Abstract
Previous studies reported that coronavirus disease 2019 (COVID‐19) was likely to result in liver injury. However, few studies investigated liver injury in patients with COVID‐19 with chronic liver diseases. We described the clinical features in patients with COVID‐19 with nonalcoholic fatty liver disease (NAFLD). Confirmed patients with COVID‐19 from hospitals in 10 cities of Jiangsu Province, China, were retrospectively included between January 18, 2020, and February 26, 2020. The hepatic steatosis index (HSI) was used to defined NAFLD. A total of 280 patients with COVID‐19 were enrolled. Eighty‐six (30.7%) of 280 patients with COVID‐19 were diagnosed as NAFLD by HSI. One hundred (35.7%) patients presented abnormal liver function on admission. The median alanine aminotransferase (ALT) levels (34.5 U/L vs. 23.0 U/L; P < 0.001) and the proportion of elevated ALT (>40 U/L) (40.7% vs. 10.8%; P < 0.001) were significantly higher in patients with NAFLD than in patients without NAFLD on admission. The proportion of elevated ALT in patients with NAFLD was also significantly higher than patients without NAFLD (65.1% vs. 38.7%; P < 0.001) during hospitalization. Multivariate analysis showed that age over 50 years (odds ratio [OR], 2.077; 95% confidence interval [CI], 1.183, 3.648; P = 0.011) and concurrent NAFLD (OR, 2.956; 95% CI, 1.526, 5.726; P = 0.001) were independent risk factors of ALT elevation in patients with COVID‐19, while the atomized inhalation of interferon α‐2b (OR, 0.402; 95% CI, 0.236, 0.683; P = 0.001) was associated with a reduced risk of ALT elevation during hospitalization. No patient developed liver failure or death during hospitalization. The complications and clinical outcomes were comparable between patients with COVID‐19 with and without NAFLD. Conclusion: Patients with NAFLD are more likely to develop liver injury when infected by COVID‐19. However, no patient developed severe liver‐related complications during hospitalization.
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- ARDS
acute respiratory distress syndrome
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
confidence interval
- CLD
chronic liver disease
- COVID‐19
corona virus disease 2019
- CT
computed tomography
- DM
diabetes mellitus
- FBG
fasting blood glucose
- GGT
gamma‐glutamyl transpeptidase
- HSI
hepatic steatosis index
- ICU
intensive care unit
- IFN
interferon
- IQR
interquartile range
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- Tbil
total bilirubin
In December 2019, a novel respiratory infection disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) emerged in Wuhan, China, and rapidly spread from Wuhan to other regions.( 1 , 2 ) As of June 16, 7,941,791 laboratory confirmed cases and 434,796 deaths had been reported.( 3 )
The clinical characteristics of corona virus disease 2019 (COVID‐19) have been reported in several studies.( 1 , 4 , 5 , 6 , 7 , 8 ) Despite atypical pneumonia being the primary symptom of COVID‐19, liver impairment has also been commonly observed in patients with COVID‐19.( 8 ) Chen et al.( 4 ) reported that 28% and 35% of patients with COVID‐19 had increased alanine aminotransferase (ALT) and increased aspartate aminotransferase (AST), respectively, on admission. In the study by Guan et al.,( 7 ) which analyzed clinical characteristics of 1,099 patients with confirmed COVID‐19 in China, 21.3% and 22.2% of patients presented elevated ALT and AST, respectively. These studies indicated that liver injury is common in patients with COVID‐19.
It is reported that 2%‐11% of patients with COVID‐19 had comorbidities of chronic liver diseases (CLDs).( 8 ) Currently, nonalcoholic fatty liver disease (NAFLD), which affects a quarter of the global population, has emerged as the most common CLD.( 9 ) However, the clinical features of patients with COVID‐19 with NAFLD are not clear. This study investigated the clinical features and liver injury in patients with COVID‐19 with NAFLD in a multicenter cohort of patients with COVID‐19 in Jiangsu Province, China.
Patients and Methods
Patients
A total of 342 consecutive patients with confirmed COVID‐19 were retrospectively enrolled from 10 designated hospitals in 10 cities of Jiangsu Province, China, between January 18, 2020, and February 26, 2020. The follow‐up data of clinical outcomes were obtained up to February 29, 2020. The diagnosis of COVID‐19 was based on guidance provided by the World Health Organization (WHO).( 10 ) All patients with COVID‐19 tested positive for SARS‐CoV‐2 in throat swab samples by real‐time polymerase chain reaction in accordance with guidance provided by the WHO.( 11 )
Patients with the following comorbidities were excluded: viral hepatitis (defined by positive serum hepatitis B surface antigen and/or hepatitis C antibody and/or a known history of chronic hepatitis B or chronic hepatitis C), significant alcohol consumption (defined by >30 g/day in men and >20 g/day in women), autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, or any other CLD. Our study was approved by the ethics committee of these hospitals, with a waiver of informed consent.
