Abstract
Background
COVID‐19 convalescent plasma (CCP) represents an appealing approach to the treatment of patients with infections due to SARS‐CoV‐2. We endeavored to quickly establish a sustainable CCP transfusion program for a regional network of health care facilities.
Study design and methods
A regional collaborative group was activated to address the issues necessary to implementing a CCP transfusion program and making the program sustainable. A wide range of health care providers including physicians (critical care, infectious disease, transfusion medicine), nurses, pharmacists, laboratorians, and information technology (IT) specialists were required to make the program a success.
Results
The CCP implementation team initially consisted of four members but quickly grew to a group of nearly 20 participants based on different issues related to program implementation. Overall, six major implementation “themes” were addressed: (a) registration of individual hospitals and principal investigators with a national investigational new drug research protocol; (b) collaboration with a regional blood donor center; (c) targeted recruitment of convalesced donors; (d) IT issues related to all aspects of CCP ordering, distribution, and transfusion; (e) prioritization of patients to receive CCP; and (f) evaluation of CCP products including antibody characteristics and patient response to therapy.
Conclusion
Within 4 weeks of initiation, CCP was successfully transfused at multiple hospitals in our regional health care delivery system. A program infrastructure was established that will make this program sustainable into the future. This approach has broader implications for the success of multi‐institutional programs requiring rapid implementation.
Abbreviations
- CCP
COVID‐19 convalescent plasma
- IND
investigational new drug
- IT
information technology
- MVRBC
Mississippi Valley Regional Blood Center
SARS‐CoV‐2 is a novel coronavirus and is the etiologic agent of COVID‐19. 1 It is a single‐stranded RNA virus that was first identified in Wuhan, China, in December 2019. Although brought under control locally, the virus proved to be highly communicable with infection declared to have reached pandemic status by the WHO on 11 March 2020. 2 Since that time, the virus has been detected in virtually every country in the world, has caused well over 4 million cases of disease, and is responsible for hundreds of thousands of deaths. 3 The United States has not been immune to the ravages of COVID‐19 and now accounts for the highest number of cases and associated fatalities worldwide. 3
A variety of antiviral agents, and combinations of agents, are under intense evaluation for the treatment of COVID‐19. 4 In addition, multiple vaccine candidates are being evaluated. 5 However, no established, evidence‐based treatments are available at this time. COVID‐19 convalescent plasma (CCP) represents a novel approach to the treatment of COVID‐19 patients and has deep historical roots. Passive antibodies obtained from convalesced patients have shown promising results in the treatment of a variety of viral infectious diseases including influenza, measles, and SARS. 6 This strategy is also the basis for hyperimmune globulin therapy (eg, hepatitis B immune globulin). At the same time, convalescent plasma has not always proven to be a useful adjunct to more conventional therapy, as the recent Ebola virus epidemic in West Africa demonstrated. 7
Without a vaccine or effective medications to ameliorate COVID‐19, the establishment of convalescent plasma transfusion programs has taken on greater urgency. To this end, a national investigational new drug (IND) expanded access program (National Clinical Trial Identification Number 04338360), administered by the Mayo Clinic, was initiated in March 2020 to study the effectiveness of CCP in a defined patient population (those with severe or life‐threatening COVID‐19) and facilitate the provision of CCP to participating institutions. 8 Our regional health care delivery network, SSM Health, a major provider of health care services in the St Louis region, became engaged in the rapid establishment of a CCP program for its network hospitals. In this paper, our approach to the implementation of a successful CCP program is described.
1. MATERIALS AND METHODS
SSM Health is a comprehensive, not‐for‐profit health system composed of 23 hospitals, 300 physician offices, and a variety of other health care services. The system spans four states (Illinois, Missouri, Oklahoma, and Wisconsin). The St Louis network of hospitals, heretofore referred to as SSM‐STL, includes seven facilities: five community hospitals; a children's hospital; and an academic, university‐affiliated hospital. With the exception of the Oklahoma‐based hospitals, all other SSM Health facilities use Mississippi Valley Regional Blood Center (MVRBC) as the provider of blood products and related services. Importantly, no hospital has the infrastructure to collect blood products, including CCP.
