To the Editor,
Serological testing is increasingly recognized as a useful tool for management of the coronavirus disease‐2019 (COVID‐19) pandemic. 1 Cross‐sectional serosurveys provide information on exposure levels in a target population, which is helpful for designing public health strategies to blunt community transmission. 2 To maximize sensitivity, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) seroprevalence could be defined as positivity of any antibody isotype (IgG, IgM, or IgA), as detected by enzyme‐linked immunosorbent assays or chemiluminescent immunoassays (CLIA). 3 , 4 , 5 Theoretically, however, this approach may lead to false‐positive results outnumbering true‐positive ones in low prevalence settings, even when highly specific assays are used. We conducted a cross‐sectional, risk‐stratified seroepidemiological survey on healthcare workers at Hospital Clínico Universitario of Valencia, a tertiary teaching hospital with 586 beds. The study was approved by the Research Ethics Committee of University Clinic Hospital, INCLIVA, Valencia (2020‐04).
Asymptomatic healthcare workers (n = 1153) were screened for presence of SARS‐CoV‐2 IgM and IgG antibodies using the MAGLUMI 2019‐nCov IgG/IgM CLIA (Snibe, Shenzhen, China), between 13 April and 30 April 2020. According to the manufacturer's inserts, 6 the clinical sensitivities of IgM and IgG are 78.65% and 91.21%, respectively, while the specificities of IgM and IgG are 97.50% and 97.3%, respectively. The prevalence of SARS‐CoV‐2 infection in asymptomatic individuals in our Health Department was unknown at that time as only patients with COVID‐19 suspicion seeking medical attendance at the hospital were tested by reverse‐transcription polymerase chain reaction (RT‐PCR).
A total of 40 of the 1153 individuals (3.5%) tested positive for IgM and negative for IgG. Here, we aimed to gain further insight into the diagnostic value of this SARS‐CoV‐2 antibody pattern in this particular setting. Subjects (28 females and 12 males) displaying the SARS‐CoV‐2 IgM+/IgG− profile had a median age of 52 years (range, 32‐66 years). The median value of serum IgM, 1.3 AU/mL (range, 1.1‐5.3 AU/mL), was close to the positive threshold established by the manufacturer (1.1 AU/mL). These 40 individuals were screened for presence of SARS‐CoV‐2 RNA in upper respiratory tract specimens by the Abbott RealTime SARS‐CoV‐2 assay (Abbott Diagnostics, Chicago, IL) on the m2000 RT instrument, within 1 week after initial serological screening, of which only one yielded a positive result (2.5%). A follow‐up serum was available and collected from 34 out of the 40 individuals displaying the SARS‐CoV‐2 IgM+/IgG− profile, at a median of 17 days later (range, 11‐33 days), at which time all participants remained asymptomatic (Figure 1). Five sera retained IgM reactivity (median, 3.0 AU/mL; range, 1.2‐4.8 AU/mL), one pertaining to the individual testing positive by RT‐PCR, while it was lost in the remaining 29 sera. It is widely accepted that IgG can be uniformly detected by 3 to 4 weeks after SARS‐CoV‐2 infection. 6 Here, no IgG seroconversion was documented in any of the 34 individuals. It must be noted that no follow‐up serum was available from the individual testing positive by RT‐PCR. On the basis of the above, we interpreted that in all but one case (97% taking into account the 34 individuals with a follow‐up sample) the IgM+/IgG− antibody profile corresponded to a false‐positive result. This figure is significantly higher than expected for a test with a reported specificity of 97.5% of the IgM assay, 7 in a 1%‐5% prevalence setting, which is most likely reflective of our health department within the study period (positive predictive value of 25% or 62.5%, respectively). Nevertheless, we acknowledge factors that could argue against this assumption: (i) false‐negative results of initial RT‐PCR assays may occur 8 ; (ii) no repeat RT‐PCR testing was performed; (iii) IgG seroconversion may have been delayed beyond the time of follow‐up screening in some individuals in our series.
Figure 1.
