Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV2) is spreading all over the world and poses a great threat to humans. This study aimed to systematically review the current situation and public health burden associated with children infected with SARS‐CoV2.
Methods
We searched four electronic databases without language limitations. The pooled proportion or odds ratio (OR) and 95% CI confidence interval (CI) were calculated for each analysis to explore the prevalence of asymptomatic infection and coinfection, as well as to assess the sex of SARS‐CoV‐2‐infected children.
Results
We obtained data from 14 eligible studies with 410 patients for the meta‐analysis. The pooled proportion of asymptomatic infection was 40.45% (95% CI, 24.04‐56.85), while coinfection was 10.14% (95% CI, 3.97‐16.30), of which Mycoplasma pneumonia (50%; 95% CI, 28.24‐71.76) and influenza virus or parainfluenza virus (22.76%; 95% CI, 4.76‐40.77) were the most common pathogens. Both male and female children were susceptible to SARS‐CoV2 infection. And the pooled proportion of family clustering infection was 83.63% (95% CI, 77.54‐89.72).
Conclusion
A high proportion of asymptomatic infections occurs in children infected with SARS‐CoV2, who are also susceptible to coinfection regardless of sex. These data affirm the increasing public health burden arising from infected children regarding the causation of asymptomatic infection or misdiagnosis and as a significant contributor to virus spread. The public should pay more attention to children during epidemics and conduct multimethod detection to further effectively identify infected children and control the source of infection.
Keywords: asymptomatic infection, children, coinfection, SARS‐CoV2
1. INTRODUCTION
The city of Wuhan in China became the focus of global attention due to an outbreak of pneumonia of unknown etiology in late December 2019 that was epidemiologically linked to a seafood wholesale market, where the sale of live animals also occurred. The pathogen was promptly identified as a novel coronavirus, which is currently designated the 2019 novel coronavirus then officially named severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV2). 1 , 2 Although the estimated mortality rate of SARS‐CoV2 is approximately 6%, 3 which is lower than that of severe acute respiratory syndrome (SARS) (9.6%), its transmission rate is similar to that of SARS (3%), leading to an increase in mortality. At present, there are no effective medicines, and vaccines against SARS‐CoV2 are still in the trial stage. As a result of human‐to‐human transmission, SARS‐CoV2 spread very fast and caused a formidable outbreak in many cities in China and globally. As of 13th April 2020, a total of 1 773 084 cases were confirmed, with 111 562 deaths. 4 The primary reported cases were mainly middle‐aged and elderly patients, with fewer cases among children. However, both adults and children are susceptible to infection with this virus.
The first confirmed pediatric case of SARS‐CoV2 infection was reported in Shenzhen, China on January 20. 5 This first pediatric case was a ten‐year‐old asymptomatic child who had a CT scan to determine ground‐glassopacity in the lungs after contact with confirmed cases in the family. According to published statistics, there were 44 672 laboratory confirmed cases throughout China as of February 11, of which only 965 (2%) were younger than 19 years. 6 However, the number of infected children is increasing significantly with the peak of the epidemic, and more tests for pathogens are being carried out in communities. Additionally, some children have asymptomatic infections, facilitating virus evasion and diffusion, and this has gradually attracted more public attention.
The outbreak of SARS‐CoV2 is catastrophic. There have been many studies on SARS‐CoV2 infection in adults, bringing surveillance precautions into much public awareness, whereas studies on infected children are still relatively scarce due to fewer cases or difficulty in conducting full‐scale detection. Therefore, it is valuable to perform a comprehensive analysis of the different published SARS‐CoV2 pediatric cases recording clinical and epidemiological features, merging and contrasting results across multiple studies and identifying the current status and public health burden of childhood infection through pooled estimates.
Thus, the present study was undertaken to evaluate the current status of SARS‐CoV2 infection in children by performing a meta‐analysis to raise awareness and concern about the growing health burden on the public arising from infected children. The findings provide some helpful information for clinicians and the public health sector for developing tracking policies and for preparing intensive strategies against SARS‐CoV2 threats.