When it became apparent that CCP represented an important therapeutic option for patients with COVID‐19, the SSM‐STL network became interested in bringing this therapeutic option to its member hospitals in St Louis. In late March 2020, a core team consisting of the regional directors of pharmacy and laboratory services, the medical director of the regional donor center (MVRBC), and a transfusion medicine specialist was convened to initiate program planning. The expressed goals of this group were stated as follows:
To have a donor available to MVRBC on the first day that they can accept a donor;
To infuse a COVID‐19 patient within 48 hours of that donation;
To make as many doses of CCP available to hospital providers as needed.
Based on these goals, this core team defined six program elements that would need to coalesce to meet the goals of the CCP transfusion project and make it sustainable. These program elements included the following:
Registration of individual hospitals and principal investigators with a national IND research protocol;
Collaboration with our regional donor center, MVRBC;
Targeted recruitment of convalesced donors;
Information technology (IT) issues related to the receipt of CCP in the hospital blood bank, clinician order of plasma, and distribution of plasma for transfusion;
Prioritization of patients to receive CCP;
Evaluation of CCP products received including antibody titers and patient response to therapy.
2. RESULTS
2.1. Registration of individual hospitals and principal investigators with a national IND research protocol
Convalescent COVID‐19 plasma is not an approved therapy for infection with the SARS‐CoV‐2 virus. As such, authorization to collect and transfuse CCP must be done under an Food and Drug Administration (FDA) IND protocol. Individual patients can be enrolled through an emergency IND, although this becomes cumbersome for an institution that envisions the enrollment of multiple patients. Fortunately, an expanded access IND was initiated by the Mayo Clinic using their centralized institutional review board to oversee the process. On the patient transfusion side, this requires enrollment of the institution, registration of a principal investigator for that institution, and enrollment of individual patients who are candidates for CCP. With respect to the latter, patient follow‐up is required at proscribed time‐frames (4 hours, 7 days, 30 days) and serious adverse events must be reported. All of this is handled electronically through the Mayo COVID‐19 plasma website (uscovidplasma.org). The hospitals in the SSM‐STL network were quickly registered with the Mayo IND with the exception of the children's hospital, because the Mayo IND only allows for CCP transfusion to adults (≥18 years of age). Convalescent plasma at the children's hospital, if needed, would be undertaken with a patient‐specific emergency IND. Once hospitals were registered, a clinician from each member hospital was identified to serve as principal investigator and that individual was registered with the Mayo site. These individuals were largely infectious disease or critical care physicians.
2.2. Collaboration with our regional donor center, MVRBC
Although the transfusion of CCP must be undertaken under an appropriate IND, the collection of CCP and its processing does not differ, in any material way, from the routine collection of blood products. In short, donors of convalescent plasma are required to meet all donor eligibility criteria with the exception that they have positive nucleic acid testing (NAT) for SARS‐CoV‐2. In addition, the donor must have been asymptomatic for a period of at least 28 days. The FDA originally made an exception for donors in the 14‐ to 27‐day period of asymptomatic convalescence, but qualification in this time period would require repeat negative molecular testing for SARS‐CoV‐2. However, this requirement was rescinded on 1 May 2020. Despite this change, MVRBC, in conjunction with the SSM‐STL CCP collaborative group, decided to retain the 28‐day period of convalescence reasoning that antibody levels would likely be higher in this time frame. Of note, before CCP is distributed to hospitals by MVRBC, it is assessed for antibody activity using the Abbott Diagnostics SARS‐CoV‐2 IgG assay.
