Abstract
Introduction
Coronavirus Disease 2019 (COVID‐19) has spread worldwide, and it has reached to more than 14.5 million cases. Although Hubei province is the epicenter of China, little is known about epidemiological and clinical features of COVID‐19 in other areas in Hubei province around Wuhan. In addition, the virological data, particularly the factors associated with viral shedding of COVID‐19 has not been well described.
Objective
To describe the epidemiological and clinical features of patients with COVID‐19 in Tianmen city, and identify risk factors associated with prolonged viral shedding of COVID‐19.
Methods
Inpatients with COVID‐19 admitted before February 9, 2020 were included. Characteristics were compared between patients with early and late viral RNA shedding. Multivariate cox regression model was used to investigate variables associated with prolonged viral shedding.
Results
One hundred and eighty‐three patients were included. About 8.2% patients were categorized as critical degree of severity. All patients received antiviral therapy, with arbidol and interferon being the commonest. About 38.3% and 16.9% patients were treated with corticosteroid and immunoglobulin, respectively. Time from onset to admission (HR = 0.829, P < 0.001), and administration of corticosteroid (HR = 0.496, P = 0.002), arbidol (HR = 2.605, P = 0.008) and oseltamivir (HR = 0.416, P < 0.001) were independently associated with duration of viral shedding.
Conclusion
Symptoms of patients from Tianmen are relatively mild. Treatment should be started as early as possible, but corticosteroid and oseltamivir should be initiated with caution. In addition, clinical trials on arbidol should be conducted to demonstrate its effectiveness.
Keywords: characteristics, COVID‐19, treatments, viral shedding
1. INTRODUCTION
On March 11, 2020, the outbreak of coronavirus disease 2019 (COVID‐19) was characterized as a pandemic by the World Health Organization (WHO). 1 First reported in December 2019, COVID‐19 has spread worldwide, leading to more than 14.5 million cumulative infections and more than 607 000 fatalities as of July 21, 2020. 2
To date, clinical features and epidemiological characteristics of the disease were reported. Fever and cough were common clinical manifestations, and most patients were supposed to have a favorable prognosis, except those with older age and underlying comorbidities. 3 , 4 , 5 , 6 , 7 Although most patients of COVID‐19 in China were treated in Wuhan, the capital of Hubei province, little is known about patients with COVID‐19 in other areas of Hubei province outside Wuhan. Whether the clinical manifestation and epidemiological features of patients in other areas of Hubei province are similar to those in Wuhan is unclear.
In addition, compare with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), the symptoms of COVID‐19 are relatively mild, 8 , 9 , 10 but that does not mean the duration of viral shedding is short. As Hubei province is the epicenter of China, the virological data of patients in Hubei province is of great importance in public health policy making. Here, we reviewed medical records of patients with laboratory‐confirmed COVID‐19 in Tianmen, a city in Hubei province adjacent to Wuhan, to describe the clinical features, epidemiological characteristics and risk factors associated with prolonged viral shedding of COVID‐19.
2. MATERIALS AND METHODS
2.1. Study design and participants
Medical records of inpatients diagnosed with COVID‐19 in the First People’s Hospital of Tianmen, the largest designated hospital for COVID‐19 in Tianmen city, Hubei province, were retrospectively reviewed. Patients were admitted from January 18, 2020 (ie, the admission date of the first‐confirmed case of COVID‐19 in Tianmen city) to February 9, 2020. COVID‐19 was diagnosed according to the interim guidance from the WHO. 11 All patients were laboratory‐confirmed infection of SARS‐CoV‐2. Throat‐swab samples were obtained from each patient, and tested by real‐time reverse transcriptase‐polymerase chain reaction in three designated institutions (Center for Disease Control and Prevention of Tianmen city, First People’s Hospital of Tianmen and Wuhan KingMed Diagnostics) certified by the Health Commission of Hubei province.