2. METHODS
2.1. Search strategy
The present review adhered to the Systematic Review and Meta‐Analysis's Preferred Reporting Project (PRISMA) statement. 7 In the second week of April 2020, a search was conducted in four electronic databases, including PubMed, EMBASE, Web of Science and the Chinese databases China National Knowledge Infrastructure (CNKI), without language limitations. The following search terms (MeSH) were used in “All fields” to identify relevant published articles:
-
1.
“SARS‐CoV‐2” OR “SARS2” OR “COVID19” OR “COVID‐19 pandemic” OR “COVID‐19 virus infection” OR “coronavirus disease‐19” OR “2019 novel coronavirus infection” OR “2019‐nCoV infection” OR “coronavirus disease 2019” OR “2019‐nCoV disease” OR “COVID‐19 virus disease” OR “2019‐nCoV” OR “2019 novel coronavirus disease”
-
2.
“child” OR “children” OR “infant” OR “infants”
-
3.
1 AND 2.
2.2. Study selection
The published studies were retrieved with no restrictions of language. The included studies were required to meet the following eligibility criteria: (a) studies focused on pediatric patients infected with SARS‐CoV2 whose nucleic acid test or CT scan were positive; (b) retrospective observational studies, case reports or research articles describing the epidemiological, demographic, and clinical features of confirmed cases, which allowed stratification; and (c) a minimum size of patients (n > 3) to conduct a meta‐analysis. Reports published as review articles, editorials, press, managements or guidance were excluded. We also excluded repetitive cases by searching the hospitals where the data were collected because they contained the same information. All titles were independently screened by two authors in accordance with the search strategy mentioned above (BJ and HW). A total of 747 records were acquired from the 4 electronic databases. After removing duplicate records, two authors independently performed an abstract review of potentially relevant records to assess their eligibility based on the inclusion/exclusion criteria. Differences were resolved through discussion until a consensus was reached. Then, a full text search of the remaining 27 records was conducted, and 13 records were eliminated due to case repetition and difficulty in stratifying (Figure 1).
Figure 1.
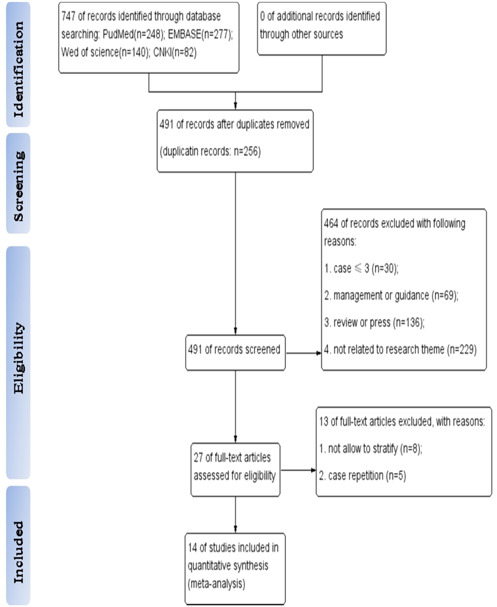
Flow diagram of study selection [Color figure can be viewed at wileyonlinelibrary.com]
2.3. Data extraction and analysis
We stratified the information to evaluate the current status of pediatric infection by asymptomatic infection, sex, coinfection, and family clustering. Meta‐analysis of proportions was also calculated for asymptomatic infection, coinfection and family clustering in children. Pooled proportions were calculated by single‐arm meta‐analysis. As sex was considered a dichotomous variable, we used the binomial distribution to calculate odds ratio (OR) and 95% confidence interval (CI) for the included studies. A pooled OR not equal to 1 indicated a difference between subjects. Heterogeneity was assessed with the I 2 statistics for each analysis, with the significance level set at P < .05. 8 If significant heterogeneity between studies was detected, a random effects model was used to combine OR estimates, or the studies were divided into specific subgroups based on different factors. 9 Subgroup analysis was performed based on clinical symptoms and coinfecting pathogens. A forest plot was used to illustrate the distribution of the outcome and effect size obtained from each published study. All statistical analyses were conducted using STATA 12.0 (STATA Corp, College Station, TX).
