking and a history of smoking, on the clinical severity of COVID‐19.
2. METHODS
2.1. Search strategy and selection criteria
This systematic review and meta‐analysis adhered to PRISMA guidelines 16 and was AMSTAR 2 compliant (Appendix pp8‐12). 13 Two authors independently searched MEDLINE, Embase, CENTRAL, and Web of Science for studies published between 1 December 2019 and 2 June 2020. The search strategy is provided in the Appendix (p13). No language restrictions were applied. COVID‐19 resource centers of The Lancet, The Lancet Respiratory Medicine, The New England Journal of Medicine, and The BMJ were also hand searched up to 5 July 2020. Reference lists of included studies and previous systematic reviews were additionally screened for their relevance.
To capture all available relevant evidence, randomized, and observational studies reporting the smoking status of hospitalized patients presenting with different severities of disease and/or at least one clinical endpoint of interest were deemed eligible for inclusion. Smoking history included current and former tobacco smokers or e‐cigarette users. Disease severity, including severe or critical cases, was defined a priori and based on the COVID‐19 diagnostic criteria issued by the Chinese National Health Commission (Appendix p13). 17 Other acceptable criteria included the Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) criteria for severe community‐acquired pneumonia. 18 Clinical endpoints of disease progression, intensive care unit (ICU) admission, mechanical ventilation requirement, and/or mortality were used as surrogate markers for in‐hospital severity. We excluded studies on other coronaviruses or if there was insufficient information to distinguish disease severity based on smoking status. Case series involving less than 20 patients, review articles, editorials, conference abstracts, and nonclinical studies were also excluded. Preprints were not assessed for eligibility due to their preliminary nature.
Two authors (WNC, AS) independently screened the titles and abstracts of retrieved studies, with full‐texts of all potentially eligible papers subsequently assessed for inclusion. Any discrepancy was resolved by consensus discussion with the senior author (AK).
2.2. Data analysis
Data from studies that fulfilled our inclusion criteria were extracted independently by three authors (WNC, AS, and RKR). Main data‐points included: study details (author, journal, date, country, study design, study period, and funding), total numbers of patients, and their clinical outcomes by smoking status.
Two authors (AS and AD) independently assessed the quality of included studies using the Newcastle‐Ottawa Scale modified for case series, cohort studies, and cross‐sectional studies. 19 Scores were then classified by the Agency for Healthcare Research and Quality standards as good, fair, or poor. Any discrepancies in quality assessment were resolved by a third author (WNC).
As per our prespecified analysis plan, random‐effects meta‐analyses of pooled raw data were employed using the DerSimonian and Laird method for each outcome with sufficient data to account for anticipated differences across countries and study design over time. Current smokers were compared to former and never‐smokers, and patients with a smoking history were compared to never‐smokers. Where available, adjusted effect estimates were combined and in the absence of adjustment for confounders, raw effect estimates were combined. The results are presented in forest plots as risk ratios (RR) and corresponding 95% confidence intervals (CI) for each outcome. I 2 estimates of heterogeneity, representing the variability across studies, are classified as low (<30%), moderate (30%‐60%), or high (>60%). Sensitivity analyses included only good‐quality studies and, for severity outcomes, studies using the COVID‐19‐specific criteria for grading severity. Subgroup analyses were completed by country. Funnel plots were used to check for publication bias and tested for asymmetry using Harbord's test, 20 with studies with no events in either exposed or unexposed arms excluded from this analysis. P values <.05 were considered significant.
Data were analyzed using Stata (version 15). The study protocol was prospectively registered with PROSPERO, number CRD42020180920. 21
2.3. Role of the funding source
This study received no funding. All authors had full access to all of the data and took responsibility for the decision to submit for publication.
3. RESULTS
The search identified 1038 papers, of which 339 were duplicates. After screening the titles and abstracts of the remaining 699 papers, 350 full‐texts were reviewed. Overall, 35 studies met the inclusion criteria, with a further 12 identified from the references of included studies or by the reviewer team (Figure 1). The 47 included studies 4 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 represented a total of 32 849 hospitalized COVID‐19 patients: 8417 (25.6%) with any reported smoking history, comprising 1501 current smokers, 5676 former smokers, and 1240 unspecified smokers; 22 420 (68.3%) never‐smokers; and a further 2012 (6.1%) patients who did not currently smoke, though it was unclear whether they were former or never‐smokers (Table 1).
Figure 1.
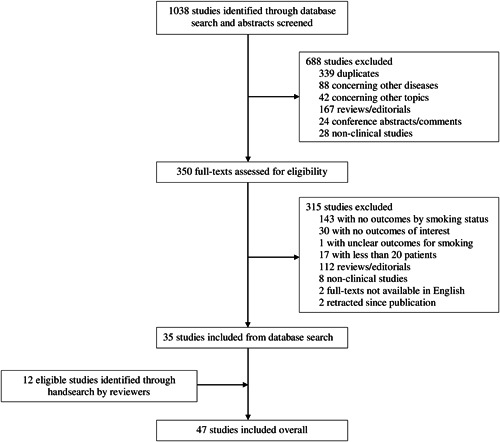
Flow diagram of selection of included studies
Table 1.