2.2. Treatment choice
Treatment protocol for each patient was made by a team of trained physicians from the First People’s Hospital of Tianmen, according to the Chinese management guideline for COVID‐19 (version 5.0). 12 All patients received general treatment, including proper bed rest, supportive treatments and monitoring of vital signs. Although there were no specific antiviral drugs for COVID‐19, most patients in our study received arbidol, α‐interferon and lopinavir/ritonavir, except some without enough antiviral agents in the earliest stage of the epidemic. Patients with high fever, and systemic syndromes such as headache and myalgia, were treated with oseltamivir empirically before virological test results. Patients with increased white blood cell count or procalcitonin, or presented with purulent sputum during hospitalization were considered to be treated with antibiotics. Those with rapid lesion progression on thoracic CT, hypoxemia (SpO2 < 93% or oxygen index ≤ 300 mmHg) or respiratory distress (respiratory rate ≥ 30/min) during hospitalization were considered to use corticosteroid. Patients with decreased lymphocyte count were recommended to use intravenous immunoglobulin. For patients with hypoxemia (SpO2 < 93% or oxygen index ≤ 300 mmHg), oxygen therapy was administered to improve hypoxemic conditions. If there was no improvement after 1 to 2 hours, noninvasive mechanical ventilation (NMV) and invasive mechanical ventilation (IMV) would be considered. No patients in our study were treated with extracorporeal membrane oxygenation.
2.3. Data collection
Epidemiological, clinical, radiological, laboratory, treatment and outcome data were independently collected by two members from electronic medical records. Disagreement in interpretation were resolved by consultation with all team members. The present study was approved by the ethics committee of the First People’s Hospital of Tianmen and was granted a waiver of inform consent (No. 2020001).
Information was collected on hospital admission. Demographic data, exposure history, underlying comorbidities, signs, symptoms and date of onset were collected through interview with each patient. After admission, laboratory tests, including a complete blood test, coagulation, serum biochemistry (including liver and renal function, electrolytes, creatine kinase [CK] and lactate dehydrogenase [LDH]), and high sensitive C‐reaction protein (hs‐CRP) and thoracic CT scan were conducted. These examinations were repeated, and the frequency was determined by the team of physicians. The result of laboratory tests on admission and the images of thoracic CT scan during hospitalization were extracted and analyzed. Information of treatment (antibiotics, antiviral agents, corticosteroid and immunoglobulin), the most intense level of oxygen support (nasal cannula, mask, NMV or IMV) and outcome were recorded from electronic medical records.
Fever was defined as the highest temperature prior to hospital admission of at least 37.3°C. Exposure history and illness severity were defined according to the Chinese management guideline for COVID‐19 (version 5.0). 12 Radiographic extent was measured by counting the infiltrated lobes, in which the lingular lobe was not considered as a separate lobe. Corticosteroid treatment was defined as administration of an average dose equivalent to ≥20 mg of methylprednisolone per day.
2.4. Follow‐up and outcome
Throat‐swab samples were obtained every 2 days during hospitalization, and virus was considered eradicated after two consecutive negative tests for SARS‐CoV‐2 RNA. Duration of viral shedding was defined as the number of days from symptom onset to the first negative test for SARS‐CoV‐2 RNA, without a positive test afterward. The outcome of our study was the eradication of SARS‐CoV‐2 RNA in throat‐swab.
2.5. Statistical analysis
The statistical package SPSS, version 22.0, was used for the statistical analysis. Continuous variables were presented as median and interquartile range (IQR), and categorical variables were presented as absolute numbers and percentages. Continuous variables were compared by the Mann‐Whitney U test and the categorical variables were compared by chi‐square tests or Fisher’s exact tests as appropriate. The Kaplan‐Meier method was used for the evaluation of prognosis. Two‐sided P values of less than 0.05 were considered to be statistically significant.
The multivariate cox proportional hazard regression survival model was used to investigate the variables with a P value of less than 0.05 in the univariate analysis and met the proportional hazard assumption. All the preselected variables were entered simultaneously into the final model. Hazard ratios (HRs) were calculated for the independent variables. In the multivariate analysis, two‐sided P values of less than 0.05 were considered to be statistically significant.