3. RESULTS
3.1. Characteristics of the included studies
The flow diagram of the search and study selection process was shown in Figure 1. A total of 14 eligible studies 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 with 410 patients (Table 1) were included. Of the 14 selected studies, 1 was from Spain and 13 from China, 2 of which were from the same hospital (Wuhan Children's Hospital); however, they were included because the cases were collected at different times. There were seven retrospective studies, six case reports, and one research study. All the included studies were published from January to April 2020, after the initial outbreak of SARS‐CoV2 occurred at the end of December 2019. The numbers of cases in the included studies ranged from 5 to 115 cases, with 232 males and 178 females. The overall average age (±standard error [SE]) of the pediatric patients was 5.27 ± 2.4 years (Table 1).
Table 1.
Characteristics of the included studies
| Author | Country (city/province) | Year/month | All | Male | Female | Age (Mean [y]) | Study type |
|---|---|---|---|---|---|---|---|
| Jie et al, 10 | China, Wuhan | 2020/3 | 91 | 56 | 35 | 4 | Retrospective study |
| Yaoling et al, 11 | China, Wuhan | 2020/4 | 115 | 73 | 42 | … | Retrospective study |
| Liu et al, 12 | China, Shandong | 2020/3 | 10 | 3 | 7 | 5.1 | Cases report |
| Ya‐nan et al, 13 | China, Xian | 2020/2 | 7 | 4 | 3 | 1.3 | Cases report |
| Jiehao et al, 14 | China, Shanghai | 2020/2 | 10 | 4 | 6 | 6.1 | Cases report |
| Kai et al, 15 | China, Shenzhen | 2020/4 | 15 | 5 | 10 | 7 | Retrospective study |
| Wei et al, 16 | China, Zhuhai | 2020/3 | 5 | 4 | 1 | 3.4 | Cases report |
| Mengqi et al, 17 | China, Chongqing | 2020/2 | 5 | 4 | 1 | 6.6 | Cases report |
| Xuegong et al, 18 | China, Autonomous region | 2020/4 | 31 | 15 | 16 | 7.1 | Retrospective study |
| Haiyan et al, 19 | China, Zhejiang | 2020/3 | 36 | 23 | 13 | 8.3 | Retrospective study |
| Qinxue et al, 20 | China, Changshao | 2020/3 | 9 | 3 | 6 | 7.5 | Cases report |
| Tagarro et al, 21 | Spain, Madrid | 2020/3 | 41 | 18 | 23 | 1 | Retrospective study |
| Yi et al, 22 | China, Guangzhou | 2020/3 | 10 | 6 | 4 | 8.1 | Research study |
| Fang et al, 23 | China, Hubei | 2020/3 | 25 | 14 | 11 | 3 | Retrospective study |
| Total | 410 | 232 | 178 | 5.27 ± 2.4 |
3.2. The manifestations of SARS‐CoV2 infection in children
After infection, the children might appear to be asymptomatic, though with a positive result using nucleic acid diagnosis with a nasopharyngeal or rectal swab. The estimated proportion of asymptomatic patients ranged from 10% to 80%, with the pooled proportion reaching 40.45% (95% CI, 24.04‐56.85; Figure 1A), with significant heterogeneity. Subgroup analysis of the clinical manifestations in the symptomatic children is shown in Figure 2B, revealing that the most prevalent clinical symptoms were fever (48.70%; 95% CI, 36.77‐60.63) and dry cough (35.09%; 95% CI, 23.62‐46.57), followed by sore throat (12.24%; 95% CI, 4.02‐20.45), diarrhea or/and vomiting(11.30%; 95% CI, 4.82‐17.79). Heterogeneity among the estimated clinical symptoms was significant, with I 2 values ranging from 50.40% to 80.9%.We also analyzed the risk of sex in the pediatric patients, showing that males did not tend to be more susceptible to infection than females, with a pooled OR among males compared with females of 1.15 for the line crossing the null hypothesis (95% CI, 0.63‐2.08, Figure 3); the I 2 value was 59.8%.
Figure 2.