Data Collection
The epidemiologic, clinical, laboratory, radiologic, treatment, and outcomes data were collected from medical records of all the patients. All relevant data were entered into a computerized database and checked to avoid errors. The upper limit of normal (ULN) of ALT, AST, total bilirubin (Tbil), gamma‐glutamyl transpeptidase (GGT), and alkaline phosphatase (ALP) were 40 U/L, 40 U/L, 20 μmol/L, 50 U/L, and 150 U/L, respectively. Abnormal liver function was defined as any parameter (ALT, AST, GGT, ALP, or Tbil) higher than the ULN.
Definition of NAFLD
We defined NAFLD using the published hepatic steatosis index (HSI) in the absence of other causes of CLD.( 12 ) This index has been validated and used in several studies,( 13 , 14 , 15 , 16 ) and was calculated by using the following equation: HSI = 8 × ( ALT/AST ratio) + body mass index (BMI) (+2, if a female patient; +2, if diabetic).( 12 ) Serum ALT and AST results of the first test after admission were used for the calculation of the HSI. A cutoff of 36 was used to define the presence of NAFLD; this value has been validated and used in several studies.( 12 , 13 , 14 , 15 )
Statistical Analysis
All data were analyzed using IBM SPSS, version 22.0 (IBM, Armonk, NY). Continuous data were presented as median (interquartile range [IQR]). Categorical data were shown as counts and percentages. Continuous variables between the two groups were analyzed using two‐sample t tests or the Mann‐Whitney U test, as appropriate, while categorical variables were analyzed by chi‐square tests or Fisher’s exact tests. Logistic regression was performed to select independent risk factors of elevated ALT (>40 U/L). Variables having P < 0.05 in univariate analysis were used for multivariate input logistic regression analysis. P < 0.05 was considered statistically significant.
Results
Demographic Characteristics and Onset Symptoms
Of the 342 patients with COVID‐19, 34 patients without BMI data and 7 patients with insufficient biochemistry data were excluded; 7 patients with alcohol abuse and 14 patients with other CLDs, such as chronic hepatitis B, were also excluded (Fig. 1). A total of 280 patients with COVID‐19 were included for the final analysis; 52.1% of the patients were men, and the median age was 43.0 (IQR, 32.0‐56.0) years. The median BMI was 24.2 (IQR, 21.9‐26.2) kg/m2, and 38 (13.6%) of 280 patients met the diagnostic criteria of obesity (BMI ≥28 kg/m2). The median time from symptom onset to admission was 5.0 (IQR, 2.0‐8.0) days.
Fig. 1.
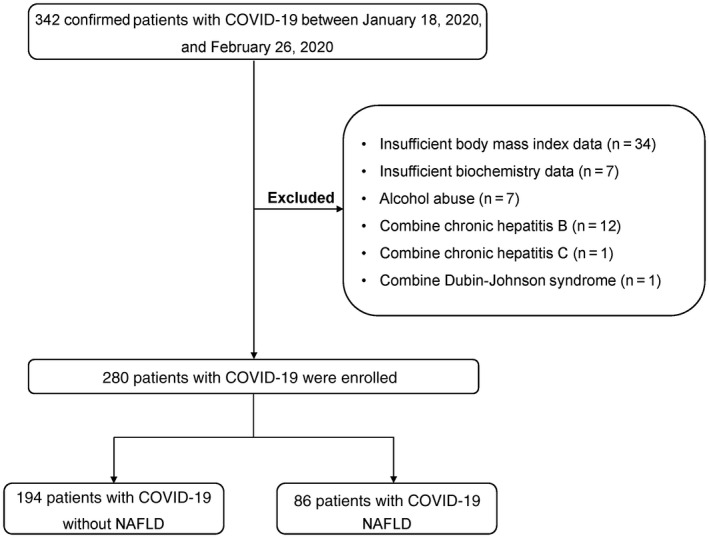
Flow chart of patient selection.
Fever (187 [66.8%]) and cough (156 [55.7%]) were the most common symptoms, followed by fatigue (58 [20.7%]), sore throat (32 [11.4%]), muscle ache (28 [10.0%]), shortness of breath (23 [8.2%]), and headache (19 [6.8%]). Seventy‐one (25.4%) patients had one or more underlying medical conditions, including hypertension (45 [16.1%]), diabetes (21 [7.5%]), chronic lung diseases (10 [3.6%]), and malignant tumor (4 [1.4%]). More than half of the patients (58.6%) had contact with suspected or confirmed patients within 2 weeks of onset symptoms (Table 1).
Table 1.
