Abstract
This is a case report of a 60‐year‐old male, without any cardiovascular risk factor and no cardiac history admitted to hospital with a diagnosis of interstitial pneumonia caused by coronavirus disease 2019 (COVID‐19). After 7 days, the blood tests showed a significant rise of inflammatory and procoagulant markers, along with a relevant elevation of high‐sensitivity Troponin I. Electrocardiogram and transthoracic echocardiogram (TTE) were consistent with a diagnosis of infero‐posterolateral acute myocardial infarction and the patient was transferred to the isolated Cath Lab for primary percutaneous coronary intervention (PCI). The angiography showed an acute massive thrombosis of a dominant right coronary artery without clear evidence of atherosclerosis. Despite the optimal pharmacological therapies and different PCI techniques, the final TIMI flow was 0/1 and after 3 hr the clinical condition evolved in cardiac arrest for pulseless electric activity. Acute coronary syndrome–ST‐elevation myocardial infarction is a relevant complication of COVID‐19. Due to high levels of proinflammatory mediators, diffuse coronary thrombosis could occur even in patients without cardiac history or comorbidities. This clinical case suggests that coronary thrombosis in COVID‐19 patients may be unresponsive to optimal pharmacological (GP IIb–IIIa infusion) and mechanical treatment (PCI).
Keywords: acute myocardial infarction, coronary thrombosis, COVID‐19, primary PCI
1. INTRODUCTION
In February 2020 the World Health Organization (WHO) officially termed coronavirus disease 2019 (COVID‐19), the infective severe acute respiratory syndrome caused by the novel coronavirus (SARS‐CoV‐2). From December 2019, when the first cases of COVID‐19 were documented in Wuhan, China, the outbreak spread rapidly to various continents, becoming a pandemic and causing the most serious world health emergency of the past years.
As reported in the previous viral epidemics data, the previous viral epidemics data (SARS, MERS, and H1N1 influenza) describe several cardiovascular complications, including myocarditis, bradi and tachyarrhythmias, heart failure, sudden cardiac death, and acute coronary syndrome (ACS), all significantly affecting the overall mortality of these patients. 1
Our Hospital is located in Brescia, Lombardy (Italy) the Northern region where the COVID‐19 outbreak has been more violent and widespread. In the past month, the Lombardy region has reported an unexpectedly high rate of infection (10 million inhabitants, 70,145 ascertained infections, and 12,940 virus‐related deaths as of April 23, 2020).
1.1. Case report
On the 20th of March 2020, a 60‐year‐old man with a 7‐day history of fever, cough, and radiological diagnosis of interstitial pneumonia was transferred from another hospital to our institution. The patient claimed he had been previously in contact with a colleague positive to COVID‐19. A nasopharyngeal swab for COVID‐19 detection was collected, and after 48 hr the test result confirmed the active infection.
The patient's symptoms started with low‐grade fever, dry cough, asthenia, and muscle pain on March 10. After a couple of days, the fever became higher and unresponsive to paracetamol. The patient had no history of other preexisting pathological conditions except amoxicillin allergy.
The results of physical examination on March 20 revealed blood pressure of 130/85 mmHg, heart rate of 101 bpm, body temperature of 36°C, oxygen saturation (SpO2) of 85% while breathing ambient air, respiratory rate of 18 breath/min; the SpO2 reached 94% after oxygen supplement by Venturi mask at 8 L/min, and FiO2 of 40%.
Routine blood tests at admission revealed normal white blood cell count (6,500 μl) with 84% neutrophil and 9.9% lymphocyte, normal platelet count (146 × 103 μl), normal hemoglobin concentration, high levels of C‐reactive protein (PCR 134 mg/L), and slight increase of lactate dehydrogenase (LDH 372 U/L) normal levels of BNP (proBNP 43 pg/ml). The serum creatinine was 1.09 mg/dl and eGFR at 70 ml/min (Table 1).
TABLE 1.