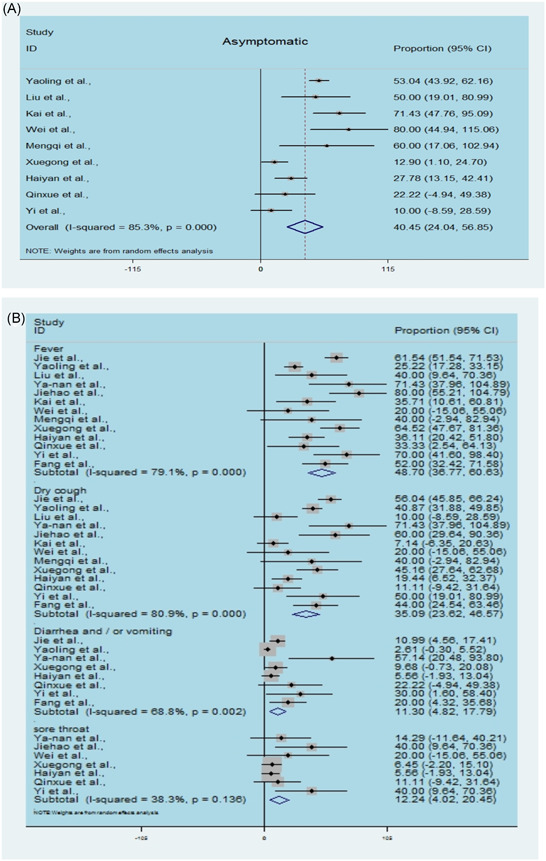
Estimated asymptomatic infection and clinical symptoms in children by SARS‐CoV2. A, Single‐arm meta‐analysis of the proportion of asymptomatic infection in enrolled cases. B, Single‐arm meta‐analysis of the proportion of clinical symptoms in enrolled cases. SARS‐CoV2, severe acute respiratory syndrome coronavirus 2 [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3.
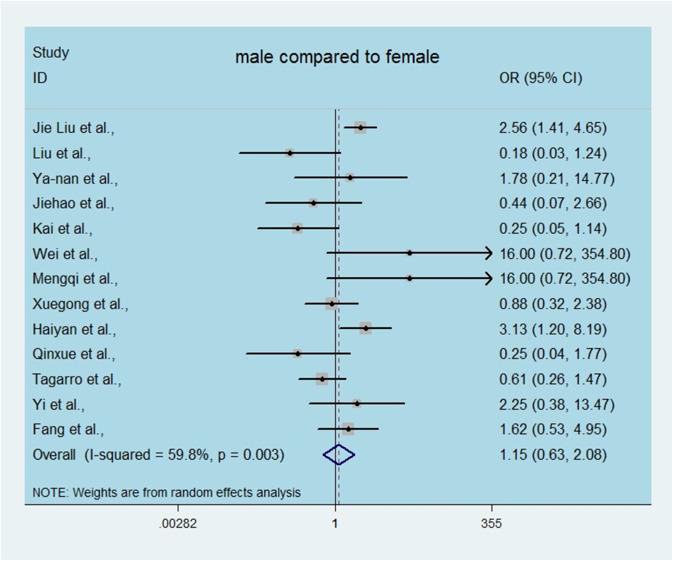
Odds ratio (OR) of SARS‐CoV2‐infected children among male compared with female. SARS‐CoV2, severe acute respiratory syndrome coronavirus 2 [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Coinfection situation in SARS‐CoV2‐infected children
There were four articles demonstrated the coinfection situation of children infected with SARS‐CoV2. As shown in Figure 4A, the proportion of coinfection ranged from 4.88% to 24%, with a pooled estimated of 10.14% (95% CI, 3.97‐16.30; Figure 4A). Furthermore, subgroup analysis of types of coinfected diseases showed the pooled proportion of Mycoplasma pneumonia coinfection to be as high as 50% (95% CI, 28.24‐71.76; Figure 4B), followed by influenza virus or parainfluenza virus (22.76%; 95% CI, 4.76‐40.77). The heterogeneity in the estimated coinfected pathogen was insignificant, with an I 2 value of 0.
Figure 4.