Characteristics of included studies
| Setting | Study design | Number of centers | Study period | Number of patients, current smokers vs former/never‐smokers | Number of patients, any smoking history vs never‐smokers | Study quality | |
|---|---|---|---|---|---|---|---|
| Azar et al 22 | United States | Cohort | 24 | Jan‐Apr | 10 vs 216 | 73 vs 153 | Fair |
| Bhargava et al 23 | United States | Cohort | 1 | Mar‐Apr | 11 vs 186 | ⋯ | Good |
| Bi et al 24 | China | Cohort | 1 | Jan‐Mar | 8 vs 105 | ⋯ | Good |
| Brenner et al 25 | International | Cohort | 1+ | ‐Apr | 11 vs 150 | ⋯ | Poor |
| Buckner et al 26 | United States | Case series | 3 | Mar‐May | ⋯ | 22 vs 64 | Poor |
| CDC COVID‐19 Response Team 27 | United States | Cohort | 1+ | Feb‐Mar | 27 vs 1467 | 105 vs 1389 | Poor |
| Chen et al 28 | China | Case series | 1 | Jan‐Mar | ⋯ | 15 vs 130 | Poor |
| Chen et al 29 | China | Cohort | 575 | ‐Jan | ⋯ | 111 vs 1479 | Good |
| Chen et al 30 | China | Case series | 1 | Jan‐Feb | 12 vs 262 | ⋯ | Poor |
| Docherty et al 31 | UK | Cohort‡ | 208 | Feb‐May | 852 vs 13 332 | 5216 vs 8968 | Good |
| Feng et al 32 | China | Cohort | 3 | Jan‐Mar | ⋯ | 44 vs 410 | Good |
| Goyal et al 33 | United States | Case series | 2 | Mar‐Apr | 20 vs 373 | 98 vs 295 | Poor |
| Guan et al 34 | China | Cohort | 552 | Dec‐Jan | 137 vs 948 | 158 vs 927 | Poor |
| Hu et al 35 | China | Case series | 1 | Jan‐Mar | ⋯ | 38 vs 285 | Good |
| Huang et al 36 | China | Case series‡ | 1 | Dec‐Jan | 3 vs 38 | ⋯ | Poor |
| Huang et al 37 | China | Cohort | 1 | Jan‐Mar | 56 vs 288 | ⋯ | Good |
| Huang et al 38 | China | Case series | 8 | Jan‐Feb | ⋯ | 16 vs 186 | Good |
| Hur et al 39 | United States | Cohort | 10 | Mar‐Apr | 16 vs 470 | 163 vs 323 | Good |
| Inciardi et al 40 | Italy | Cohort | 1 | Mar‐Mar | ⋯ | 17 vs 82 | Poor |
| Ji et al 41 | China | Cohort | 2 | Jan‐Mar | ⋯ | 19 vs 189 | Good |
| Kalligeros et al 42 | United States | Cohort | 3 | Feb‐Apr | 12 vs 91 | 48 vs 55 | Good |
| Klang et al 43 | United States | Cohort | 5 | Mar‐May | ⋯ | 793 vs 2613 | Good |
| Kuderer et al 44 | Internationala | Cohort | 1+ | Mar‐May | 25 vs 406 | 226 vs 205 | Fair |
| Li et al 45 | China | Cohort† | 1 | Jan‐Mar | 41 vs 503 | 92 vs 452 | Good |
| Li et al 46 | China | Case series | 1 | Jan‐Feb | ⋯ | 7 vs 18 | Poor |
| Liu et al 47 | China | Cohort | 3 | Dec‐Jan | ⋯ | 5 vs 73 | Good |
| Petrilli et al 49 | United States | Cohort‡ | 4 | Mar‐May | 141 vs 2145 | 702 vs 1584 | Good |
| Qin et al 48 | China | Cohort | 1 | Jan‐Feb | ⋯ | 7 vs 445 | Poor |
| Rastrelli et al 50 | Italy | Case series | 1 | ⋯ | 1 vs 30 | 12 vs 19 | Poor |
| Shi et al 51 | China | Cohort | 2 | Jan‐Mar | ⋯ | 16 vs 290 | Good |
| Shi et al 52 | China | Cohort | 1+ | ‐Feb | ⋯ | 40 vs 434 | Good |
| Sun et al 53 | China | Cohort | 1 | Feb‐Mar | ⋯ | 12 vs 45 | Good |
| Toussie et al 54 | United States | Cohort | 1+ | Mar‐Mar | ⋯ | 29 vs 94 | Fair |
| Wan et al 55 | China | Case series‡ | 1 | Jan‐Feb | 9 vs 126 | ⋯ | Poor |
| Wang et al 56 | China | Cohort† | 1 | ⋯ | 41 vs 503 | 92 vs 452 | Poor |
| Wang et al 57 | China | Cohort | 1 | Jan‐Feb | 16 vs 109 | 16 vs 109 | Poor |
| Yang et al 58 | China | Cohort | 1 | Dec‐Feb | ⋯ | 2 vs 50 | Poor |
| Yao et al 59 | China | Cohort | 1 | Jan‐Mar | 4 vs 104 | ⋯ | Good |
| Yu et al 60 | China | Cohort | 24 | Jan‐Mar | 13 vs 408 | ⋯ | Good |
| Yu et al 61 | China | Cross‐sectional | 2 | Jan‐Feb | ⋯ | 5 vs 65 | Good |
| Yu et al 62 | China | Cohort | 1 | Jan‐Mar | ⋯ | 16 vs 76 | Poor |
| Yu et al 63 | China | Cohort | 1+ | Dec‐Feb | ⋯ | 26 vs 265 | Fair |
| Zhang et al 64 | China | Case series | 1 | Jan‐Feb | 2 vs 138 | 9 vs 131 | Poor |
| Zhang et al 65 | China | Cohort | 1 | Jan‐Feb | 6 vs 114 | ⋯ | Fair |
| Zheng et al 66 | China | Cohort‡ | 3 | Jan‐Feb | 8 vs 58 | ⋯ | Fair |
| Zheng et al 67 | China | Case series | 1 | Jan‐Feb | 8 vs 65 | 8 vs 65 | Poor |
| Zhou et al 4 | China | Cohort | 2 | Dec‐Jan | 11 vs 180 | ⋯ | Good |
Note: All studies are retrospective except: †ambispective (includes prospective and retrospective components) and ‡prospective.