Demographic and Epidemiologic Characteristics of Patients With COVID‐19 With and Without NAFLD
| Variables (n [%] or Median [IQR]) | All Patients (N = 280) | Non‐NAFLD (n = 194) | NAFLD (n = 86) | P Value |
|---|---|---|---|---|
| Age (years) | 43.0 (32.0‐56.0) | 42.5 (31.8‐57.3) | 43.5 (32.8‐53.3) | 0.924 |
| Age range | 0.747 | |||
| ≤50 years | 185 (66.1) | 127 (65.5) | 58 (67.4) | |
| >50 years | 95 (33.9) | 67 (34.5) | 28 (32.6) | |
| Sex | 0.181 | |||
| Male | 146 (52.1) | 96 (49.5) | 50 (58.1) | |
| Female | 134 (47.9) | 98 (50.5) | 36 (41.9) | |
| BMI (kg/m2) | 24.2 (21.9‐26.2) | 23.1 (21.0‐24.8) | 27.1 (25.3‐29.7) | <0.001 |
| BMI range | <0.001 | |||
| <28 kg/m2 | 242 (86.4) | 191 (98.5) | 51 (59.3) | |
| ≥28 kg/m2 | 38 (13.6) | 3 (1.5) | 35 (40.7) | |
| Onset signs and symptoms | ||||
| Fever | 187 (66.8) | 132 (68.0) | 55 (64.0) | 0.503 |
| Cough | 156 (55.7) | 109 (56.2) | 47 (54.7) | 0.812 |
| Fatigue | 58 (20.7) | 42 (21.6) | 16 (18.6) | 0.562 |
| Sore throat | 32 (11.4) | 22 (11.3) | 10 (11.6) | 0.944 |
| Muscle ache | 28 (10.0) | 21 (10.8) | 7 (8.1) | 0.49 |
| Shortness of breath | 23 (8.2) | 16 (8.2) | 7 (8.1) | 0.976 |
| Headache | 19 (6.8) | 16 (8.2) | 3 (3.5) | 0.144 |
| Comorbidities | ||||
| Any comorbidity | 71 (25.4) | 44 (22.7) | 27 (31.4) | 0.122 |
| Hypertension | 45 (16.1) | 27 (13.9) | 18 (20.9) | 0.118 |
| Diabetes | 21 (7.5) | 11 (5.7) | 10 (11.6) | 0.081 |
| Chronic lung diseases | 10 (3.6) | 8 (4.1) | 2 (2.3) | 0.454 |
| Malignant tumor | 4 (1.4) | 2 (1.0) | 2 (2.3) | 0.4 |
| Exposure history | ||||
| Contact with suspected or confirmed patients | 164 (58.6) | 114 (58.8) | 50 (58.1) | 0.922 |
| Contacted with people from Wuhan or non‐Wuhan areas of Hubei Province | 102 (36.4) | 77 (39.7) | 25 (29.1) | 0.088 |
| Visited Wuhan or non‐Wuhan areas of Hubei Province | 97 (34.6) | 67 (34.5) | 30 (34.9) | 0.955 |
| Time from symptom onset to admission (days) | 5.0 (2.0‐8.0) | 5.0 (3.0‐8.0) | 4.5 (2.0‐8.0) | 0.258 |
Eighty‐six (30.7%) of 280 patients with COVID‐19 were diagnosed as having NAFLD by HSI in our study. BMI levels (median, 27.1 kg/m2 vs. 23.1 kg/m2; P < 0.001) and the proportion of obesity (40.7% vs. 1.5%; P < 0.001) in patients with NAFLD were significantly higher than patients without NAFLD; however, age and sex were comparable between the two groups. There were also no significant differences in symptoms, comorbidities, and exposure history between the two groups (Table 1).
Laboratory and Radiology Examination
On admission, the baseline white blood cells and lymphocyte levels were 4.9 (IQR, 3.9‐6.2) × 109/L and 1.2 (IQR, 0.9‐1.6) × 109/L, respectively. Leukopenia and lymphopenia were observed in 25.4% and 26.8% of patients, respectively. One hundred (35.7%) patients presented with abnormal liver function on admission. Serum ALT (56 [20.0%]) was the most frequent abnormal parameter, followed by GGT (42 [15.0%]), AST (38 [13.6%]), Tbil (26 [9.3%]), and ALP (7 [2.5%]) on admission. The median baseline levels of ALT, AST, GGT, Tbil, ALP, fasting blood glucose, triglyceride, and total cholesterol were 25.0 (IQR, 19.0‐37.0) U/L, 24.8 (IQR, 20.0‐32.0) U/L, 25.0 (IQR, 15.0‐39.0) U/L, 10.2 (IQR, 7.1‐15.0) μmol/L, 63.0 (IQR, 51.0‐75.0) U/L, 5.7 (IQR, 5.0‐6.4) mmol/L, 1.2 (IQR, 0.9‐1.6) mmol/L, and 3.8 (IQR, 3.3‐4.5) mmol/L, respectively. A total of 255 (91.1%) patients presented abnormal chest computed tomography (CT) images (Table 2).
Table 2.