3. RESULTS
3.1. Demographic and clinical characteristics
As of February 9, 2020, 190 patients with COVID‐19 were hospitalized in the First People’s Hospital of Tianmen. Ten patients died during hospitalization and 180 were discharged. In seven non‐survivors, SARS‐CoV‐2 RNA was continuously detectable until death. The remaining 183 patients with confirmed duration of viral shedding were included in the final analysis (Table 1). The median age was 49.0 years (IQR 39.0‐55.0), ranging from 24 to 83 years and 114 (62.3%) patients were male. The most common self‐reported symptoms on admission were fever and cough, followed by sputum production, dyspnea and fatigue. Hemoptysis was not presented in any patient. Hypertension was the most common comorbidities, followed by diabetes. One hundred and thirty‐five (73.8%) patients were categorized as normal degree of severity, 33 (18.0%) and 15 (8.2%) patients were categorized as severe and critical degree of severity, respectively. The median time from illness onset to hospital admission was 7.0 days (IQR 5.0‐9.0), and the median duration of viral shedding was 20.0 days (IQR 16.0‐25.0), ranging from 9 to 39 days.
TABLE 1.
Demographic and clinical characteristics
| Characteristics | Duration of viral shedding | |||
|---|---|---|---|---|
| Total (n = 183) | <20 days (n = 86) | ≥20 days (n = 97) | P value | |
| Age, years | 49.0 (39.0‐55.0) | 49.0 (35.0‐55.0) | 50.0 (40.0‐56.0) | 0.111 |
| Gender | 0.861 | |||
| Male | 114 (62.3%) | 53 (61.6%) | 61 (62.9%) | |
| Female | 69 (37.7%) | 33 (38.4%) | 36 (37.1%) | |
| Exposure history | 118 (64.5%) | 57 (66.3%) | 61 (62.9%) | 0.632 |
| Smoking history | 6 (3.3%) | 3 (3.5%) | 3 (3.1%) | 1.000 |
| Initial symptoms | ||||
| Fever | 162 (88.5%) | 74 (86.0%) | 88 (90.7%) | 0.322 |
| Highest temperature | 38.4 (38.0‐38.9) n=135 | 38.3 (38.0‐38.8) n=61 | 38.5 (38.0‐39.0) n=74 | 0.157 |
| Cough | 134 (73.2%) | 52 (60.5%) | 82 (84.5%) | <0.001 |
| Sputum | 41 (22.4%) | 13 (15.1%) | 28 (28.9%) | 0.026 |
| Dyspnea | 28 (15.3%) | 12 (14.0%) | 16 (16.5%) | 0.634 |
| Fatigue or myalgia | 21 (11.5%) | 11 (12.8%) | 10 (10.3%) | 0.599 |
| Hemoptysis | 0 | |||
| Diarrhea | 4 (2.2%) | 2 (2.3%) | 2 (2.1%) | 1.000 |
| Comorbidities | ||||
| Hypertension | 20 (10.9%) | 8 (9.3%) | 12 (12.4%) | 0.507 |
| Diabetes | 12 (6.6%) | 4 (4.7%) | 8 (8.2%) | 0.327 |
| Cardiovascular disease | 3 (1.6%) | 0 | 3 (3.1%) | 0.249 |
| Chronic lung disease | 3 (1.6%) | 1 (1.2%) | 2 (2.1%) | 1.000 |
| Chronic kidney disease | 2 (1.1%) | 1 (1.2%) | 1 (1.0%) | 1.000 |
| Nervous system disease | 1 (0.5%) | 0 | 1 (1.0%) | 1.000 |
| Endocrine disease | 4 (2.2%) | 2 (2.3%) | 2 (2.1%) | 1.000 |
| Tumor | 0 | |||
| Time from illness onset to hospital admission, days | 7.0 (5.0‐9.0) | 5.0 (3.0‐7.0) | 8.0 (6.0‐11.0) | <0.001 |