In addition, to implementing a system for qualifying donors (eg, standard operating procedure changes), the blood center had to validate additional apheresis instruments for the collection of convalescent plasma, assure an adequate supply and availability of apheresis kits, and provide those resources to the St Louis region for plasma collection (the primary site of operation for MVRBC is Davenport, Iowa). As part of their implementation, the blood center decided that plasma would be collected from eligible donors by apheresis technology rather than through whole blood donation. The benefits of this approach are that (a) up to an 800‐mL collection could serve as the source for multiple 200‐mL CCP units and (b) donors could be recruited at a more frequent interval for repeat donations. In contrast, only a single CCP unit could be obtained from a whole blood donation, the red cells would likely not be deemed acceptable for transfusion, and the interval between donations would be the standard 56‐day temporary deferral. Finally, MVRBC required assistance from their hospital clients to identify eligible convalesced donors to successfully launch their CCP collection program.
2.3. Targeted recruitment of convalesced donors
As opposed to making a broad regional appeal for convalescent donors, it became evident that the process could be jump‐started by targeting recruitment to patients known to have a positive molecular test for SARS‐CoV‐2. Fortunately, the SSM‐STL network has a regional clinical microbiology facility that implemented a laboratory developed NAT for SARS‐CoV‐2. Nearly 200 patients who tested positive were identified. A stepwise process was implemented to connect these potential donation candidates to MVRBC for assessment. First, the potential donor's medical record was interrogated to determine if they were suitable for donation. As an example, patients who were deceased, less than 18 or more than 80 years, or currently hospitalized were excluded from immediate consideration. The potential donors were then contacted to determine their interest in donating plasma and their willingness to be contacted by MVRBC to discuss donation. For those who were interested and agreed to contact, an online MVRBC physician referral form was completed. Thereafter, a representative from MVRBC contacted the potential donor, explained the process of donation, and conducted a brief screen to determine suitability. Appropriate donors were then scheduled for donation. This was mostly based on the time at which they would reach the 28‐day threshold of convalescence. Additional COVID‐19 patients (approx. 100) were identified through an interrogation of the Epic electronic health record, to identify patients who tested positive for the virus in a facility other than the SSM‐STL centralized microbiology laboratory. These patients were contacted in the same manner described above. Finally, a system was implemented so that hospitalized COVID‐19 patients who recovered would be asked about their interest in donating convalescent plasma as part of a routine discharge follow‐up call. In sum, this targeted approach to donor recruitment facilitated the identification of over 130 suitable candidates who agreed to be contacted about plasma donation (see Figure 1 for recruitment details and Table 1 for characteristics of this potential donor population).
FIGURE 1.
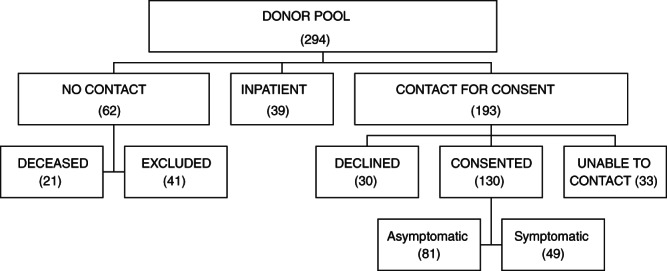
Patients with confirmed COVID‐19 were evaluated for their suitability to be approached by MVRBC for CCP donation. Patients were not contacted if they were deceased or had severe underlying clinical conditions making them unsuitable donors or were hospital inpatients. The remaining patients were contacted, the majority of whom consented to be approached by MVRBC for plasma donation
TABLE 1.
Characteristics of COVID‐19 patients who agreed to be contacted about convalescent plasma donation (n = 130)
| Characteristic | Value |
|---|---|
| Age (y) | |
| Mean | 48 |
| Median | 49 |
| Range | 20‐78 |
| Sex | |
| Male | 59 |
| Female | 71 |
| Ethnicity a | |
| Black | 64 |
| White | 59 |
| Other | 1 |
| Declined | 6 |
| CCP Donors (total) | 36 |
| Repeat donors b | 11 |
No potential donors were of Hispanic or Latino origin.
Four additional donors attempted to donate but were deferred.