Clinical laboratory results
| Measure | Reference range | Day 1 | Day 7 |
|---|---|---|---|
| Red blood cell count | 4.2–5.4 × 106 μl | 4.62 | 4.66 |
| Hemoglobin | 14–18 g/dl | 13.6 | 13.9 |
| Hematocrit | 40–52% | 39.7 | 40.6 |
| White blood cell count | 4.0–10.8 × 103 μl | 6.5 | 12.30 |
| Neutrophil count % | 40–75% | 84 | 86 |
| Lymphocyte count % | 20–50% | 9.9 | 6.3 |
| Platelet count | 130–430 × 103 μl | 146 | 289 |
| Creatinine | 0.72–1.18 mg/dl | 1.09 | 1.1 |
| eGFR | >90 ml/min/1.73 m2 | 69 | 61 |
| LDH | 0–248 U/L | 372 | 761 |
| C‐reactive protein | 0.0–7.0 mg/L | 134 | 359 |
| Ferritin | 24–336 ng/ml | 210 | 1,629 |
| d‐dimer | 0–270 ng/ml | 190 | 1,392 |
| Pro BNP | 0–100 pg/ml | 43 | N/A |
| High sensitivity troponin I | 0–19.8 ng/L | 15 | 12,990 |
Abbreviations: eGFR, estimated glomerular filtration rate; LDH, lactate dehydrogenase; N/A, not applicable; Pro BNP, pro‐brain natriuretic peptide.
The arterial gas analysis showed a pH of 7.41, oxygen partial pressure of 71 mmHg, carbon dioxide partial pressure of 38 mmHg, and bicarbonate level of 22 mmol/L.
The chest X‐ray revealed evidence of pneumonia with bilateral multiple interstitial ill‐defined patchy opacities (Figure 1); a 12‐lead electrocardiogram (ECG) showed normal sinus rhythm and normal ST segment (Figure 2a).
FIGURE 1.
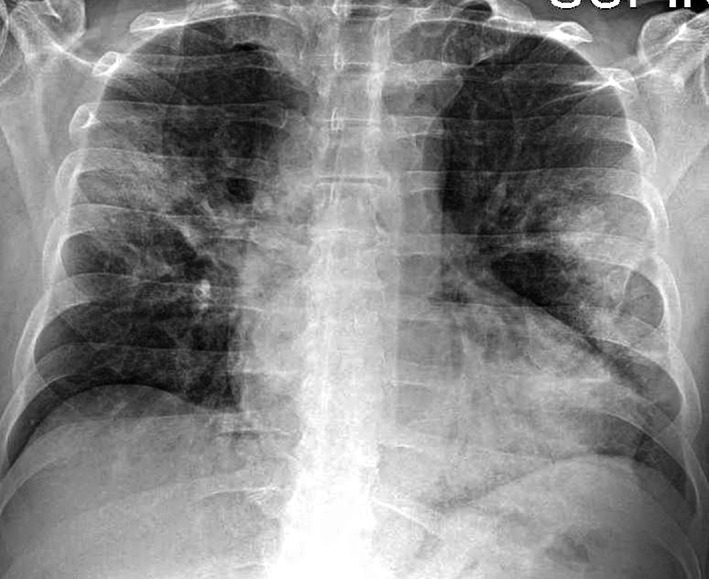
Chest radiography at presentation: bilateral multiple interstitial ill‐defined patchy opacity
FIGURE 2.
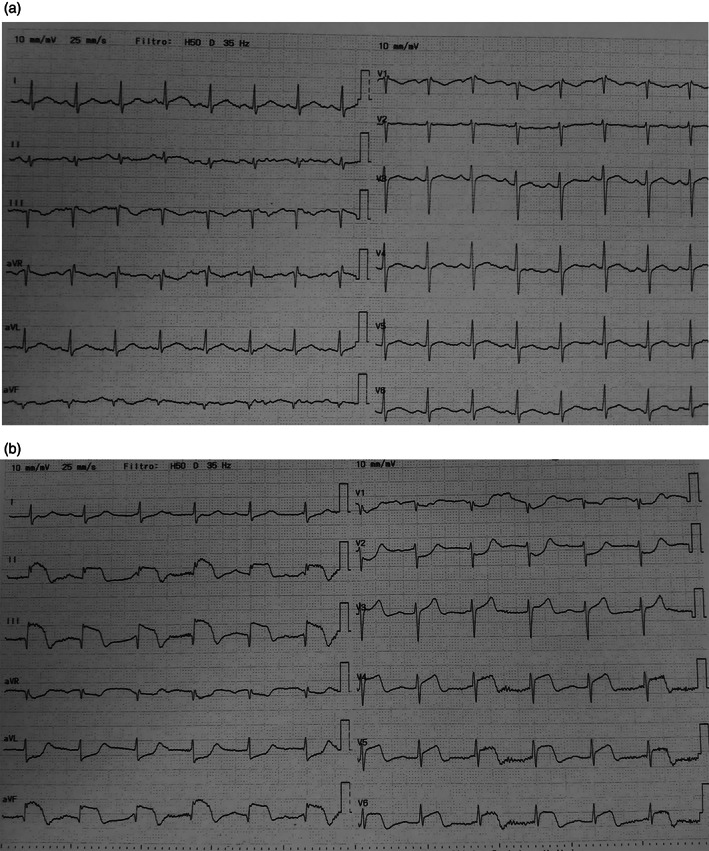
(a) ECG at presentation: normal morphology. (b) ECG at seventh day: ST elevation in the inferior and in V4–V6 and ST depression in aVL and V1–V2 leads
When admitted, the patient was treated with dexamethasone (12 mg iv), hydroxychloroquine (200 mg twice daily), antiviral drugs (lopinavir/ritonavir—2 tablets 200/50 mg twice daily), oxygen support (Venturi mask FiO2 40%), antibiotic prophylaxis with ceftriaxone (2 g iv), and venous thromboembolic (VTE) prophylaxis with enoxaparin (4,000 U.I. sc). After 48 hr, n‐acetylcysteine (600 mg twice daily) and furosemide (20 mg iv twice daily) were administered.