| Duration of viral shedding, days | 20.0 (16.0‐25.0) | 15.0 (14.0‐18.0) | 24.0 (22.0‐28.0) | <0.001 |
| Illness severity status | <0.001 | |||
| Normal | 135 (73.8%) | 76 (88.4%) | 59 (60.8%) | |
| Severe | 33 (18.0%) | 8 (9.3%) | 25 (25.8%) | |
| Critical | 15 (8.2%) | 2 (2.3%) | 13 (13.4%) | |
3.2. Radiologic and laboratory findings
Data of radiologic and laboratory findings are shown in Table 2. All patients had at least one positive thoracic CT result during the entire disease course. One hundred and three (56.3%) patients showed pulmonary infiltration in five lobes at the severest stage of disease. On admission, 54 (29.5%) patients had leukopenia (<4 × 109/L), 38 (20.8%) had neutropenia (<2 × 109/L) and 32 (17.5%) had lymphocytopenia (<0.8 × 109/L). Leukocytosis (>10 × 109/L) was presented in four (2.2%) patients. The median neutrophil‐lymphocyte ratio was 2.51 (IQR 1.60‐3.79). Thrombocytopenia (<100 × 109/L) was presented in 10 (5.5%) patients. Fourty‐one (22.4%) and 46 (25.1%) patients had elevated alanine aminotransferase (ALT) (>50 U/L) and aspartate aminotransferase (AST) (>40 U/L), respectively. Twenty‐four patients had elevated plasma serum creatinine (>97 µmol/L). About half patients (82 of 173) presented with elevated LDH (>250 U/L). Twelve of 173 (6.9%) patients had elevated CK (>310 U/L) and 9 of 175 (5.1%) patients had elevated creatine kinase muscle‐brain isoform (CK‐MB) (>24 U/L). More than three‐quarter of patients (143 of 183, 78.1%) had elevated hs‐CRP (>6 mg/L).
TABLE 2.
Radiologic and laboratory findings
| Characteristics | Duration of viral shedding | |||
|---|---|---|---|---|
| Total (n = 183) | <20 days (n = 86) | ≥20 days (n = 97) | P value | |
| Radiographic extent | 0.002 | |||
| 1 | 12 (6.6%) | 9 (10.5%) | 3 (3.1%) | |
| 2 | 18 (9.8%) | 12 (14.0%) | 6 (6.2%) | |
| 3 | 22 (12.0%) | 17 (19.8%) | 5 (5.2%) | |
| 4 | 28 (15.3%) | 7 (8.1%) | 21 (21.6%) | |
| 5 | 103 (56.3%) | 41 (47.7%) | 62 (63.9%) | |
| Laboratory findings | ||||
| Red blood cell count (×1012/L) | 4.36 (4.02‐4.76) | 4.46 (4.08‐4.79) | 4.30 (4.00‐4.66) | 0.160 |
| Hemoglobin (g/L) | 133.0 (120.0‐144.0) | 135.0 (120.8‐144.0) | 132.0 (119.5‐144.0) | 0.225 |
| <100 | 9/183 (4.9%) | 3/86 (3.5%) | 6/97 (6.2%) | |
| White blood cell count (×109/L) | 4.79 (3.84‐6.09) | 4.63 (3.89‐5.93) | 4.89 (3.78‐6.21) | 0.587 |
| <4 | 54/183 (29.5%) | 26/86 (30.2%) | 28/97 (28.9%) | |
| >10 | 4/183 (2.2%) | 1/86 (1.2%) | 3/97 (3.1%) | |
| Platelet (×1012/L) | 191.0 (142.0‐232.0) | 195.0 (142.5‐229.8) | 178.0 (142.0‐232.0) | 0.315 |
| <100 | 10/183 (5.5%) | 5/86 (5.8%) | 5/97 (5.2%) | |
| Neutrophil count (×109/L) | 3.01(2.11‐4.26) | 2.64 (2.10‐3.99) | 3.15 (2.12‐4.57) | 0.236 |