Baseline Laboratory Parameters and Chest CT of Patients With COVID‐19 With and Without NAFLD
| Variables (n [%] or Median [IQR]) | All Patients (N = 280) | Non‐NAFLD (n = 194) | NAFLD (n = 86) | P Value |
|---|---|---|---|---|
| WBC (×109/L) | 4.9 (3.9‐6.2) | 4.7 (3.7‐5.8) | 5.5 (4.2‐6.8) | <0.001 |
| Decreased | 71 (25.4) | 58 (29.9) | 13 (15.1) | 0.009 |
| Lymphocytes (×109/L) | 1.2 (0.9‐1.6) | 1.2 (0.9‐1.6) | 1.4 (0.9‐1.8) | 0.041 |
| Decreased | 75 (26.8) | 61 (31.4) | 14 (16.3) | 0.008 |
| ALT (U/L) | 25.0 (19.0‐37.0) | 23.0 (15.8‐30.0) | 34.5 (25.0‐50.5) | <0.001 |
| >40 U/L | 56 (20.0) | 21 (10.8) | 35 (40.7) | <0.001 |
| AST (U/L) | 24.8 (20.0‐32.0) | 24.0 (19.0‐32.0) | 26.0 (20.0‐33.3) | 0.222 |
| >40 U/L | 38 (13.6) | 24 (12.4) | 14 (16.3) | 0.378 |
| GGT (U/L) | 25.0 (15.0‐39.0) | 21.0 (14.0‐33.0) | 34.0 (19.8‐48.3) | <0.001 |
| >50 U/L | 42 (15.0) | 22 (11.3) | 20 (23.3) | 0.01 |
| Tbil (μmol/L) | 10.2 (7.1‐15.0) | 10.1 (7.0‐14.3) | 10.9 (7.8‐16.3) | 0.077 |
| >20 μmol/L | 26 (9.3) | 13 (6.7) | 13 (15.1) | 0.025 |
| ALP (U/L) | 63.0 (51.0‐75.0) | 62.0 (51.0‐77.0) | 63.0 (51.3‐72.8) | 0.617 |
| >150 U/L | 7 (2.5) | 7 (3.6) | 0 | 0.074 |
| FBG (mmol/L) | 5.7 (5.0‐6.4) | 5.6 (4.9‐6.2) | 5.7 (5.1‐7.0) | 0.116 |
| TG (mmol/L) | 1.2 (0.9‐1.6) | 1.1 (0.8‐1.5) | 1.4 (1.0‐1.9) | 0.004 |
| TC (mmol/L) | 3.8 (3.3‐4.5) | 3.7 (3.2‐4.4) | 3.9 (3.4‐4.6) | 0.078 |
| Chest CT | 0.727 | |||
| No pneumonia | 25 (8.9) | 19 (9.8) | 6 (7.0) | |
| Unilateral pneumonia | 37 (13.2) | 26 (13.4) | 11 (12.8) | |
| Bilateral pneumonia | 218 (77.9) | 149 (76.8) | 69 (80.2) |
Abbreviations: TC, total cholesterol; TG, triglyceride; WBC, white blood cells.
The proportion of leukopenia (29.9% vs. 15.1%; P = 0.009) and lymphopenia (31.4% vs. 16.3%; P = 0.008) was higher in patients without NAFLD than with NAFLD. The median ALT levels (34.5 U/L vs. 23.0 U/L; P < 0.001) and the proportion of elevated ALT (40.7% vs. 10.8%; P < 0.001) in patients with NAFLD were significantly higher than in patients without NAFLD. Patients with NAFLD also presented higher GGT levels (34.0 U/L vs. 21.0 U/L; P < 0.001) and a higher proportion of GGT elevation (22.3% vs. 11.3%; P = 0.01) than patients without NAFLD. However, there were no significant differences in the median serum AST, Tbil, and ALP levels between the two groups. The proportion of abnormalities of chest CT images was also similar between the two groups (Table 2).
Dynamic Changes of Liver Function Tests During Hospitalization
The highest levels of liver function tests for each patient during hospitalization were selected for the analysis. During hospitalization, 176 (62.9%) patients presented abnormal liver function tests, including 131 (46.8%) patients with elevated ALT, 92 (32.9%) with elevated GGT, 74 (26.4%) with elevated AST, 72 (25.7%) with elevated Tbil, and 10 (3.6%) with elevated ALP. Among these patients, 76 (43.2%) patients had normal liver function on admission. The median peak values of ALT, AST, GGT, Tbil, and ALP were 39.0 (IQR, 24.0‐66.0) U/L, 28.0 (IQR, 22.0‐42.0) U/L, 34.0 (IQR, 18.0‐60.0) U/L, 14.7 (IQR, 10.4‐20.5) μmol/L, and 68.0 (IQR, 56.0‐84.0) U/L during hospitalization, respectively.
During hospitalization, the proportion of elevated ALT in patients with NAFLD was significantly higher than in patients without NAFLD (65.1% vs. 38.7%; P < 0.001), while the proportion of elevated Tbil, AST, GGT, and ALP was comparable between the two groups. The median peak values of ALT (54.0 U/L vs. 33.0 U/L; P < 0.001) and GGT (43.0 U/L vs. 28.0 U/L; P < 0.001) were significantly higher in patients with NAFLD than in those without NAFLD during hospitalization. However, the median peak values of AST, Tbil, and ALP were comparable between the two groups (Table 3).
Table 3.