During the first 5 days of the hospital stay, the clinical conditions and the vital signs of the patient were stable (afebrile, SpO2 90–92% at 10 L/min, and FiO2 40%). On the sixth day, the patient showed a significant worsening of the shortness of breath and oxygen saturation (SpO2 87%), indeed respiratory support by CPAP machine was started (FiO2 100%, PEEP 12 cmH2O).
On the seventh day (March 27) the patient showed a significant psychomotor agitation along with slight chest discomfort. The daily blood tests showed a significant rise of pro‐coagulant and inflammatory markers d‐Dimer (1,392 ng/ml), white blood count (12,300 μl), PCR (359 mg/L), LDH (761 U/L), ferritin (1,629 ng/ml) along with a significant elevation of high sensitivity troponin I levels (12,990 ng/ml) (Table 1).
A 12‐lead ECG showed a clear ST elevation in the inferior leads and V4–V6, and an ST depression in aVL and V1–V2 (Figure 2b); Echocardiography quickly performed confirmed the ST‐elevation myocardial infarction (STEMI) diagnosis showing akinesia of the infero‐posterolateral wall with an estimated LVEF of 40%.
Following the local protocol for the management of STEMI patients during the COVID outbreak, we decided to perform an urgent coronary angiography and emergency percutaneous coronary intervention (PCI).
The patient was treated with a ticagrelor oral loading dose of 180 mg, ASA 150 mg, and heparin 4,000 U.I., and immediately transferred to the isolated Cath Lab. The total ischemic time was less than 6 hr and the time from STEMI diagnosis to PCI was less than 60 min.
The coronary angiography from the left radial approach showed no evidence of coronary atherosclerosis of left main, LAD and circumflex, and acute thrombotic occlusion of proximal‐mid right coronary artery (RCA) with TIMI 0 flow (Figure 3a,b).
FIGURE 3.
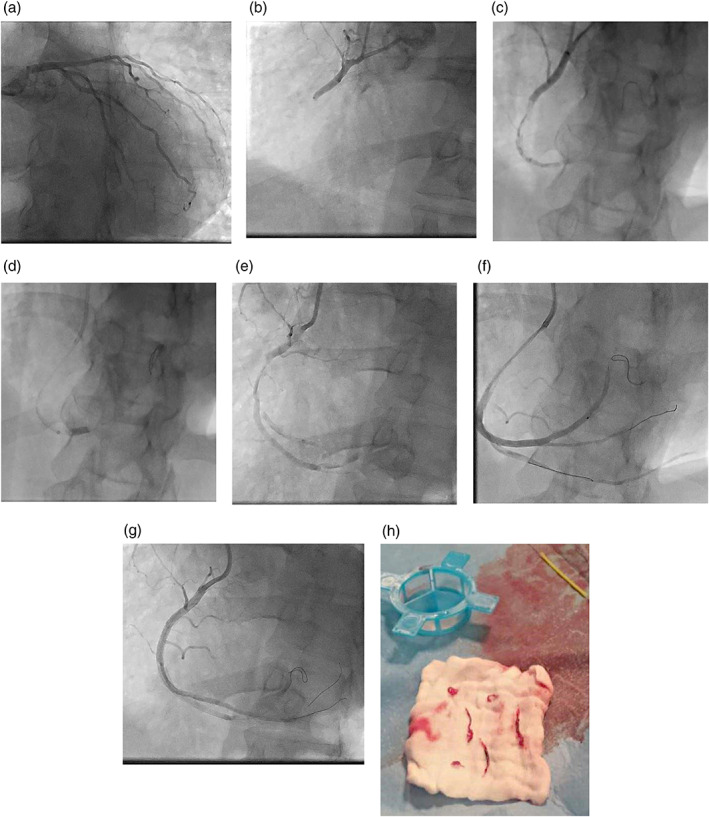
Angiography and PCI for RCA. (a) No evidence of coronary atherosclerosis of left coronary. (b) Acute thrombotic occlusion of RCA. (c) Diffuse intracoronary thrombosis of RCA. (d) Thrombus aspiration using a 6 F Guideliner. (e) Persistent diffuse intracoronary thrombosis. (f) Distal tip injection, no coronary atherosclerosis. (g) Result. (h) Thrombus removed [Color figure can be viewed at wileyonlinelibrary.com]
Angiography showed a high grade thrombus burden diffused in all RCA extensions (Figure 3c). We decide to proceed with manual thrombus aspiration, as first strategy, using a 6 Fr Eliminate aspiration catheter (Terumo Medical) without achieving any effective recanalization. Images of diffuse intracoronary thrombosis with persistent TIMI 0–1 flow was documented. Eptifibatide 180 μg/kg IV bolus was given, followed by continuous infusion of 2 μg/kg/min; the ACT value was 304 s; after some minutes we administered a second eptifibatide 180 μg/kg intracoronary bolus through the tip of Eliminate aspiration catheter, placed in distal RCA.