| <2 | 38/183 (20.8%) | 17/86 (19.8%) | 21/97 (21.6%) | |
| Lymphocyte count (×109/L) | 1.21(0.92‐1.49) | 1.28 (0.98‐1.57) | 1.16 (0.85‐1.40) | 0.038 |
| <0.8 | 32/183 (17.5%) | 13/86 (15.1%) | 19/97 (19.6%) | |
| Monocyte count (×109/L) | 0.45 (0.33‐0.57) | 0.46 (0.38‐0.57) | 0.44 (0.29‐0.57) | 0.308 |
| N/L ratio | 2.51 (1.60‐3.79) | 2.31 (1.54‐3.28) | 2.68 (1.65‐4.40) | 0.073 |
| Total bilirubin (μmol/L) | 11.2 (8.6‐15.4) | 11.3 (8.5‐14.8) | 11.2 (8.5‐15.6) | 0.908 |
| >23 | 10/183 (5.5%) | 6/86 (7.0%) | 4/97 (4.1%) | |
| Albumin (g/L) | 37.0 (34.2‐40.2) | 37.9(34.8‐41.0) | 36.5 (34.1‐39.5) | 0.046 |
| <40 | 135/183 (73.8%) | 59/86 (68.6%) | 76/97 (78.4%) | |
| Globulin (g/L) | 30.0 (27.8‐31.9) | 30.0(28.3‐32.5) | 30.0 (27.3‐31.7) | 0.347 |
| <20 | 0 | 0 | 0 | |
| ALT (U/L) | 24.3 (15.1‐43.8) | 25.3 (14.7‐44.4) | 24.0 (15.8‐43.8) | 0.871 |
| >50 | 41/183 (22.4%) | 20/86 (23.3%) | 21/97 (21.6%) | |
| AST (U/L) | 27.3 (20.1‐40.2) | 25.8 (19.9‐39.7) | 29.5 (20.5‐40.8) | 0.304 |
| >40 | 46/183 (25.1%) | 21/86 (24.4%) | 25/97 (25.8%) | |
| Creatinine (μmol/L) | 68.5 (56.8‐83.5) | 69.4 (54.9‐79.9) | 68.4 (57.7‐83.7) | 0.400 |
| >97 | 24/183 (13.1%) | 10/86 (11.6%) | 14/97 (14.4%) | |
| LDH(U/L) | 243.0 (205.5‐334.0) n=173 | 233.0 (190.5‐306.0) n=85 | 251.0 (210.0‐343.0) n=88 | 0.178 |
| >250 | 82/173 (47.4%) | 38/85 (44.7%) | 44/88 (50.0%) | |
| CK (U/L) | 68.0 (41.0‐115.0) n=173 | 63.0 (40.0‐109.0) n=85 | 75.0 (42.3‐119.0) n=88 | 0.181 |
| >310 | 12/173 (6.9%) | 3/85 (3.5%) | 9/88 (10.2%) | |
| CK‐MB (U/L) | 9.8 (7.0‐13.6) n=175 | 9.9 (7.3‐13.8) n=85 | 9.8 (6.6‐13.5) n=90 | 0.633 |
| >24 | 9/175 (5.1%) | 3/85 (3.5%) | 6/90 (6.7%) | |
| Hs‐CRP (mg/L) | 33.4 (7.3‐70.3) | 23.7 (5.5‐55.1) | 38.1 (11.8‐78.3) | 0.010 |
| >6 | 143/183 (78.1%) | 62/86 (72.1%) | 81/97 (83.5%) | |
| PT (s) | 12.3 (12.0‐12.7) n = 177 | 12.2 (12.0‐12.6) n = 85 | 12.4 (12.0‐12.8) n = 92 | 0.364 |
| >14 | 7/177 (4.0%) | 2/85 (2.4%) | 5/92 (5.4%) | |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase; CK, creatine kinase; CK‐MB, creatine kinase muscle‐brain isoform; Hs‐CRP, high sensitive C‐reaction protein; LDH, lactate dehydrogenase; PT, prothrombin time.
3.3. Treatments
All patients received antiviral therapy, including arbidol (173, 94.5%), α‐interferon (162, 88.5%), lopinavir/ritonavir (159, 86.9%), oseltamivir (117, 63.9%) and ribavirin (6, 3.3%). One hundred and thirty‐seven (74.9%) patients received antibiotics. Corticosteroid and immunoglobulin were used to 70 (38.3%) and 31 (16.9%) patients, respectively. Oxygen support was required for 169 (92.3%) patients, with nasal cannula (139, 76.0%) being the most common. Mechanical ventilation was applied for 11 (6.0%) patients, including 4 (2.2%) patients with IMV. (Table 3)
TABLE 3.