Laboratory Parameters in Patients With COVID‐19 Patients With and Without NAFLD During Hospitalization
| Variables (n [%] or Median [IQR]) | All Patients (N = 280) | Non‐NAFLD (n = 194) | NAFLD (n = 86) | P Value |
|---|---|---|---|---|
| ALT | ||||
| >40 U/L | 131 (46.8) | 75 (38.7) | 56 (65.1) | <0.001 |
| Median peak levels (U/L) | 39.0 (24.0‐66.0) | 33.0 (22.0‐58.8) | 54.0 (31.7‐78.0) | <0.001 |
| AST | ||||
| >40 U/L | 74 (26.4) | 51 (26.3) | 23 (26.7) | 0.936 |
| Median peak levels (U/L) | 28.0 (22.0‐42.0) | 27.5 (22.0‐42.0) | 31.0 (23.0‐42.0) | 0.255 |
| GGT | ||||
| >50 U/L | 92 (32.9) | 57 (29.4) | 35 (40.7) | 0.063 |
| Median peak levels (U/L) | 34.0 (18.0‐60.0) | 28.0 (16.0‐54.8) | 43.0 (31.2‐66.5) | <0.001 |
| Tbil | ||||
| >20 μmol/L | 72 (25.7) | 49 (25.3) | 23 (26.7) | 0.793 |
| Median peak levels (μmol/L) | 14.7 (10.4‐20.5) | 14.8 (10.3‐20.4) | 14.7 (10.8‐20.9) | 0.569 |
| ALP | ||||
| >150 U/L | 10 (3.6) | 9 (4.6) | 1 (1.2) | 0.148 |
| Median peak levels (U/L) | 68.0 (56.0‐84.0) | 67.5 (55.0‐84.8) | 68.0 (58.0‐81.5) | 0.905 |
Risk Factors of Elevated ALT During Hospitalization
Logistic regression analysis was performed to identify the risk factors of elevated ALT (>40 U/L) in patients with COVID‐19. Univariate analysis revealed that age >50 years (odds ratio [OR], 1.847; 95% confidence interval [CI], 1.120, 3.047; P = 0.016), male sex (OR, 1.753; 95% CI, 1.090, 2.819; P = 0.021), BMI ≥28 kg/m2 (OR, 2.467; 95% CI, 1.205, 5.052; P = 0.014), concurrent NAFLD (OR, 2.962; 95% CI, 1.745, 5.028; P < 0.001), severe illness (OR, 6.133; 95% CI, 2.259, 16.652; P < 0.001), intensive care unit (ICU) admission (OR, 4.338; 95% CI, 1.391, 13.530; P = 0.011), and atomized inhalation of interferon (IFN)α‐2b (OR, 0.389; 95% CI, 0.239, 0.633; P < 0.001) were associated with elevated ALT levels (Table 4). Further multivariate analysis showed that age over 50 years (OR, 2.077; 95% CI, 1.183, 3.648; P = 0.011) and concurrent NAFLD (OR, 2.956; 95% CI, 1.526, 5.726; P = 0.001) were two independent risk factors of ALT elevation, while atomized inhalation of IFNα‐2b (OR, 0.402; 95% CI, 0.236, 0.683; P = 0.001) was related to a reduced risk of ALT elevation.
Table 4.
Risk Factors of Elevated ALT (>40 U/L) During Hospitalization in Patients With COVID‐19
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Age | |||||
| ≤50 years | Reference | ||||
| >50 years | 1.847 (1.120, 3.047) | 0.016 | 2.077 (1.183, 3.648) | 0.011 | |
| Sex | |||||
| Female | Reference | ||||
| Male | 1.753 (1.090, 2.819) | 0.021 | 1.646 (0.975, 2.778) | 0.062 | |
| BMI | |||||
| <28 kg/m2 | Reference | ||||
| ≥28 kg/m2 | 2.467 (1.205, 5.052) | 0.014 | 0.901 (0.360, 2.258) | 0.825 | |
| NAFLD | |||||
| No | Reference | ||||
| Yes | 2.962 (1.745, 5.028) | <0.001 | 2.956 (1.526, 5.726) | 0.001 | |
| Severe illness | |||||
| No | Reference | ||||
| Yes | 6.133 (2.259, 16.652) | <0.001 | 4.009 (0.966, 16.638) | 0.056 | |
| ICU | |||||
| No | Reference | ||||
| Yes | 4.338 (1.391, 13.530) | 0.011 | 0.931 (0.175, 4.946) | 0.933 | |
| Hypertension | |||||
| No | Reference | ||||
| Yes | 0.576 (0.297, 1.116) | 0.102 | |||
| Diabetes | |||||
| No | Reference | ||||
| Yes | 0.842 (0.343, 2.067) | 0.708 | |||
| Atomized inhalation of IFNα‐2b | |||||
| No | Reference | ||||
| Yes | 0.389 (0.239, 0.633) | <0.001 | 0.402 (0.236, 0.683) | 0.001 | |
| Lopinavir/ritonavir | |||||
| No | Reference | ||||
| Yes | 1.244 (0.733, 2.111) | 0.418 | |||
| Arbidol | |||||
| No | Reference | ||||
| Yes | 1.218 (0.761, 1.949) | 0.411 | |||
Treatment and Clinical Prognosis
Atomized inhalation of IFNα‐2b, lopinavir/ritonavir, and arbidol were the most commonly used antiviral drugs in our study; these account for 57.1%, 72.5%, and 49.3% of the patients, respectively (Table 5), while 73.6% of patients received empirical antibiotic treatment. In addition, 73 (26.1%) patients were given corticosteroids, and 35 (12.5%) patients were treated with gamma globulin. Up to February 29, 2020, a total of 22 (7.9%) patients developed respiratory failure and 4 (1.4%) patients progressed to acute respiratory distress syndrome (ARDS). However, no patient developed liver failure and death. Twenty‐eight (10.0%) patients were transferred to the ICU during hospitalization. As of February 29, 2020, 69 (24.6%) patients were still hospitalized. The treatment drugs, complications, and clinical outcomes were comparable between patients with and without NAFLD.