Contains data from the United States, Canada, and Spain.
There were 25 multicentre studies (three prospective 31 , 49 , 66 and 22 retrospective) 4 , 22 , 25 , 26 , 27 , 29 , 32 , 33 , 34 , 38 , 39 , 41 , 42 , 43 , 44 , 47 , 51 , 52 , 54 , 60 , 61 , 63 and 22 single‐centre studies (two prospective, 36 , 55 two with prospective and retrospective components, 45 , 56 and 18 retrospective 23 , 24 , 28 , 30 , 35 , 37 , 40 , 46 , 48 , 50 , 53 , 57 , 58 , 59 , 62 , 64 , 65 , 67 ). The majority of studies investigated a Chinese population (32/47, 68%), with the United States contributing 10 studies. Overall, study quality was good in 22 studies, fair in six and poor in 19 (Appendix p17). Of 38 studies disclosing funding status, 28 received funding.
Three studies 32 , 56 , 64 reported smoking index or pack‐years by outcome of interest. Six studies 23 , 25 , 39 , 49 , 54 , 61 reported outcomes for tobacco smokers, including one 25 that had pooled outcomes with those of e‐cigarette users. The remaining studies did not specify the substance of smoking.
Disease severity was graded according to the Chinese COVID‐19‐specific criteria in 14 studies, 24 , 28 , 32 , 35 , 38 , 46 , 48 , 53 , 55 , 57 , 63 , 64 , 65 , 66 the IDSA/ATS criteria in three studies 34 , 45 , 59 and a locally devised criteria in one study (Appendix p13). 54 Two studies 52 , 62 did not specify the criteria utilized.
Current smokers, whose outcomes were evaluated in 27 studies, had an overall prevalence of 6.2% (specifically, China: 8.7%, United States: 4.6%). They had a significantly increased risk of presenting with severe disease (RR: 1.80; 95% CI: 1.14‐2.85; P = .012; I 2 = 76%; Figure 2A), as well as severe or critical disease (RR: 1.98; 95% CI: 1.16‐3.38; P = .012; I 2 = 87%; Figure 2B), compared to former or never‐smokers. Effects were consistent when only analyzing studies using the COVID‐19‐specific criteria (Appendix p22). On sensitivity analysis, including only good‐quality studies resulted in these effects becoming nonsignificant. There were no significant effects on in‐hospital outcomes, including disease progression (RR: 1.54; 95% CI: 0.52‐4.58; P = .439; I 2 = 81%; Appendix p19), ICU admission (RR: 0.72; 95% CI: 0.42‐1.24; P = .237; I 2 = 40%; Appendix p20), mechanical ventilation requirement (RR: 1.13; 95% CI: 0.75‐1.72; P = .561; I 2 = 32%; Appendix p21) or mortality (RR: 1.46; 95% CI: 0.83‐2.60; P = .192; I 2 = 81%; Figure 2C). There were no differences in outcomes by country of origin (Appendix p23). A meta‐analysis was not performed for critical disease alone as only one study reported this outcome.
Figure 2.

A, Forest plot showing the effect of current smoking on severe COVID‐19. B, Forest plot showing the effect of current smoking on severe or critical COVID‐19. C, Forest plot showing the effect of current smoking on mortality. COVID‐19, coronavirus disease 2019
The overall prevalence of a smoking history, including current, former, and/or unspecified smokers, was 26.9% (specifically, China: 10.3%, United States: 23.6%). Their outcomes were investigated in 35 studies. Compared to never‐smokers, a history of smoking significantly increased the risk of presenting with severe disease (RR: 1.31; 95% CI: 1.12‐1.54; P = .001; I 2 = 12%; Figure 3A), as well as severe or critical disease (RR: 1.35; 95% CI: 1.19‐1.53; P < .0001; I 2 = 19%; Figure 3B). However, only the effect on severe or critical disease remained significant when limiting the analysis to only studies using the COVID‐19‐specific criteria for grading severity (Appendix p29). The effect on critical disease alone was not statistically significant (RR: 1.44; 95% CI: 0.95‐2.17; P = .085; I 2 = 0%; Appendix p25). However, a smoking history significantly increased mortality risk (RR: 1.26; 95% CI: 1.20‐1.32; P < .0001; I 2 = 0%; Figure 3C) in addition to other in‐hospital outcomes, such as disease progression (RR: 2.18; 95% CI: 1.06‐4.49; P = .035; I 2 = 69%; Appendix p26) and mechanical ventilation requirement (RR: 1.20; 95% CI: 1.01‐1.42; P = .043; I 2 = 0%; Appendix p28). There was no statistically significant difference in ICU admission (RR: 1.12; 95% CI: 0.96‐1.31; P = .157; I 2 = 0%; Appendix p27). Sensitivity analyses excluding lower‐quality studies supported the primary analyses for all outcomes of interest (Appendix p29). Only the mortality analysis facilitated comparison by country, in which significant detrimental effects were observed in publications from China, United States, and the UK, but not Italy, which contributed one study only for this outcome (Appendix p29).