Treatments in hospital
| Treatments | Duration of viral shedding | |||
|---|---|---|---|---|
| Total (n = 183) | <20 days (n = 86) | ≥20 days (n = 97) | P value | |
| Antibiotics | 137 (74.9%) | 54 (62.8%) | 83 (85.6%) | <0.001 |
| Antiviral treatment | ||||
| Arbidol | 173 (94.5%) | 85 (98.8%) | 88 (90.7%) | 0.020 |
| Oseltamivir | 117 (63.9%) | 41 (47.7%) | 76 (78.4%) | <0.001 |
| Lopinavir/Ritonavir | 159 (86.9%) | 72 (83.7%) | 87 (89.7%) | 0.232 |
| α‐interferon | 162 (88.5%) | 78 (90.7%) | 84 (86.6%) | 0.385 |
| Ribavirin | 6 (3.3%) | 1 (1.2%) | 5 (5.2%) | 0.216 |
| Corticosteroid | 70 (38.3%) | 18 (20.9%) | 52 (53.6%) | <0.001 |
| Duration (d)† | 4.0 (3.0‐6.0) | 4.5 (1.8‐7.0) | 4.0 (3.0‐6.0) | |
| Dose (mg/d)† | 43.3 (40.0‐74.4) | 60.0 (40.0‐80.0) | 40.0 (40.0‐63.2) | |
| Immunoglobulin | 31 (16.9%) | 5 (5.8%) | 26 (26.8%) | <0.001 |
| Oxygen support | 0.074 | |||
| Nasal cannula | 139 (76.0%) | 68 (79.1%) | 71 (73.2%) | |
| Mask | 19 (10.4%) | 8 (9.3%) | 11 (11.3%) | |
| NMV | 7 (3.8%) | 1 (1.2%) | 6 (6.2%) | |
| IMV | 4 (2.2%) | 1 (1.2%) | 3 (3.1%) | |
†For 70 patients who received corticosteroid; duration and dose were count before the first negative result of SARS‐CoV‐2 RNA; IMV, invasive mechanical ventilation; NMV, noninvasive mechanical ventilation.
3.4. Risk factors for prolonged viral shedding
In univariable analysis, patients with a duration of viral shedding of less than 20 days (ie, the median duration of viral shedding of 183 patients) were compared with those without (Tables 1, 2, 3). Patients with prolonged viral shedding were older, but the difference was not significant (P = 0.111). Patients with prolonged viral shedding were more likely to present with cough (P < 0.001) and sputum (P = 0.026). During hospitalization, severe and critical patients were more common in those with prolonged viral shedding (P < 0.001). Time from illness onset to hospital admission (P < 0.001), radiographic extent (P = 0.002), lymphocyte count (P = 0.038), albumin (P = 0.046), hs‐CRP (P = 0.010), and prescription of antibiotics (P < 0.001), arbidol (P = 0.020), oseltamivir (P <0.001), corticosteroid (P < 0.001) and immunoglobulin (P < 0.001) were also associated with prolonged viral shedding.
In the Cox regression model, cough and sputum were not included in the model because of unconfirmed accuracy. Therefore, illness severity status, radiographic extent, lymphocyte count, hs‐CRP, albumin, time from illness onset to admission, antibiotics, arbidol, oseltamivir, corticosteroid and immunoglobulin were chosen into the final model. Among all the above variables, time from illness onset to admission (HR = 0.829, P < 0.001) (Figure 1), and administration of corticosteroid (HR = 0.496, P = 0.002) (Figure 2), arbidol (HR = 2.605, P = 0.008) (Figure 3) and oseltamivir (HR = 0.416, P < 0.001) (Figure 4) were independently associated with duration of viral shedding (Table 4).
FIGURE 1.