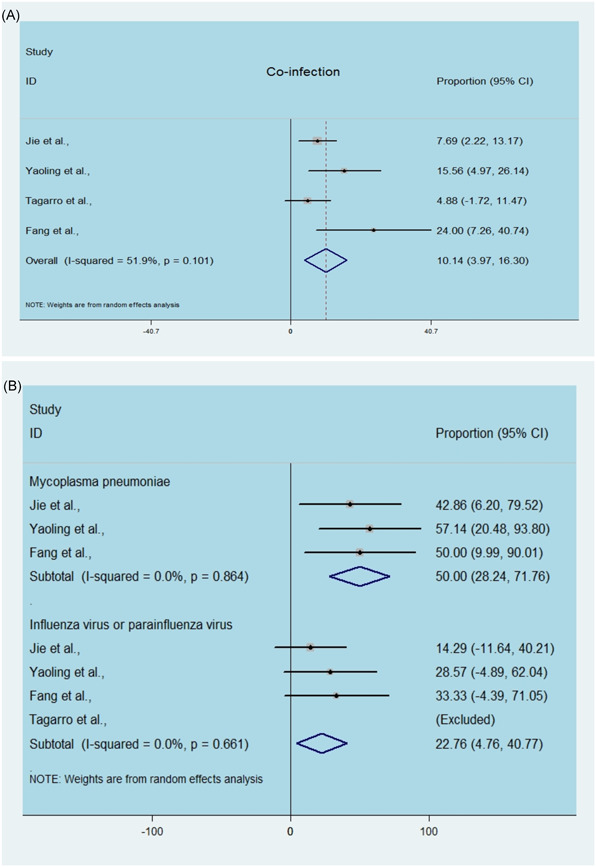
Estimated coinfection and coinfected pathogens in children by SARS‐CoV2. A, Single‐arm meta‐analysis of the proportion of coinfection in enrolled cases. B, Single‐arm meta‐analysis of the proportion of coinfected pathogens in enrolled cases. SARS‐CoV2, severe acute respiratory syndrome coronavirus 2 [Color figure can be viewed at wileyonlinelibrary.com]
3.4. The source of infection in SARS‐CoV2‐infected children
As reported, in adults the routes of infection were diverse, however, the presentation of symptoms in most of the infected children started after a confirmed diagnosis in family members. As shown in Figure 5, the proportion of family clustering in children highly ranged from 64% to 91.30%, with a pooled estimated of 83.63% (95% CI, 77.54‐89.72; Figure 5).
Figure 5.
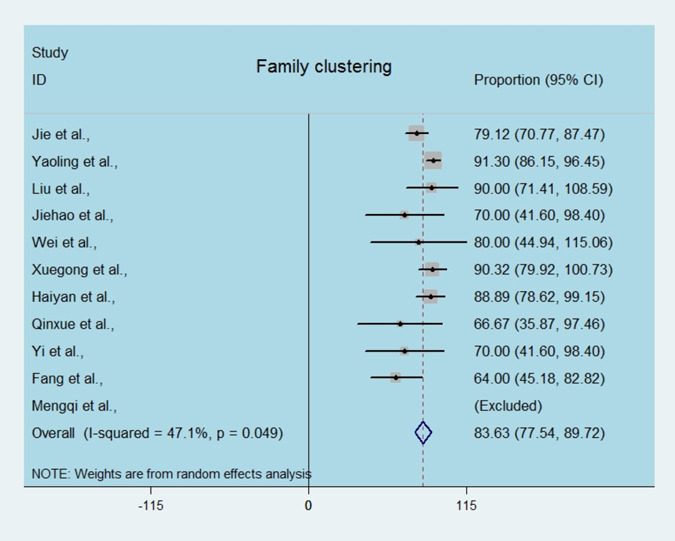
Estimated family clustering infection in children by SARS‐CoV2. Single‐arm meta‐analysis of the proportion of family cluster in enrolled cases. SARS‐CoV2, severe acute respiratory syndrome coronavirus 2 [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
SARS‐CoV2 is a newly discovered coronavirus with a genetic structure that is 82% similar to SARS‐CoV. 24 This origin of this new coronavirus is wild animals, and it can be transmitted through droplets or contact or through the fecal‐oral route, with high incidence and rapid spread, posing a huge threat to global public health. 25
Children are also susceptible to viral infection because of their immature immune system. Although the number of infected cases in children is comparably fewer than that in adults, there is no doubt that the number of confirmed pediatric cases is showing a tendency to increase significantly as the epidemic continues. However, the current status and characteristics of pediatric infections have not been fully described due to the small number of scattered cases. Hence, we collected qualified reported cases of children for systematic review and meta‐analysis, aiming to evaluate the features and situation of the children infected with SARS‐CoV2 and their possibly increasing health burden on the public.