Figure 3.
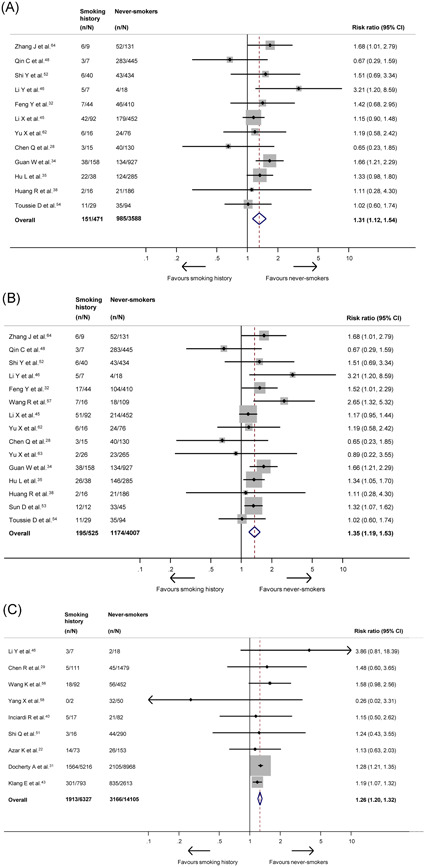
A, Forest plot showing the effect of a smoking history on severe COVID‐19. B, Forest plot showing the effect of a smoking history on severe or critical COVID‐19. C, Forest plot showing the effect of a smoking history on mortality. COVID‐19, coronavirus disease 2019
Overall, there was a moderate‐to‐high degree of heterogeneity between studies evaluating the effects of current smoking and a low degree of heterogeneity between studies investigating a history of smoking. The potential for publication bias was only detected in the comparison of disease progression in patients with a smoking history (Appendix p26), though heterogeneity was high for this outcome.
4. DISCUSSION
To our knowledge, this is the largest meta‐analysis amongst peer‐reviewed literature assessing the effect of smoking tobacco on the severity of COVID‐19. Principally, the present analysis found that current smokers have an increased risk of presenting to hospital with severe COVID‐19 and are approximately twice as likely to experience severe or critical COVID‐19 as former or never‐smokers. While this risk became nonsignificant following sensitivity analysis of good‐quality studies only, there were only two studies for each outcome and none graded disease severity by COVID‐19‐specific criteria, thus precluding meaningful interpretation. Overall, there was a high degree of heterogeneity amongst studies evaluating current smoking, even when analyzing good‐quality studies only. For patients with a smoking history, there is an increased risk of presentation to hospital with severe, as well as severe or critical, COVID‐19 and subsequent increased risk of in‐hospital mortality. Additionally, these patients were more likely to experience disease progression and require mechanical ventilation. That all outcomes remained significant on inclusion of only good‐quality studies suggests these analyses represent true effects. A high level of heterogeneity was only observed in assessing the effect of smoking history on disease progression, which is likely secondary to substantial inter‐study variation in defining progression. This outcome also displayed potential for publication bias, however, none was found in other analyses, indicating the low impact of publication bias on our results.