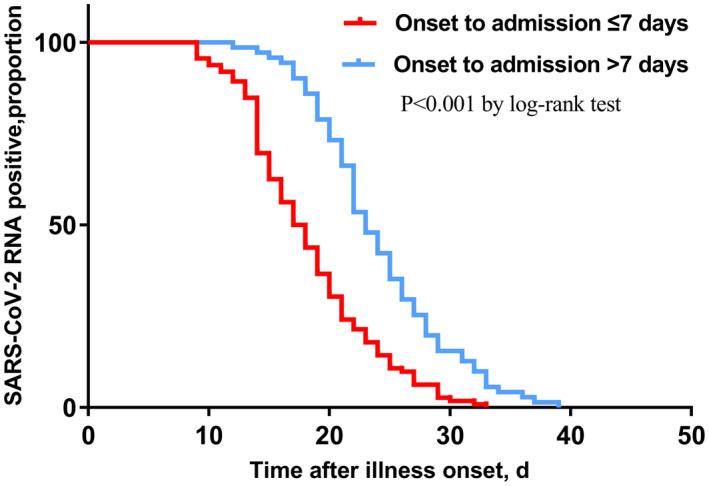
Cumulative proportion of patients with detectable SARS‐CoV‐2 RNA by day after illness onset between patients who admitted to the hospital of less than 7 days and those who did not. (P < 0.001 by log‐rank test)
FIGURE 2.
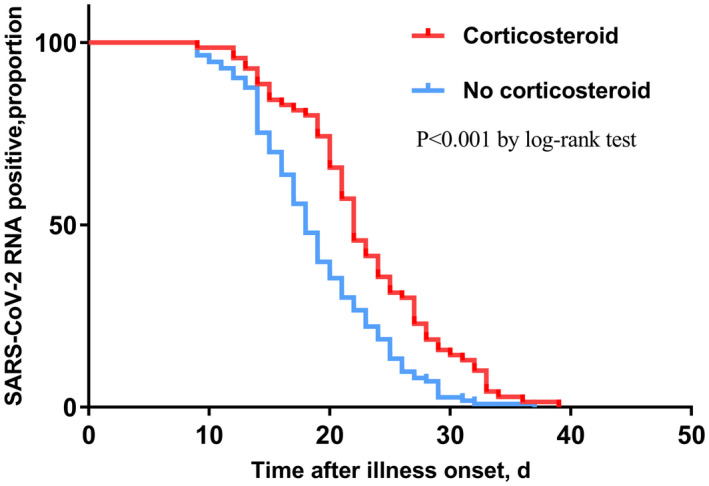
Cumulative proportion of patients with detectable SARS‐CoV‐2 RNA by day after illness onset between patients who received corticosteroid and those who not. (P < 0.001 by log‐rank test)
FIGURE 3.
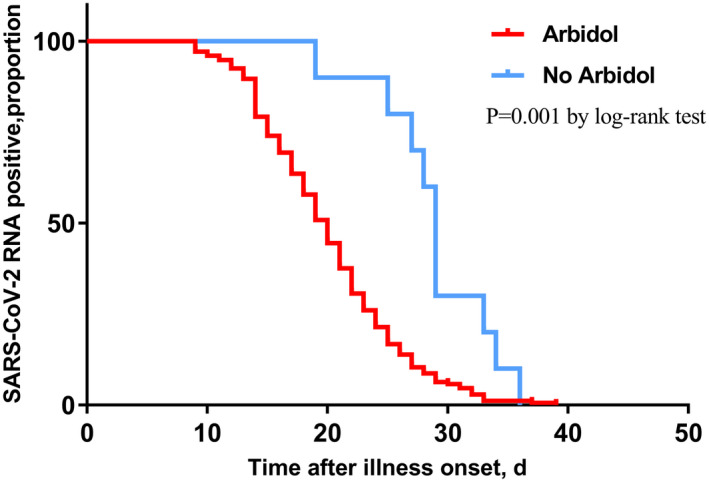
Cumulative proportion of patients with detectable SARS‐CoV‐2 RNA by day after illness onset between patients who received arbidol and those who not. (P = 0.001 by log‐rank test)
FIGURE 4.