This review included 14 studies encompassing 410 pediatric cases. In all the included articles, there is no record of the prognosis of pediatric patients, but according to the statistics, the prevalence of infected children ranged from 1.2% to 5% in different countries, and children constituted less than 1% of all US hospitalizations. Additionally, the prevalence of severe and critical disease was 10.6% in children aged <1 at diagnosis, 1 to 5 years (7.3%), 6 to 10 years (4.2%), 11 to 15 years (4.1%) and 16 to 17 years (3.0%), indicating that children under 1 year old have a high incidence of severe illness. 26 We conducted a single‐arm meta‐analysis to analyze the prevalence of clinical symptoms in infected children. However, we found that the clinical manifestations of the infected children were not as typical as those of adults, which might include asymptomatic, fever and dry cough, or upper respiratory tract symptoms such as stuffy nose, runny nose, and sore throat. Gastrointestinal symptoms such as abdominal discomfort, nausea, vomiting, abdominal pain, and diarrhea might also occur. Furthermore, gastrointestinal symptoms are the first manifestation in neonates. 27 These symptoms of SARS‐COV2 are similar to SARS with the predominant and most consistent symptom is fever. Other symptoms include rhinitis, cough, and diarrhea. 28 While children infected by Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) with more typical symptoms, ranging from asymptomatic infection to severe respiratory distress leading to death. 29
In our study, most cases were of preschool age, and the prevalence of asymptomatic cases highly reached 40%. Additionally, the most common clinical symptoms of children with manifestations were fever and dry cough, followed by sore throat and diarrhea or vomiting, but few children experienced symptoms of dyspnea and chest tightness, as adults do. Compared with adult cases, the condition of pediatric cases was mostly mild, which maybe related to the underdeveloped respiratory system. One study showed that SARS‐CoV2 can utilize multiple homologous isoforms of angiotensin‐converting enzyme II (ACE2) to effectively enter human respiratory tract cells. 30 The weak function of ACE2 receptors or their low expression in the underdeveloped respiratory system can limit viral invasion to some extent. Nonetheless, a concern is that many infected children were asymptomatic but positive for viral nucleic acid, and they might become the most dangerous group of virus transmission in the public. Du et al 31 estimated that the basic reproduction number (R0) of SARS‐CoV2 was 2.56. Kucharski et al 32 believed that R0 fluctuated between 1.5 and 4, suggesting that SARS‐CoV2 has a strong ability to spread. Furthermore, the viral load of the upper respiratory tract in asymptomatic patients is roughly equivalent to that in patients with obvious pneumonia. 33 Collectively, as found from our study, the proportion of asymptomatic children was surprisingly large, suggesting that infected children are an important source of virus spread leading to outbreaks and difficulty in control, as children are easily overlooked by the public due to their social status and stereotype.
Based on our findings, sex was not a risk factor associated with these SARS‐CoV2‐infected children because the pooled OR of males compared to females was close to 1 and crossed the invalid line, indicating that boys are not more susceptible than girls. As shown in Figure 4, approximately 10% of confirmed pediatric cases had coinfections, and the most common coinfecting pathogens were M. pneumonia (50%) and influenza virus or parainfluenza virus (22%), according to our statistics. Moreover, we found some other coinfecting viruses, such as adenovirus, syncytial virus and Epstein‐Barr virus, but statistics were missing due to the small number of cases or few hospitals conducted coinfection detection. We believed that this number would increase if attention had not been just focused on SARS‐CoV2 detection and ignored the detection of other pathogens. It is unknown whether children with coinfection are more likely to be immunologically susceptible to coronavirus infection. Considering the early stages of the outbreak, if the contact history of suspicious child cases was unclear, along with the routine respiratory pathogen test being conducted and found to be positive, it was easy to overlook the detection of SARS‐CoV2 nucleic acid, resulting in misdiagnosis as ordinary bronchopneumonia and a large amount of viral transmission among the public. Additionally, whether coinfections with SARS‐CoV2 result in sequelae and their effects on subsequent growth and public burden are still unknown.