2.4. IT issues related to the receipt of CCP in the hospital blood bank, clinician order of plasma, and distribution of plasma for transfusion
While donors were being recruited for CCP donation, it became critical to establish the IT infrastructure that would support the plasma program at the hospital level. This would allow for the tracking of units through all phases of the transfusion process including plasma receipt into the blood bank, order for CCP preparation and transfusion by the clinical provider, distribution of the product to the patient, and documentation of transfusion in the blood product administration module of the Epic electronic health record. Aside from tracking individual units, a robust IT infrastructure would allow for the easy retrieval of program data from the individual hospitals in the SSM‐STL network. In addition to the more standard procedural elements described above, unique features were built into the ordering process to meet the needs of the Mayo Clinic IND protocol. These included links to the Mayo consent and the patient registration forms (Figure 2). Once a patient is registered in the Mayo system, a unique numerical identifier is provided to the principal investigator for the site. The inclusion of this number, as a required field in the CCP ordering process, was a critical addition related to blood product billing. Specifically, MVRBC does not charge the ordering hospital for CCP as long as the patient is registered with the Mayo protocol and the patient's unique identifier is provided to the blood center. The cost for the CCP units is borne by the Biomedical Advanced Research and Development Authority (BARDA), a sponsor of this research protocol along with the FDA. In sum, a robust IT infrastructure has allowed us to closely monitor all aspects of the transfusion process and has provided us with a resource for rapid data extraction. This contrasts with facilities that track CCP using manual downtime procedures.
FIGURE 2.
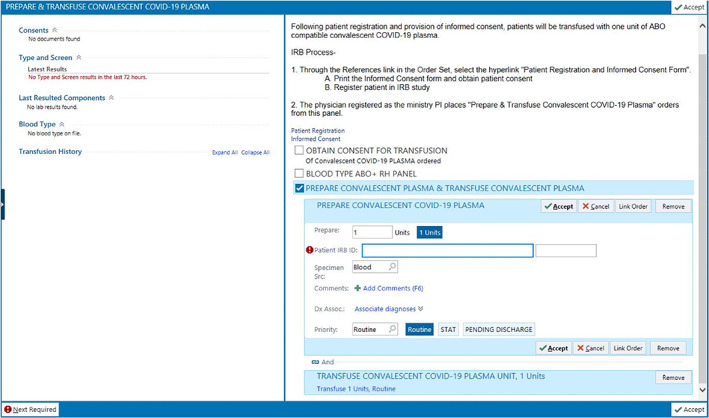
A screenshot depicting the ordering process for CCP in the Epic electronic health record. Note information concerning the Mayo Clinic IND protocol, links to patient registration and the informed consent form, and the required field for the patient‐specific identification number [Color figure can be viewed at wileyonlinelibrary.com]
2.5. Prioritization of patients to receive CCP
Approximately 2 weeks into the development of our comprehensive, multi‐institutional CCP program, it became obvious that, at least early on, the demand for CCP would far outstrip the availability of the product. Because of this, the principal investigators at each site determined that it would be critical for them, as a group, to discuss patients considered to be appropriate for CCP and prioritize patients prior to their registration with the Mayo IND. The Mayo IND provides specific criteria for patient inclusion in the protocol, namely, that they have positive molecular testing for SARS‐CoV‐2, are an adult (≥18 years of age), and have met defined clinical criteria qualifying them as having severe or life‐threatening COVID‐19. 8 These inclusion criteria were a useful starting point for our clinicians, but it was felt that the additional consideration of significant comorbid conditions and an objective measure of disease severity (SOFA scoring system; Sequential Organ Failure Assessment) would assist in prioritizing patients for CCP. To this end, our team of principal investigators developed criteria, approved by the SSM‐STL clinical practice group, to rank patients in a tiered prioritization scheme (see Appendix S1 and Emanuel et al 9 ). The overriding ethical principle driving prioritization was maximal survival benefit—that is, with a scarce resource such as CCP, patients most likely to benefit by this therapeutic intervention should have priority in receiving it. Related to this, patient age was not included as a criterion for prioritization though special consideration was given to pregnant patients and health care workers. Initially, the principal investigator team met daily to determine the prioritization of patients who were candidates for CCP. After a week of daily meetings, weekly meetings were held as the clinical team developed confidence in the group members' ability to identify appropriate patients and move the process forward successfully (ie, patient registration, CCP ordering, etc.).