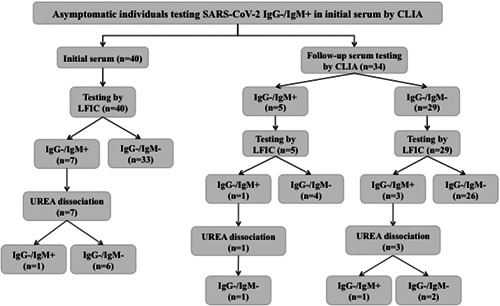
Flow chart depicting the results of analyses of sera from asymptomatic health care workers displaying a SARS‐CoV‐2 IgM+/IgG− antibody pattern on initial screening by a chemiluminescent immunoassay (MAGLUMI 2019‐nCov IgG/IgM CLIA; Snibe, Shenzhen, China) (n = 40). Qualitative assessment of SARS‐CoV‐2 IgM avidity was carried out using the LFIC AllTest 2019‐nCoV IgG/IgM Rapid Test Cassette (Hangzhou AllTest Biotech Co, Ltd, Hangzhou, China). A volume of 10 µL of serum was diluted into 1 mL of sample buffer before depositing (100 µL) into the appropriate location of the cassette (Test T‐hole). When the fluid was about to reach the absorbent pad, 100 µL of sample buffer containing 6 M urea was added to the T hole on the card. Serum specimens were run in parallel in the absence of urea treatment. Each reading was carried out independently by two observers after 20 minutes incubation. Appearance of either strong or weak sharp bands at the T line was recorded as a positive result. Absence of discernible lines was recorded as negative. Complete disappearance of reactive lines after urea treatment was interpreted as presence of low‐avidity antibodies, whereas their persistence was taken to indicate high‐avidity antibody presence
Urea dissociation antibody test performed on a lateral flow immunochromatography (LFIC) matrix has proven helpful in reducing false‐positive IgM results in COVID‐19 patients due to the presence of rheumatoid factor (RF). 9 We previously adapted the AllTest 2019‐nCoV IgG/IgM Rapid Test Cassette (Hangzhou AllTest Biotech Co., Ltd. Hangzhou, China) 10 for qualitative assessment of SARS‐CoV‐2‐specific antibody avidity. 11
When tested by LFIC, only 7 of the initial 40 sera returned an identical profile (IgM+/IgG), whereas the remaining 33 were IgM−/IgG−; as for the 34 follow‐up sera, 4 tested IgM+/IgG− and 30 IgM−/IgG−. Thus, overall 40 out of 74 sera yielded discordant results, consistent with previously reported discrepancies between SARS‐CoV‐2 IgM results provided by CLIA and LFIC assays. 12 Specifically, positive agreements for SARS‐CoV‐2 IgMs between 4 LFIC assays and a CLIA test were found to vary between 16.7% to 83.3%. 12
IgMs were eluted after urea treatment in 9 of 11 sera testing positive by LFIC. The two sera testing positive for IgM after urea dissociation belonged to the single RT‐PCR‐positive individual.
Initial sera were tested for the presence of RF, detecting above normal (>10 IU/mL) levels in 7 of the 40 sera (25%), with a median of 17 UI/mL (12‐21 IU/mL). Two of the 10 RF‐positive sera tested IgM+ by LFIC and CLIA, but IgM reactivity of these two sera disappeared following urea dissociation. In summary, detection of isolated SARS‐CoV‐2 IgM in asymptomatic individuals seldom represents true SARS‐CoV‐2 infection. False‐positive IgM reactivity can be eliminated after urea treatment using an LFIC device. The results reported herein should be taken into consideration to enhance data interpretation in seroepidemiological surveys conducted in low‐prevalence settings.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
AV, IT, DH, MJA, EA, CSdL, CG, and JF performed serological and RT‐PCR assays; RO designed the seroepidemiological survey; AV, IT, and DN analyzed and interpreted the data; DN wrote the manuscript.
ACKNOWLEDGMENTS
We are grateful to all personnel who work at Clinic University Hospital, in particular, to staff at the Microbiology laboratory for their commitment in the fight against COVID‐19.
REFERENCES
- 1. Krammer F, Simon V. Serology assays to manage COVID‐19. Science. 2020;368:1060‐1061. [DOI] [PubMed] [Google Scholar]
- 2. Bryant JE, Azman AS, Ferrari MJ, et al. Serology for SARS‐CoV‐2: apprehensions, opportunities, and the path forward. Sci Immunol. 2020;5:eabc6347. [DOI] [PubMed] [Google Scholar]
- 3. Garcia‐Basteiro AL, Moncunill G, Tortajada M, et al. Seroprevalence of antibodies against SARS‐CoV‐2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11:3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Korth J, Wilde B, Dolff S, et al. SARS‐CoV‐2‐specific antibody detection in healthcare workers in Germany with direct contact to COVID‐19 patients. J Clin Virol. 2020;128:104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen LH, Drew DA, Joshi AD, et al. Risk of COVID‐19 among frontline healthcare workers and the general community: a prospective cohort study. MedRxiv preprint. 2020. 10.1101/2020.04.29.20084111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26:845‐848. 10.1038/s41591-020-0897-1 [DOI] [PubMed] [Google Scholar]
- 7. MAGLUMI 2019‐nCov IgG/IgM CLIA . 271 2019‐nCoV IgM, V2.0, 2020‐03/272 2019‐nCoV IgG, V1.2, 2020‐02. Snibe, Shenzhen, China.
- 8. Arevalo‐Rodriguez I, Buitrago‐Garcia D, Simancas‐Racines D, et al. False‐negative results of initial RT‐PCR assays for Covid‐19: a systematic review. MedRxiv preprint. 2020. 10.1101/2020.04.16.20066787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Q, Du Q, Guo B, et al. A method to prevent SARS‐CoV‐2 IgM false positives in gold immunochromatography and enzyme‐linked immunosorbent assays. J Clin Microbiol. 2020;58:e00375‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Package Insert. AllTest 2019‐nCoV IgG/IgM rapid test cassette. Hangzhou, China: Hangzhou AllTest Biotech Co, Ltd. https://www.siloambio-tech.com/en/product_1297294.html
- 11. Valdivia A, Torres I, Huntley D, et al. Qualitative assessment of SARS‐CoV‐2‐specific antibody avidity by lateral flow immunochromatographic IgG/IgM antibody assay. J Med Virol. 2020. 10.1002/jmv.26344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saenz‐Flor KV, Santafe LM. Concordance of "rapid" serological tests and IgG and IgM chemiluminescence for SARS‐COV‐2. MedRxiv Preprint. 2020. 10.1101/2020.06.01.20114884 [DOI] [Google Scholar]