It is worth noting that one of the included studies revealed real‐time RT‐PCR positivity using rectal swabs for eight of ten pediatric patients, which remained detectable well after nasopharyngeal swabs became negative, 22 suggesting that the gastrointestinal tract may shed virus and that fecal‐oral transmission between children and adults is possible. Positive results of rectal swabs lasting for 13 days in one of the eight patients, a 12‐year‐old girl, suggests that the discharge standard of infected children cannot rely only on nasopharyngeal swabs; it needs to be combined with rectal swab detection and prolonged detection time.
Family cluster is one of the epidemic characteristics of SARS‐CoV2. In our analysis, up to 80% of infected children come from family clustering infections, indicating close contact with the confirmed members in family is the key to a cluster of outbreaks. As reported, most cases of mortality involved children under 1 year old or newborns. 10 Interestingly, SARS‐CoV2 was not detected in serum or throat swab by RT‐PCR in any of the newborns of six mothers with confirmed SARS‐CoV2. However, virus‐specific antibodies were detected in neonatal blood sera samples. 34 One of the possible explanations is antibody‐dependent enhancement (ADE) of SARS‐CoV2 because ADE can induce the immune response and elicit sustained inflammation, lymphopenia, and/or cytokine storms, one or all of which have been documented in severe cases and deaths. It has been also reported in the literature that the cytokine storm may be related to the severity of COVID‐19. 35
Our study had some limitations. We did not analyze the incubation period of children because it was difficult to stratify for meta‐analysis due to different descriptions in some different studies. As of 11 February 2020, after analyzing 44 672 laboratory confirmed cases, a study reported that 965 cases were younger than 19. However, only 410 pediatric cases were enrolled in our study because we deleted some duplicate cases, which also resulted in a reduction in case number. Only four articles were selected for analyzing coinfection because it was not assessed or reported in the remaining studies. Most clinicians directly detected coronavirus and ignored other coinfections if they found suspicious symptoms early in the outbreak. It was estimated that the proportion of coinfection in children maybe increase once the implementation of multiple detection technologies. Even thought the number of published articles was limited, we would like to alert the public to coinfection in children based on our findings. Whether the coinfected virus will cause increased coronavirus virus virulence or facilitate mutation or severe unknown complications is still unclear.
5. CONCLUSIONS
In our study, we found that the proportion of asymptomatic infections in children was high; both males and females were susceptible to SARS‐CoV2. Children may have coinfection, and the pathogens of coinfection were diverse; of which M. pneumoniae and influenza virus or parainfluenza virus were the main coinfecting pathogens, implying that early misdiagnosis or a single detection method would overlook the virus and facilitate rapid spread in the public. Overall, an increasing public health burden indeed arises from the infected children, and we cannot ignore this. Nucleic acid detection of both nasopharyngeal swabs and rectal swabs along with lung CT examination should be actively conducted for children with unknown contact history or in families with diagnosed cases. In addition, misdiagnosis at the early stage should be avoided, which is of great significance for controlling the epidemic.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supporting information
Zheng B, Wang H, Yu C. An increasing public health burden arising from children infected with SARS‐CoV2: A systematic review and meta‐analysis. Pediatric Pulmonology. 2020;55:3487–3496. 10.1002/ppul.25008
Contributor Information
Hui Wang, Email: twanghzzm@jnu.edu.cn.
Cuixiang Yu, Email: yucx@mail.sysu.edu.cn.
REFERENCES
- 1. The 2019‐nCoV Outbreak Joint Field Epidemiology Investigation Team , Li Q. Notes from the field: an outbreak of NCIP (2019‐nCoV) infection in China‐Wuhan, Hubei Province, 2019–2020. China CDC Weekly 2020;2:79‐80. [PMC free article] [PubMed] [Google Scholar]
- 2. Tan WJ, Zhao X, Ma XJ, Wang WL, Niu PH, Xu WB. A novel coronavirus genome identified in a cluster of pneumonia cases—Wuhan, China 2019‐2020. China CDC Weekly. 2020;2:61‐62. [PMC free article] [PubMed] [Google Scholar]
- 3. Dadax . COVID‐19 coronavirus outbreak, Available at: https://www.worldometers.info/coronavirus
- 4. World Health Organization . Novel coronavirus(2019‐nCoV): situation report—84. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200413-sitrep-84-covid-19.pdf?sfvrsn=44f511ab_2. Accessed 13 April 2020.