Our finding that current smoking is associated with increased disease severity in COVID‐19 patients validates previous findings from several smaller meta‐analyses in a much larger patient population, achieved through a more rigorous, prospectively registered methodology. 9 , 10 , 11 , 12 , 21 The finding that patients with a smoking history are at increased risk of more severe disease, and increased mortality, also confirms previous findings of a smaller meta‐analysis. 11 The association of both current smoking and smoking history with greater severity of COVID‐19 is biologically plausible for a wealth of reasons. Smoking tobacco is the primary cause of preventable disease, disability, and death in the United States, and is responsible for over 8 million deaths worldwide per year. 68 Smoking is a major risk factor for adverse respiratory and cardiovascular outcomes, in addition to a wide range of malignant and nonmalignant disease. 68 In addition, smoking increases severity and mortality of both bacterial and viral infections through the induction of mechanical and structural changes in the respiratory tract and alteration of cell‐ and humoral‐mediated immune responses. 69 , 70 In the context of respiratory viruses, smoking has been reported to cause increased hospital and ICU admissions with influenza infection, greater severity with respiratory syncytial virus bronchiolitis and increased mortality with viral pneumonia. 71 , 72 , 73
With regard to coronaviruses, in particular, smoking is associated with increased susceptibility and mortality in MERS‐CoV infection, potentially due to upregulation of dipeptidyl peptidase‐IV, the host receptor for MERS‐CoV, in smokers. 74 , 75 The angiotensin‐converting enzyme‐2 (ACE‐2), previously shown to be the host receptor for SARS‐CoV, has also been proven to be the host receptor for SARS‐CoV‐2, facilitating initial intracellular entry via interactions with viral spike glycoproteins. 76 Subsequent studies have confirmed that ACE‐2 expression is upregulated in human lung tissue samples taken from both current and past smokers, likely mediated by the α‐7 subtype of the nicotinic acetylcholine receptor. 77 , 78 , 79 , 80 , 81 In a series of elegant in vitro experiments, Smith et al 80 report a consistent correlation between smoking history and increased ACE‐2 expression that was dose‐dependent, detectable in both bulk and single‐cell analyses, and remained significant after multivariate linear regression controlling for age, sex, race, and body mass index. It is, therefore, plausible that smokers are exposed to higher SARS‐CoV‐2 loads as a result of increased expression of ACE‐2, which may provide a mechanistic explanation for the increased risk of severe disease and mortality associated with smoking in COVID‐19 patients that we report. Moreover, the inhibition of SARS‐CoV‐2 progression in vitro by human recombinant soluble ACE‐2, a neutralizing agent, holds therapeutic potential and is currently in phase II clinical trials (ClinicalTrials.gov Identifier: NCT04335136). 82 However, to complicate matters, previous studies also report that postentry viral‐mediated downregulation of ACE‐2 played a major role in the pathogenesis of SARS‐CoV‐associated acute lung injury. 83 , 84 Smoking itself has been postulated as having varying, organ‐specific effects on ACE‐2 levels, with specific cigarette components, such as nicotine, potentially exerting a different effect to whole smoke. 80 Therefore, further studies characterizing the complex relationship of smoking and ACE‐2 in COVID‐19 are warranted.
That smoking history is associated with a significantly increased risk of in‐hospital mortality in COVID‐19 patients, whilst current smoking is not, is a surprising finding. Reductions in morbidity and all‐cause mortality following smoking cessation are well characterized and thus former smokers would be expected to have better baseline health status and improved outcomes. 68 A systematic review assessing prevalence of current smokers who were hospitalized for COVID‐19 reported a pooled prevalence of 6.5% and propose that in view of the lower than expected prevalence of current smokers compared to population estimates, current smoking is not a predisposing factor for hospitalization and smoking and/or nicotine may exert a protective effect against severe COVID‐19. 85 The idea that smoking and/or nicotine may be protective against COVID‐19 is echoed by several preprint studies that gained widespread media attention. 14 Although these hypotheses may explain the nonsignificant association of current smoking and increased mortality that we report, since the majority of included studies did not statistically adjust the effect of smoking for baseline covariates, it is not appropriate to compare the prevalence of smoking in hospitalized COVID‐19 patients with overall population estimates, as the two populations are inherently different with regards to demographic factors. We believe there are far more credible reasons for the nonsignificant association between current smoking and mortality that we report and the low prevalence of smoking among patients with COVID‐19 in published studies.
Predominantly, in the context of a global pandemic, accurately recording smoking history is likely to be of low priority for frontline clinicians whose principal focus is stabilizing severely and critically ill patients. Therefore, patients may have been too acutely unwell to answer questions or clinicians may not enquired directly about smoking status, leading to misclassification of smokers as nonsmokers. Similarly, collateral history collected from family members or referring clinicians is likely to be less accurate than ascertainment of patient‐reported smoking status. Additionally, in an example of reverse causality, hospitalized patients are more likely to have quit smoking on admission, resulting in additional potential misclassification of current smokers as former or nonsmokers. Given the well‐known scarcity of ICU resources such as ventilators, it is also possible that social desirability bias may have contributed to patients not reporting current smoking for fear of being denied access to such interventions, further exacerbating misclassification bias. 14 , 86 Finally, given the association of smoking with lower socioeconomic status, 87 it is possible that current smokers are exhibiting worse health‐seeking behaviors and either self‐treating or deteriorating in the community. Thus, they would not be accounted for in the reported studies which assessed hospitalized patients, leading to survivorship bias and lower event rates for in‐hospital mortality. Due to these factors, the summary estimate for in‐hospital mortality we report has likely been biased towards a null result for current smokers. Similarly, the twofold increase in risk of severe or critical disease for current smokers is likely an underestimate of the effect size.
With no targeted therapies against COVID‐19 currently available, as a scientific community, we must focus on prevention, particularly for those at risk of severe or critical disease. Frontline clinicians must conscientiously record accurate smoking histories in all confirmed COVID‐19 patients, both for triage of vulnerable patients and to support future research efforts. During the current pandemic, independent surveys have reported increased smoking frequency in current smokers and high rates of relapse in former smokers, 88 , 89 , 90 which is unsurprising given the stress, isolation, and other adverse psychosocial repercussions of life during a global pandemic. 91 , 92 Considering our finding that current smoking and smoking history are associated with increased COVID‐19 severity, urgent public health measures emphasizing smoking cessation advice, support and pharmacotherapy must be provided to reduce overall disease burden, despite a currently altered social landscape. Good‐quality studies have proven the benefits of mobile phone‐based interventions, 93 highlighting that even during periods of social distancing and self‐isolation, remote methods of smoking cessation may be feasible and efficacious. Furthermore, as countries begin easing lockdown restrictions, it is imperative that governments and policymakers protect vulnerable populations, such as current and former smokers, through adequate shielding measures and appropriate legislation.