Table 5.
Treatment, Complications, and Outcomes of Patients With COVID‐19 With and Without NAFLD
| Variables (n [%]) | All Patients (N = 280) | Non‐NAFLD (n = 194) | NAFLD (n = 86) | P Value |
|---|---|---|---|---|
| Drug treatment | ||||
| Atomized inhalation of IFNα‐2b | 160 (57.1) | 116 (59.8) | 44 (51.2) | 0.178 |
| Lopinavir/ritonavir | 203 (72.5) | 144 (74.2) | 59 (68.6) | 0.331 |
| Arbidol | 138 (49.3) | 92 (47.4) | 46 (53.5) | 0.349 |
| Antibiotic | 206 (73.6) | 141 (72.7) | 65 (75.6) | 0.612 |
| Glucocorticoid | 73 (26.1) | 47 (24.2) | 26 (30.2) | 0.291 |
| Gamma globulin | 35 (12.5) | 21 (10.8) | 14 (16.3) | 0.203 |
| Complications | ||||
| Respiratory failure | 22 (7.9) | 12 (6.2) | 10 (11.6) | 0.118 |
| ARDS | 4 (1.4) | 2 (1.0) | 2 (2.3) | 0.4 |
| Liver failure | 0 | 0 | 0 | |
| Outcomes | ||||
| Remained in hospital | 69 (24.6) | 46 (23.7) | 23 (26.7) | 0.587 |
| Hospital discharge | 211 (75.4) | 148 (76.3) | 63 (73.3) | 0.587 |
| Severe illness | 28 (10.0) | 16 (8.2) | 12 (14.0) | 0.142 |
| Admission to ICU | 18 (6.4) | 13 (6.7) | 5 (5.8) | 0.78 |
| Death | 0 | 0 | 0 |
Discussion
In our study, 30.7% of patients with COVID‐19 had NAFLD. The global prevalence of NALFD is 6.3%‐45% in the general population.( 9 , 17 ) In most Asian countries, the prevalence of NAFLD is above 25%.( 9 ) A recent meta‐analysis of NAFLD in Mainland China reported that the overall prevalence of NAFLD is about 30%.( 18 ) Thus, the rate of concurrent NAFLD in patients with COVID‐19 in our study is consistent with the prevalence of NALFD in the general population in China, indicating that concurrent NAFLD may not be a risk factor of SARS‐CoV‐2 infection.
Consistent with other studies,( 6 , 7 ) the most common symptoms were fever (66.8%) and cough (55.7%). However, the common symptoms were not significantly different between NAFLD and non‐NAFLD groups. The most common laboratory abnormalities that we observed were leukopenia and lymphopenia. Fewer patients with COVID‐19 with NAFLD had leukopenia and lymphopenia compared with patients without NAFLD. These findings need to be validated in the future, and the mechanisms deserve further investigation.
We found that 37.3% of patients presented abnormal liver function on admission; the most common included elevated ALT, AST, and GGT. Previous research also reported that 14%‐53% of COVID‐19 cases reported abnormal levels of ALT and AST.( 8 ) In the study by Zhang et al.,( 8 ) elevated GGT was observed in 30 (54%) of 56 patients with COVID‐19. Thus, the proportions of elevated ALT, AST, and GGT in our study were consistent with reported data. However, we found that median levels of ALT and GGT as well as the proportion of elevated ALT and GGT were higher in patients with NAFLD compared to patients without NAFLD on admission. Furthermore, patients with NAFLD had a higher proportion of elevated ALT levels and higher peak ALT levels during hospitalization compared to those without NAFLD. Logistic regression analysis also confirmed that NAFLD was associated with elevated ALT in our study. These results indicate that patients with NAFLD are more likely to develop abnormal liver function after infection by SARS‐CoV‐2. However, the levels of elevated ALT, AST, and GGT were generally not high on admission or during hospitalization in our study. All patients with NAFLD with abnormal liver function tests showed mild to moderate liver damage; severe liver injury and liver failure were not observed in patients with or without NAFLD. Previous studies also reported that few patients developed severe liver‐related complications.( 7 , 19 )
Currently, the mechanisms of COVID‐19‐related liver injury are not clear. It is reported that SARS‐CoV‐2 may directly cause liver injury through angiotensin converting enzyme 2 (ACE2) expressed in cytomembranes.( 20 ) Both liver cells and bile duct cells express ACE2.( 20 ) Thus, both hepatocyte and cholangiocyte injury may occur in COVID‐19. In our study, besides ALT and AST, increased GGT, which is a diagnostic biomarker for cholangiocyte injury, was also observed. Drug‐induced liver injury may be another possible contributing factor to the observed abnormal liver function test because some patients developed liver function abnormalities after therapeutics began.( 19 ) However, mild elevations of liver enzymes, such as ALT, are also common in NAFLD.( 21 ) Further investigations are needed to understand the mechanisms of liver injury caused by the interaction between existing NAFLD and COVID‐19.