2.6. Evaluation of CCP products received including antibody titers and patient response to therapy
The overriding goals of the Mayo IND for CCP are to determine the safety and efficacy of the product in treating severely and critically ill COVID‐19 patients while facilitating availability to eligible patients. This aspect of the project is being coordinated through the American Red Cross, but participating hospitals are free to obtain plasma through their local blood supplier (eg, MVRBC). Unfortunately, because of the likely variability of the antibody content and composition of the plasma products and the almost certain variability of the patients who ultimately receive CCP, a determination of efficacy will be challenging. To gain a better understanding of the utility of CCP, it was reasoned that it would be important to analyze the serologic characteristics of each unit of CCP transfused along with an evaluation of the recipient. This would provide a more nuanced understanding of efficacy. To this end, serum aliquots from each donor of convalescent plasma will be evaluated through a variety of research assays and a serologic assay that was released by the FDA under emergency use authorization. Research assays include IgG and IgM ELISA assays for antibody to SARS‐CoV‐2 nucleocapsid antigen, spike proteins (S1 + S2), and spike protein binding domain. The Abbott Diagnostics SARS‐CoV‐2 IgG assay will also be evaluated at the same time. Most importantly, convalescent serum samples will be assessed in a viral neutralization assay, to determine if a donor's immune response actually has viral neutralizing capability. With Saint Louis University institutional review board approval, patients receiving CCP will also be evaluated, primarily through electronic medical record review, to determine if the transfusion of convalescent plasma had a beneficial effect, clinically and through laboratory value interrogation. These studies may prove to identify the types of patients who might benefit most by CCP therapy and the CCP donors with the greatest likelihood of having plasma capable of delivering a beneficial therapeutic effect.
The time span from project initiation to the successful transfusion of a unit of CCP was 30 days. The chief reason for the delay in transfusion resided in the fact that we were waiting for our donor pool to reach the 28‐day asymptomatic threshold required for donation. All other elements of our comprehensive, multi‐institutional CCP program were in place.
3. DISCUSSION
Convalescent plasma has a long history of utilization for patients suffering from a variety of infectious diseases including influenza viruses (more typical influenza A and B as well as H1N1 and H7N9 strains), Ebola virus, and non–SARS‐CoV‐2 coronavirus infections (SARS and MERS). 6 , 7 Early evidence suggests that it may have utility in augmenting severe COVID‐19 disease. 10 , 11 Although theoretically appealing, all interventions to date have suffered from a lack of rigorous randomized controlled trials that clearly demonstrate clinical efficacy. Most often, the products transfused are not well characterized with respect to viral neutralization properties and the treated patient populations are heterogeneous. 12 Thus, optimal donors and patients are generally not understood. In addition, even routine plasma transfusions are not without some risk including both infectious and noninfectious adverse effects of transfusion (eg, transfusion‐associated circulatory overload, transfusion‐related acute lung injury, and allergic reactions of varying degrees of severity). 12 More specifically, convalescent plasma may be associated with immunologic complications including a blunting of the patient's own immune response and the possibility of antibody‐dependent enhancement of disease. 13 , 14 , 15 Nevertheless, convalescent plasma utilization is more compelling when a disease has no proven therapeutic interventions, such as COVID‐19.