The present analysis has several limitations, principally the use of aggregate data for our meta‐analysis, which precludes adjustment for certain covariates reported to be predictive of disease severity, such as age, gender, and comorbidities, 3 , 4 , 5 and prevents examination of heterogeneity and subgroup analysis at the patient level. The use of individual patient data may have addressed this, however, considering the urgency of our research question and direct applicability to patient care, the considerable time burden associated with conducting an individual patient data meta‐analysis was deemed inappropriate. Also, with most studies reporting on Chinese populations, we cannot exclude the possibility of ethnic differences in smoking and susceptibility to severe COVID‐19 caused by smoking, which may have confounded our analysis. However, this reflects the current landscape of peer‐reviewed literature, which at the present time consists mainly of data from China. We were also unable to assess the effect of e‐cigarettes on COVID‐19 as no studies collected separate data on their usage, which would have been informative considering rises in popularity of these products. Finally, as discussed, the high likelihood of misclassification bias concerning current smoking status across included studies suggests that our analysis potentially underestimates the impact of current smoking on both disease severity and mortality, creating an even more compelling argument for urgent public health measures to support smoking cessation during the present time.
In conclusion, in the largest meta‐analysis available amongst peer‐reviewed literature, we report that both current smoking and a smoking history significantly increased COVID‐19 severity, whilst smoking history also significantly increased mortality risk. Due to problems with potential misclassification of current smokers among included studies, the analysis likely underestimates the likelihood of severity in this patient population. As the COVID‐19 pandemic continues to burden societies worldwide, our analysis suggests that smoking represents one of the most immediately modifiable risk factors to reduce the substantial morbidity associated with the disease. In light of this finding, governments, policymakers, and other key stakeholders must ensure that appropriate measures are taken to support and maintain smoking cessation to protect vulnerable populations and reduce the strain placed on healthcare systems working at full capacity during this global crisis.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
AK conceptualized the work. RKR, WNC, AS, AD, PTS, and AK were responsible for acquisition, analysis, and interpretation of data. RKR, WNC, and AK drafted the manuscript. AS, AD, PTS, and AK provided critical revisions. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Supporting information
Supporting information
ACKNOWLEDGMENTS
PTS is partly funded by King's Health Partners Institute of Women and Children's Health, Tommy's (Registered charity no. 1060508), and by ARC South London (NIHR). The views expressed are not necessarily those of KHP, Tommy's, the NHS, the NIHR, or the Department of Health.
Reddy RK, Charles WN, Sklavounos A, Dutt A, Seed PT, Khajuria A. The effect of smoking on COVID‐19 severity: A systematic review and meta‐analysis. J Med Virol. 2021;93:1045–1056. 10.1002/jmv.26389
Rohin K. Reddy and Walton N. Charles are co‐first authors and contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. WHO . Coronavirus disease (COVID‐19) Situation Report ‐ 190. July 28 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200728-covid-19-sitrep-190.pdf?sfvrsn=fec17314_2. Accessed July 29, 2020.
- 2. Johns Hopkins University and Medicine Coronavirus Resource Center . COVID‐19 World Map. July 29 2020. https://coronavirus.jhu.edu/map.html. Accessed July 29, 2020.
- 3. Wu Z, McCoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention [published online ahead of print February 24, 2020]. JAMA. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID‐19: a rapid systematic review and meta‐analysis. PLoS One. 2020;15:e0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Zyl‐Smit RN, Richards G, Leone FT. Tobacco smoking and COVID‐19 infection. Lancet Respir Med. 2020;8:664‐665. 10.1016/S2213-2600(20)30239-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vardavas CI, Nikitara K. COVID‐19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020;18:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID‐19). Eur J Intern Med. 2020;75:107‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of COVID‐19: a systemic review and meta‐analysis [published online ahead of print April 15, 2020]. J Med Virol. 10.1002/jmv.25889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect. 2020;81:16. 10.1016/j.jinf.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patanavanich R, Glantz S. Smoking is associated with COVID‐19 progression: a meta‐analysis [published online ahead of print May 13, 2020]. Nicotine Tob Res. 10.1093/ntr/ntaa082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karanasos A, Aznaouridis K, Latsios G, et al. Impact of smoking status on disease severity and mortality of hospitalized patients with COVID‐19 infection [published online ahead of print June 20, 2020]. Nicotine Tob Res. 10.1093/ntr/ntaa107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson C. Smokers are actually at a higher risk of dying from covid‐19. New Sci. 2020;246:8‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. WHO . WHO statement: Tobacco use and COVID‐19. May 11 2020. https://www.who.int/news-room/detail/11-05-2020-who-statement-tobacco-use-and-covid-19. Accessed July 18, 2020.
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Health Commission & National Administration of Traditional Chinese Medicine . Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J. 2020;133:1087‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community‐acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2020;200:e45‐e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. GA Wells BS, O'Connell D, Peterson, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2019. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed July 18, 2020.