In order to maximize the thrombectomy effect, we proceeded with a manual thrombus aspiration using a 6 F guide extension catheter Guideliner (Teleflex Medical) as described in previously published case series. 2
The Guideliner was carefully advanced to the distal part of RCA, and aspiration with a 50 cc syringe via a Y connector through the side port was performed during slow retrieval of the catheter (Figure 3d).
Despite removing a large amount of thrombi from the RCA, the antegrade flow was still compromised (TIMI 1). We observed no angiographic improvement after several gentle dilatations with a compliant balloon (Trek 3.0 × 15 mm; Abbott Vascular), bailout implant of DES (Xience Sierra 3.5 × 33 mm; Abbott Vascular) on the mid‐RCA where a more extensive thrombus was present and even after adenosine and nitroprusside intracoronary injection (Figure 3e–h).
At the end of the procedure, TIMI flow at was still 1–2, Myocardial Blush Grade (MBG) 0. The arterial pressure was 120/60 mmHg, while the final ECG showed a persistent ST elevation in the infero‐lateral leads similar to the pre‐PCI ECG, confirming that the myocardial revascularization was ineffective.
The patient was then retransferred to the intensive care unit (ICU) with Eptifibatide infusion of 2 μg/kg/min and respiratory support by CPAP.
After 3 hr, the patient developed significant oxygen desaturation (SpO2 70%), dyspnea, and psychomotor agitation during C‐PAP; the vital signs were PA 100/60 mmHg; HR 90 bpm; the ECG was unchanged (persistent inferolateral ST elevation). Endotracheal intubation with rapid sequence induction and invasive ventilation was immediately performed. The clinical condition evolved after 15 min in severe hemodynamic instability, hypotension, wide QRS, and cardiac arrest for pulseless electric activity (PEA); the advanced cardiopulmonary resuscitation maneuvers were immediately started according with advanced cardiopulmonary life support (ACLS) algorithm and were prolonged for more than 30 min without return of spontaneous circulation (ROSC).
2. DISCUSSION
Previous studies 3 , 4 , 5 , 6 have linked acute infections with an increased risk for acute myocardial infarction (AMI). In fact, the host inflammatory response to the infection often results in the release of proinflammatory cytokines and activation of platelets, leukocytes, and endothelial cells that can activate procoagulant pathways with prothrombotic status and consequently higher risk of acute cardiovascular events such as AMI. 3 , 4
Many possible biological factors related to the COVID‐19 infection could be involved in the physiopathological cascade that could precipitate cardiovascular complications, especially ACS 7 :
High levels of systemic proinflammatory cytokine and mediators of atherosclerosis may determine the plaque rupture through local inflammation.
Procoagulant effects of systemic inflammation may cause coronary thrombosis: plaque thrombosis, stent thrombosis, and spontaneous thrombosis.