The current COVID‐19 pandemic represents a unique opportunity to put convalescent plasma to the test, as a prophylactic intervention, a disease‐modulating agent (eg, to decrease the likelihood of disease progression), and as a therapeutic for severe or life‐threatening clinical disease. It is this use that is the basis of the Mayo Clinic IND protocol. Although not a randomized controlled study, this protocol provided the infrastructure to initiate a convalescent plasma transfusion program in the SSM‐STL network, which is the basis for this report. Our approach is unique in that it is comprehensive, involving all aspects of the transfusion process from donor recruitment to infusion of the plasma product. Further, it includes a research arm that will help to answer important issues regarding the utility of CCP including a definition of the serological characteristics of each unit transfused and a determination of efficacy based on clinical disease manifestations and the evaluation of laboratory variables. This may provide insight into the patients most likely to benefit from this therapy (ie, who to transfuse and when to transfuse in the course of their disease).
Our CCP program was initiated with a core group of four individuals who quickly developed program goals and determined the programmatic elements that would have to be in place to make the program robust and sustainable. The input of a much larger team of individuals, including pharmacy and laboratory staff members, IT specialists, nurses, and physicians, was required at different points in the program development process. In the end, when CCP became available a fully mature program was already established. We were able to jump‐start a convalescent plasma donor pool through the targeted recruitment of appropriate donors. We benefited directly by this approach in creating a sustained supply of CCP. Our approach can serve as a model for others wishing to embark on establishing a CCP program for a multihospital system. More than this, though, we hope that our general approach may be of benefit to any organization faced with the need to rapidly establish a complex program requiring the collaboration of a variety of individuals over multiple institutions. It can be done.
CONFLICTS OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGMENTS
The authors acknowledge the outstanding contributions made by regional pharmacy staff for the recruitment of convalescent plasma donors, regional blood bank staff for processing and distributing convalescent plasma, information technology staff for developing the system to manage all aspects of the hospital transfusion process, and the staff at Mississippi Valley Regional Blood Center for rapidly implementing a system to collect convalescent plasma. Most importantly, the authors acknowledge and thank the COVID‐19 survivors who donated their convalescent plasma.
Blackall D, Wulff S, Roettger T, et al. Rapid establishment of a COVID‐19 convalescent plasma program in a regional health care delivery network. Transfusion. 2020;60:2203–2209. 10.1111/trf.16026
REFERENCES
- 1. Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Coronavirus disease 2019 (COVID‐19) situation summary. https://www.cdc.gov/coronavirus/2019‐ncov/cases‐updates/summary.html. Accessed April 20, 2020.
- 3. Johns Hopkins University and Medicine . New cases of COVID‐19 in world countries. https://coronavirus.jhu.edu/data/new-cases. Accessed May 10, 2020.
- 4. Zhang Y, Xu Q, Sun Z, Zhou L. Current targeted therapeutics against COVID‐19: based on first‐line experience in China. Pharmacol Res. 2020;157:104854. 10.1016/j.phrs.2020.104854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhama K, Sharun K, Tiwari R, et al. COVID‐19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020;18:1–7. 10.1080/21645515.2020.1735227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sullivan HC, Roback JD. Convalescent plasma: therapeutic hope or hopeless strategy in the SARS‐CoV‐2 pandemic. Transfus Med Rev. 2020. 10.1016/j.tmrv.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mayo Clinic . COVID‐19 expanded access program. https://www.uscovidplasma.org/. Accessed April 10, 2020.
- 9. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid‐19. N Engl J Med. 2020;382:2049–2055. 10.1056/NEJMsb2005114 [DOI] [PubMed] [Google Scholar]
- 10. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 convalescent plasma. JAMA. 2020;323:1582–1589. 10.1001/jama.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dzik S. COVID‐19 convalescent plasma: now is the time for better science. Transfus Med Rev. 2020. 10.1016/j.tmrv.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katzelnick LC, Gresh L, Holloran E, et al. Antibody‐dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fleming AB, Raabe V. Current studies of convalescent plasma therapy for COVID‐19 may underestimate risk of antibody‐dependent enhancement. J Clin Virol. 2020;127:104388. 10.1016/j.jcv.2020.104388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smatti MK, Al Thani AA, Yassine HM. Viral‐induced enhanced disease illness. Front Microbiol. 2018;9:2991. 10.3389/fmicb.2018.02991 [DOI] [PMC free article] [PubMed] [Google Scholar]