We analyzed the risk factors of liver injury in COVID‐19. Age over 50 years was a risk factor of ALT elevation. Thus, more attention should be paid to elderly patients. We found atomized inhalation of IFNα‐2b to be related to a reduced risk of ALT >40 U/L. One interpretation may be that atomized inhalation of IFNα‐2b suppresses SARS‐CoV‐2 and reduces liver impairment caused by the virus. However, further studies are needed to confirm our hypothesis.
In our study, only 7.9% of patients developed respiratory failure and 1.4% progressed to ARDS; 6.4% of patients were transferred to the ICU during hospitalization, but no patient died. However, the complications and clinical outcomes were comparable between patients with and without NAFLD. Although more patients with NAFLD presented abnormal liver function, concurrent NAFLD is not associated with adverse clinical outcomes in patients with COVID‐19. Most of our patients were young, and the proportion of comorbidities was relatively low in our study, which may be associated with the good prognosis of our cohort. Ji et al.( 22 ) reported the prevalence of NAFLD in progressive patients with COVID‐19 was higher than stable patients (87.2% vs. 25.8%); however, the median age and comorbidities in progressive patients with COVID‐19 were also significantly higher than stable patients. Another study revealed that concurrent NAFLD increased the risk of progression to severe COVID‐19 in patients without diabetes, but the sample size was relatively small.( 23 ) Thus, the impacts of NAFLD on the prognosis of COVID‐19 deserve further investigation.
In general, the prevalence of diabetes mellitus (DM) is higher in patients with NAFLD than those without NAFLD.( 24 ) We found the incidence of DM in patients with NAFLD (11.6%) to be higher than those without NAFLD (5.7%). However, there was no significant difference of incidence of DM between these two groups (P = 0.081). A possible reason may be the relatively small sample size with only 21 patients (7.5%) having DM in our study. We found fasting blood glucose (FBG) levels to be comparable between patients with NAFLD and those without NAFLD. However, all patients were diagnosed with DM before SARS‐CoV‐2 infection. These patients have been treated with hypoglycemic drugs, which may partially interpret the comparable FBG levels between the two groups.
This study has some limitations. First, using the HSI to define NAFLD in the absence of known liver disease may misclassify and under/overestimate the presence of NAFLD. However, HSI was proposed by Lee et al.( 12 ) as a diagnostic tool for NAFLD and has been validated by several studies with acceptable diagnostic accuracy.( 12 , 25 , 26 , 27 ) Due to the emergency situation, liver histology and/or radiologically for the diagnosis of NAFLD was not available in our study. Although liver histology is considered to be the gold standard for the diagnosis of NAFLD, it cannot be conducted on the general population or for patients with COVID‐19. Second, patients with NAFLD have been reported to have a greater chance of developing drug‐induced hepatotoxicity, and this may have contributed to the greater AST/ALT values and impact the HSI scores.( 28 , 29 , 30 , 31 ) Third, the fibrosis stages of patients were not assessed in our cohort. FibroScan is a promising measurement to assess the presence of NAFLD and fibrosis stages in CLDs.( 32 , 33 ) However, FibroScan is not a routine test for patients with COVID‐19, and the information was not available for our study. Prospective studies using imaging or even histology to determine the presence of NAFLD and fibrosis stages of patients are needed to confirm the impacts of NAFLD on COVID‐19.
In conclusion, patients with NAFLD are more likely to develop liver injury when infected by SARS‐CoV‐2. However, no patient with COVID‐19 with NAFLD developed severe liver injury during hospitalization. Further prospective studies are needed to confirm our findings.
Supported by the Fundamental Research Funds for the Central Universities (No. 14380459).
Potential conflict of interest: Nothing to report.
Contributor Information
Chuanwu Zhu, Email: zhuchw@126.com.
Chao Wu, Email: dr.wu@nju.edu.cn, Email: zhuchw@126.com.
References
Author names in bold designate shared co‐first authorship.