- 20. Harbord RM, Egger M, Sterne JAC. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3433‐57. [DOI] [PubMed] [Google Scholar]
- 21. Khajuria A, Charles W, Sklavounos A, Reddy R The effects of smoking on COVID‐19 severity: a systematic review and meta‐analysis. April 27 2020. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=180920. Accessed July 18, 2020. [DOI] [PMC free article] [PubMed]
- 22. Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities in outcomes among COVID‐19 patients in a large health care system In California. Health Aff (Millwood). 2020;39:1253‐1262. 10.1377/hlthaff.2020.00598 [DOI] [PubMed] [Google Scholar]
- 23. Bhargava A, Fukushima EA, Levine M, et al. Predictors for severe COVID‐19 infection [published online ahead of print May 30, 2020]. Clin Infect Dis. 10.1093/cid/ciaa674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bi X, Su Z, Yan H, et al. Prediction of severe illness due to COVID‐19 based on an analysis of initial Fibrinogen to Albumin Ratio and Platelet count. Platelets. 2020;31:674‐679. 10.1080/09537104.2020.1760230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID‐19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;S0016‐5085(20):30655‐30657. 10.1053/j.gastro.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buckner FS, McCulloch DJ, Atluri V, et al. Clinical features and outcomes of 105 hospitalized patients with COVID‐19 in Seattle, Washington [published online ahead of print May 22, 2020]. Clin Infect Dis. 10.1093/cid/ciaa632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. CDC COVID‐19 Response Team . Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12‐March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:283‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen Q, Zheng Z, Zhang C, et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID‐19) in Taizhou, Zhejiang, China. Infection. 2020;48:543‐551. 10.1007/s15010-020-01432-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 From a nationwide analysis in China. Chest. 2020;158:97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feng Y, Ling Y, Bai T, et al. COVID‐19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid‐19 in New York City. N Engl J Med. 2020;382:2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 COVID‐19 hospitalized patients in Wuhan, China [published online ahead of print May 3, 2020]. Clin Infect Dis. 10.1093/cid/ciaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang J, Cheng A, Lin S, Zhu Y, Chen G. Individualized prediction nomograms for disease progression in mild COVID‐19 [published online ahead of print May 5, 2020]. J Med Virol. 10.1002/jmv.25969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang R, Zhu L, Xue L, et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu Province, China: a retrospective, multi‐center study. PLoS Negl Trop Dis. 2020;14:e0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hur K, Price CPE, Gray EL, et al. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID‐19. Otolaryngol Head Neck Surg. 2020;163:170‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Inciardi RM, Adamo M, Lupi L, et al. Characteristics and outcomes of patients hospitalized for COVID‐19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821‐1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ji D, Zhang D, Xu J, et al. Prediction for progression risk in patients with COVID‐19 pneumonia: the CALL score [published online ahead of print April 9, 2020]. Clin Infect Dis. 10.1093/cid/ciaa414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kalligeros M, Shehadeh F, Mylona EK, et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity (Silver Spring). 2020;28:1200‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Morbid obesity as an independent risk factor for COVID‐19 mortality in hospitalized patients younger than 50 [published online ahead of print May 23, 2020]. Obesity (Silver Spring). 10.1002/oby.22913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID‐19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907‐1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110‐118. 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li YK, Peng S, Li LQ, et al. Clinical and transmission characteristics of Covid‐19—a retrospective study of 25 cases from a Single Thoracic Surgery Department. Curr Med Sci. 2020;40:295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133:1032‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020;71:762‐768. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rastrelli G, Di Stasi V, Inglese F, et al. Low testosterone levels predict clinical adverse outcomes in SARS‐CoV‐2 pneumonia patients [published online ahead of print May 20, 2020]. Andrology. 10.1111/andr.12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shi Q, Zhang X, Jiang F, et al. Clinical characteristics and risk factors for mortality of COVID‐19 patients with diabetes in Wuhan, China: a two‐center, retrospective study. Diabetes Care. 2020;43:1382‐1391. [DOI] [PubMed] [Google Scholar]
- 52. Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID‐19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sun DW, Zhang D, Tian RH, et al. The underlying changes and predicting role of peripheral blood inflammatory cells in severe COVID‐19 patients: a sentinel? Clin Chim Acta. 2020;508:122‐129. 10.1016/j.cca.2020.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Toussie D, Voutsinas N, Finkelstein M, et al. Clinical and chest radiography features determine patient outcomes in young and middle age adults with COVID‐19 [published online ahead of print May 14, 2020]. Radiology. 10.1148/radiol.2020201754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. J Med Virol. 2020;92:797‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang K, Zhang Z, Yu M, Tao Y, Xie M. 15‐day mortality and associated risk factors for hospitalized patients with COVID‐19 in Wuhan, China: an ambispective observational cohort study. Intensive Care Med. 2020;46:1472‐1474. 10.1007/s00134-020-06047-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang R, Pan M, Zhang X, et al. Epidemiological and clinical features of 125 Hospitalized Patients with COVID‐19 in Fuyang, Anhui, China. Int J Infect Dis. 2020;95:421‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yao Q, Wang P, Wang X, et al. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Pol Arch Intern Med. 2020;130:390‐399. [DOI] [PubMed] [Google Scholar]