- 5. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514e23‐523e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151:264‐269. [DOI] [PubMed] [Google Scholar]
- 8. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;317:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Applied statistics in the pharmaceutical industry: with case studies using S‐Plus. Springer Science & Business Media. 2013.
- 10. Liu J, Lou WJ, Deng ZH, et al. Clinical and epidemiological characteristics of 91 children conformed with COVID‐19. Chin J Nosocomiol. 2020;30:1645‐1649. [Google Scholar]
- 11. Ma YL, Xia SY, Wang M, Zhang SM, Du WH, Chen Q. Clinical features of children with SARS‐Co V‐2 infection: an analysis of 115 cases 91. Chin J Contemp Pediatr. 2020;22:290‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang L, Li Z, Xu H, et al. Epidemiological and clinical characteristic of 10 children with coronavirus disease (COVID‐19) in Jinan. J Shandong university(health science). 2020;58:1‐4. [Google Scholar]
- 13. Han Y, Feng Z, Sun L, et al. A comparative‐descriptive analysis of clinical characteristics in 2019‐Coronavirus‐infected children and adults [online ahead of print]. J Med Virol. 2020. 10.1002/jmv.25835 [DOI] [PubMed] [Google Scholar]
- 14. Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020;71:1547‐1551. 10.1093/cid/ciaa198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feng K, Yun YX, Wang XF, et al. Analysis of CT features of 15 children with 2019 novel coronavirus infection. Zhonghua er ke za zhi= Chinese journal of pediatrics. 2020;58:E007. [DOI] [PubMed] [Google Scholar]
- 16. Li W, Cui H, Li K, Fang Y, Li S. Chest computed tomography in children with COVID‐19 respiratory infection. Pediatr Radiol. 2020;50:796‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu M, Song Z, Xiao K. High‐resolution computed tomography manifestations of 5 pediatric patients with 2019 novel coronavirus. J Comput Assist Tomogr. 2020;44:311‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang D, Ju XL, Xie F, et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua er ke za zhi= Chinese journal of pediatrics. 2020;58:E011‐E274. [DOI] [PubMed] [Google Scholar]
- 19. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID‐19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:698‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen Q, Guo W, Guo T, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol. 2020;55:1424‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID‐19) in children in Madrid, Spain. JAMA Pediatr. 2020;8:e201346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS‐CoV‐2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zheng F, Liao C, Fan QH, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020;40:275‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person to person transmission: a study of a family cluster. Lancet. 2020;395:514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ludvigsson JF. Systematic review of COVID in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang J, Wang D, Chen GC, Tao XW, Zeng LK. SARS‐CoV‐2 infection with gastrointestinal symptoms as the first manifestation in a neonate. Zhongguo dang dai er ke za zhi= Chinese journal of contemporary pediatrics. 2020;22:211‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li AM, Ng PC. Severe acute respiratory syndrome (SARS) in neonates and children. Arch Dis Child Fetal Neonatal Ed. 2005;90:F461‐F465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bartenfeld M, Griese S, Uyeki T, Gerber SI, Peacock G. Middle east respiratory syndrome coronavirus and children: what pediatric health care professionals need to know. Clin Pediatr. 2017;56:187‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heurich A, Hofmann‐Winkler H, Gierer S, Jahn O, Pöhlmann S. TMPRSS2 and ADAM17 Cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Du Z, Wang L, Cauchemez S, et al. Risk of 2019 novel coronavirus importations throughout China prior to the Wuhan quarantine. Preprint. 2020. 10.1101/2020.01.28.20019299 [DOI] [Google Scholar]
- 32. Kucharski A. Preliminary analysis of transmission and control of new coronavirus[EB/OL]. The London School of Hygiene & Tropical Medicine(LSHTM)LSHTM 2020. https://www.lshtm.ac.uk/newsevents/news/2020/preliminary-analysis-transmission-and-control-new-coronavirus
- 33. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID‐19 pneumonia. JAMA. 2020;323:1848‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tetro JA. Is COVID‐19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22:72‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