- 1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al.China Novel Coronavirus Investigating and Research Team . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. Severe acute respiratory syndrome‐related coronavirus: the species and its viruses ‐ a statement of the Coronavirus Study Group. BroRxiv 2020; 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- 3. World Health Organization . Coronavirus disease (COVID‐19). Situation report ‐ 148. June 16, 2020. https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200616‐covid‐19‐sitrep‐148‐draft.pdf?sfvrsn=9b2015e9_2. Accessed June 20, 2020. [Google Scholar]
- 4. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al.; China Medical Treatment Expert Group for Covid‐19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol 2020;5:428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) Infection is suspected: interim guidance. Published January 25, 2020. https://apps.who.int/iris/bitstream/handle/10665/330854/WHO‐nCoV‐Clinical‐2020.2‐eng.pdf?sequence=1&isAllowed=y. Accessed January 31, 2020. [Google Scholar]
- 11. World Health Organization . Laboratory diagnostics for novel coronavirus. 2020. https://www.who.int/publications/i/item/laboratory‐testing‐of‐2019‐novel‐coronavirus‐(‐2019‐ncov)‐in‐suspected‐human‐cases‐interim‐guidance‐17‐january‐2020. Accessed February 6, 2020. [Google Scholar]
- 12. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010;42:503‐508. [DOI] [PubMed] [Google Scholar]
- 13. Kim D, Yoo ER, Li AA, Tighe SP, Cholankeril G, Harrison SA, et al. Depression is associated with non‐alcoholic fatty liver disease among adults in the United States. Aliment Pharmacol Ther 2019;50:590‐598. [DOI] [PubMed] [Google Scholar]
- 14. Kim D, Kim W, Adejumo AC, Cholankeril G, Tighe SP, Wong RJ, et al. Race/ethnicity‐based temporal changes in prevalence of NAFLD‐related advanced fibrosis in the United States, 2005‐2016. Hepatol Int 2019;13:205‐213. [DOI] [PubMed] [Google Scholar]
- 15. Kim D, Yoo ER, Li AA, Cholankeril G, Tighe SP, Kim W, et al. Elevated urinary bisphenol A levels are associated with non‐alcoholic fatty liver disease among adults in the United States. Liver Int 2019;39:1335‐1342. [DOI] [PubMed] [Google Scholar]
- 16. Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V, et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther 2014;40:1209‐1222. [DOI] [PubMed] [Google Scholar]
- 17. Fan JG, Wei L, Zhuang H; National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association ; Fatty Liver Disease Expert Committee, Chinese Medical Doctor Association . Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J Dig Dis 2019;20:163‐173. [DOI] [PubMed] [Google Scholar]
- 18. Wu Y, Zheng Q, Zou B, Yeo YH, Li X, Li J, et al. The epidemiology of NAFLD in Mainland China with analysis by adjusted gross regional domestic product: a meta‐analysis. Hepatol Int 2020;14:259‐269. [DOI] [PubMed] [Google Scholar]
- 19. Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID‐19‐related liver functional abnormality. Clin Gastroenterol Hepatol 2020;18:1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chai X, Hu L, Zhang Y, Han WY, Lu Z, Ke AW, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. BioRxiv 2020. 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 21. Cotrim HP, Parise ER, Oliveira CP, Leite N, Martinelli A, Galizzi J, et al. Nonalcoholic fatty liver disease in Brazil. Clinical and histological profile. Ann Hepatol 2011;10:33‐37. [PubMed] [Google Scholar]
- 22. Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, et al. Non‐alcoholic fatty liver diseases in patients with COVID‐19: a retrospective study. J Hepatol 2020;73:451‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao F, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, et al. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol 2020; doi: 10.1111/jgh.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park SK, Seo MH, Shin HC, Ryoo JH. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5‐year prospective cohort study. Hepatology 2013;57:1378‐1383. [DOI] [PubMed] [Google Scholar]
- 25. Cicero AF, D'Addato S, Reggi A, Marchesini G, Borghi C; Brisighella Heart Study . Gender difference in hepatic steatosis index and lipid accumulation product ability to predict incident metabolic syndrome in the historical cohort of the Brisighella Heart Study. Metab Syndr Relat Disord 2013;11:412‐416. [DOI] [PubMed] [Google Scholar]
- 26. Chang JW, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, et al. Hepatic steatosis index in the detection of fatty liver in patients with chronic hepatitis B receiving antiviral therapy. Gut Liv 2020; doi: 10.5009/gnl19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu L, Lu W, Li P, Shen F, Mi YQ, Fan JG. A comparison of hepatic steatosis index, controlled attenuation parameter and ultrasound as noninvasive diagnostic tools for steatosis in chronic hepatitis B. Dig Liver Dis 2017;49:910‐917. [DOI] [PubMed] [Google Scholar]
- 28. Bessone F, Dirchwolf M, Rodil MA, Razori MV, Roma MG. Review article: drug‐induced liver injury in the context of nonalcoholic fatty liver disease ‐ a physiopathological and clinical integrated view. Aliment Pharmacol Ther 2018;48:892‐913. [DOI] [PubMed] [Google Scholar]
- 29. Tarantino G, Conca P, Basile V, Gentile A, Capone D, Polichetti G, et al. A prospective study of acute drug‐induced liver injury in patients suffering from non‐alcoholic fatty liver disease. Hepatol Res 2007;37:410‐415. [DOI] [PubMed] [Google Scholar]
- 30. Lammert C, Imler T, Teal E, Chalasani N. Patients with chronic liver disease suggestive of nonalcoholic fatty liver disease may be at higher risk for drug‐induced liver injury. Clin Gastroenterol Hepatol 2019;17:2814‐2815. [DOI] [PubMed] [Google Scholar]
- 31. Kim HS, Lee SH, Kim H, Lee SH, Cho JH, Lee H, et al. Statin‐related aminotransferase elevation according to baseline aminotransferases level in real practice in Korea. J Clin Pharm Ther 2016;41:266‐272. [DOI] [PubMed] [Google Scholar]
- 32. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases . AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian‐Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]