- 60. Yu Q, Wang Y, Huang S, et al. Multicenter cohort study demonstrates more consolidation in upper lungs on initial CT increases the risk of adverse clinical outcome in COVID‐19 patients. Theranostics. 2020;10:5641‐5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yu T, Cai S, Zheng Z, et al. Association between clinical manifestations and prognosis in patients with COVID‐19. Clin Ther. 2020;42:964‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yu X, Sun S, Shi Y, Wang H, Zhao R, Sheng J. SARS‐CoV‐2 viral load in sputum correlates with risk of COVID‐19 progression. Crit Care. 2020;24:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yu X, Sun X, Cui P, et al. Epidemiological and clinical characteristics of 333 confirmed cases with coronavirus disease 2019 in Shanghai, China. Transbound Emerg Dis. 2020;67:1697‐1707. 10.1111/tbed.13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730‐1741. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 65. Zhang R, Ouyang H, Fu L, et al. CT features of SARS‐CoV‐2 pneumonia according to clinical presentation: a retrospective analysis of 120 consecutive patients from Wuhan city. Eur Radiol. 2020;30:4417‐4426. 10.1007/s00330-020-06854-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zheng KI, Gao F, Wang XB, et al. Letter to the Editor: obesity as a risk factor for greater severity of COVID‐19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zheng Y, Xiong C, Liu Y, et al. Epidemiological and clinical characteristics analysis of COVID‐19 in the surrounding areas of Wuhan, Hubei Province in 2020. Pharmacol Res. 2020;157:104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Substance Abuse and Mental Health Services Administration (US), Office of the Surgeon General (US) . Smoking Cessation: a Report of the Surgeon General. Washington (DC): US Department of Health and Human Services; 2020. [Google Scholar]
- 69. Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2006‐16. [DOI] [PubMed] [Google Scholar]
- 70. Huttunen R, Heikkinen T, Syrjänen J. Smoking and the outcome of infection. J Intern Med. 2011;269:258‐269. [DOI] [PubMed] [Google Scholar]
- 71. Han L, Ran J, Mak YW, et al. Smoking and influenza‐associated morbidity and mortality: a systematic review and meta‐analysis. Epidemiology. 2019;30:405‐17. [DOI] [PubMed] [Google Scholar]
- 72. Bradley JP, Bacharier LB, Bonfiglio J, et al. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115:e7‐e14. [DOI] [PubMed] [Google Scholar]
- 73. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA Score. Front Microbiol. 2019;10:2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Park JE, Jung S, Kim A, Park JE. MERS transmission and risk factors: a systematic review. BMC Public Health. 2018;18:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Seys LJM, Widagdo W, Verhamme FM, et al. DPP4, the Middle East respiratory syndrome coronavirus receptor, is upregulated in lungs of smokers and chronic obstructive pulmonary disease patients. Clin Infect Dis. 2018;66:45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Brake SJ, Barnsley K, Lu W, McAlinden KD, Eapen MS, Sohal SS. Smoking upregulates angiotensin‐converting enzyme‐2 receptor: a potential adhesion site for novel coronavirus SARS‐CoV‐2 (Covid‐19). J Clin Med. 2020;9:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Leung JM, Yang CX, Tam A, et al. ACE‐2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID‐19. Eur Respir J. 2020;55:2000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cai G, Bossé Y, Xiao F, Kheradmand F, Amos CI. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS‐CoV‐2. Am J Respir Crit Care Med. 2020;201:1557‐1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Smith JC, Sausville EL, Girish V, et al. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS‐CoV‐2 receptor ACE2 in the respiratory tract. Dev Cell. 2020;53:514‐529. 10.1016/j.devcel.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Russo P, Bonassi S, Giacconi, Malavolta M, Tomino C, Maggi F. COVID‐19 and Smoking: is nicotine the hidden link? Eur Respir J. 2020;55:2001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Monteil V, Kwon H, Prado P, et al. Inhibition of SARS‐CoV‐2 infections in engineered human tissues using clinical‐grade soluble human ACE2. Cell. 2020;181:905‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11:875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Farsalinos K, Barbouni A, Niaura R. Systematic review of the prevalence of current smoking among hospitalized COVID‐19 patients in China: could nicotine be a therapeutic option? [published online ahead of print May 9, 2020]. Intern Emerg Med. 10.1007/s11739-020-02355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Klein JD, Thomas RK, Sutter EJ. Self‐reported smoking in online surveys: prevalence estimate validity and item format effects. Med Care. 2007;45:691‐695. [DOI] [PubMed] [Google Scholar]
- 87. Flint AJ, Novotny TE. Poverty status and cigarette smoking prevalence and cessation in the United States, 1983‐1993: the independent risk of being poor. Tob Control. 1997;6:14‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sun Y, Li Y, Bao Y, et al. Brief Report: increased addictive internet and substance use behavior during the COVID‐19 pandemic in China. Am J Addict. 2020;29:268‐270. 10.1111/ajad.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sidor A, Rzymski P. Dietary choices and habits during COVID‐19 lockdown: experience from Poland. Nutrients. 2020;12:E1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Stanton R, To QG, Khalesi S, et al. Depression, anxiety and stress during COVID‐19: associations with changes in physical activity, sleep, tobacco and alcohol use in Australian adults. Int J Environ Res Public Health. 2020;17:4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Luo M, Guo L, Yu M, Jiang W, Wang H. The psychological and mental impact of coronavirus disease 2019 (COVID‐19) on medical staff and general public—a systematic review and meta‐analysis. Psych Res. 2020;291:113190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID‐19 pandemic: a systematic review and meta‐analysis. Brain Behav Immun. 2020;88:901‐907. 10.1016/j.bbi.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone‐based interventions for smoking cessation. Cochrane Database Syst Rev. 2020;4:CD006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