Oxygen imbalance caused by pneumonia, with an infection‐induced increase in metabolic demand and reduced cardiac reserve.
Direct viral infection of myocardial and vascular cells.
Two recent studies have reported that patients who develop myocardial injury (elevation of TnI, NT‐proBNP) show more severe inflammation (elevation of PCR, IL‐6, procalcitonin), higher incidence of ARDS, the more frequent need of assisted ventilation and considerably higher short‐term mortality. 7 , 8
Recent articles have described impaired hemostasis and prothrombotic state associated with elevated multifocal thrombosis, DIC, and even antiphospholipid antibodies 9 , 10 , 11 in patients hospitalized for COVID‐19.
In a study by Tang et al from Wuhan, 10 71% of nonsurvivors from COVID‐19 infection met the ISTH criteria for DIC compared with 0.4% of survivors. Elevated d‐dimer at admission and markedly increasing d‐dimer levels (three‐ to fourfold) over time were associated with high mortality, likely reflecting coagulation activation from infection/sepsis, cytokine storm, and impending multiorgan failure. 10 Furthermore, other reports showed that patients with COVID 19 and severe respiratory distress often develop complications such as liver dysfunction or renal failure which can be related to procoagulant status. 12 , 13 , 14
In our report, the 60 years old male developed an AMI‐STEMI just when blood tests showed an important elevation of proinflammatory and thrombotic activity mediators while these results were normal in the preceding days.
The main angiographic finding was an acute massive thrombosis of a dominant RCA without evidence of clear coronary stenosis or atherosclerotic plaques. Considering the diffuse thrombosis was resistant to pharmacologic and mechanical therapy, this case supports the hypothesis that the proinflammatory and prothrombotic status related to COVID‐19 infection was the main factor that triggered the coronary thrombosis.
2.1. Limitations
As the intracoronary imaging test was not performed (OCT‐IVUS), the limitations of this report is the lack of clear information about the pathogenetic mechanisms underlying the RCA thrombosis. Furthermore, as the autopsy was not performed, we assume that fatal outcome was due to a combination of severe oxygen desaturation, acute ischemic heart failure, and consequent severe acidosis. However, we cannot exclude concomitant pulmonary thromboembolism or thrombosis of other organs as precipitating factors of cardiac arrest.
3. CONCLUSIONS
This case report highlights the clinical impact of ACS‐STEMI in COVID‐19 patients. High levels of proinflammatory and hyperthrombotic activity could significantly affect the final outcome, even in young patients without cardiac past history or significant comorbidities.
The presence of diffuse coronary thrombosis, unresponsive to the pharmacologic and mechanical percutaneous therapies, may represent a peculiar expression of the systemic prothrombotic status, which has been described in the clinical scenario of others critically ill patients. Further evidence is needed to determine if the therapeutic research may focus on antiinflammatory mediators or conversely on effective anticoagulation which may prevent cardiovascular injury. It is likely that these two factors should be both addressed by a combination of new therapies.
Tedeschi D, Rizzi A, Biscaglia S, Tumscitz C. Acute myocardial infarction and large coronary thrombosis in a patient with COVID‐19. Catheter Cardiovasc Interv. 2021;97:272–277. 10.1002/ccd.29179
REFERENCES
- 1. Madjid M, Safavi‐Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831. 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 2. Farooq V, Serruys PW, Mustafa AH, et al. Forward and back aspiration during ST‐elevation myocardial infarction: a feasibility study. EuroIntervention. 2016;11(14):e1639‐e1648. 10.4244/EIJV11I14A31. [DOI] [PubMed] [Google Scholar]
- 3. Corrales‐Medina VF, Madjid M, Musher DM. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. 2010;10(2):83‐92. 10.1016/S1473-3099(09)70331-7. [DOI] [PubMed] [Google Scholar]
- 4. Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611‐2618. [DOI] [PubMed] [Google Scholar]
- 5. Warren‐Gash C, Hayward AC, Hemingway H, et al. Influenza infection and risk of acute myocardial infarction in England and Wales: a CALIBER self‐controlled case series study. J Infect Dis. 2012;206:1652‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory‐confirmed influenza infection. N Engl J Med. 2018;378(4):345‐353. 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 7. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802‐810. 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:811. 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844‐847. 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang T, Chen R, Liu C, et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID‐19. Lancet Haematol. 2020;7(5):e362‐e363. 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and Antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382:e38. 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 14. Kui L, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133(9):1025–1031. 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]