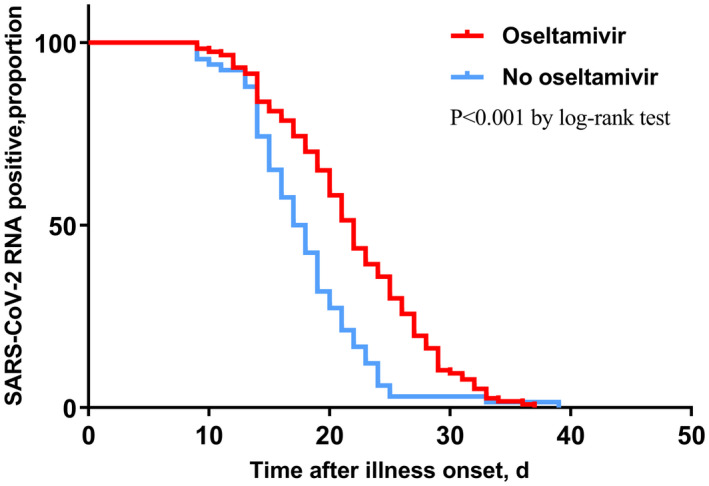
Cumulative proportion of patients with detectable SARS‐CoV‐2 RNA by day after illness onset between patients who received oseltamivir and those who not. (P < 0.001 by log‐rank test)
TABLE 4.
Independent risk factors for prolonged viral shedding
| Factors | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| HR | P value | HR | P value | |
| Illness severity status | 0.004 | 0.688 | ||
| Normal | 1 | 1 | ||
| Severe | 0.608 (0.413‐0.897) | 0.012 | 1.117 (0.716‐1.743) | 0.626 |
| Critical | 0.503 (0.294‐0.863) | 0.013 | 1.311 (0.683‐2.517) | 0.415 |
| Radiographic extent | <0.001 | 0.080 | ||
| 1 | 1 | 1 | ||
| 2 | 0.606 (0.290‐1.265) | 0.182 | 0.649 (0.306‐1.381) | 0.262 |
| 3 | 0.915 (0.453‐1.851) | 0.806 | 0.856 (0.419‐1.747) | 0.669 |
| 4 | 0.351 (0.176‐0.701) | 0.003 | 0.407 (0.198‐0.835) | 0.014 |
| 5 | 0.394 (0.214‐0.725) | 0.003 | 0.608 (0.306‐1.210) | 0.156 |
| Lymphocyte count | 1.565 (1.142‐2.145) | 0.005 | 1.226 (0.838‐1.794) | 0.295 |
| Hs‐CRP | 0.995 (0.992‐0.999) | 0.014 | 1.001 (0.996‐1.005) | 0.682 |
| Albumin | 1.036 (1.008‐1.064) | 0.012 | 0.998 (0.958‐1.041) | 0.939 |
| Time from illness onset to admission | 0.877 (0.838‐0.917) | <0.001 | 0.829 (0.784‐0.877) | <0.001 |
| Antibiotics | 0.499 (0.352‐0.706) | <0.001 | 0.779 (0.499‐1.216) | 0.272 |
| Corticosteroid | 0.554 (0.407‐0.754) | <0.001 | 0.496 (0.318‐0.773) | 0.002 |
| Arbidol | 2.720 (1.423‐5.202) | 0.002 | 2.605 (1.280‐5.302) | 0.008 |
| Oseltamivir | 0.527 (0.385‐0.722) | <0.001 | 0.416 (0.279‐0.620) | <0.001 |
| Immunoglobulin | 0.461 (0.307‐0.692) | <0.001 | 0.802 (0.487‐1.318) | 0.384 |
Abbreviation: Hs‐CRP, high sensitive C‐reaction protein.
4. DISCUSSION
In the present study, we described the epidemiological and clinical characteristics of patients in Tianmen city, Hubei province, and concluded that delayed admission, and prescription of corticosteroid and oseltamivir were significantly associated with prolonged viral shedding. On the contrary, administration of arbidol was independently related to shortened viral shedding. To date, most researches of epidemiologic and clinical features of COVID‐19 were from Wuhan, and other areas far from Wuhan. 3 , 4 , 5 , 6 , 7 , 13 , 14 , 15 , 16 , 17 , 18 , 19 Although Hubei province is the epicenter of China, little is known for patients from cities in Hubei province around Wuhan.
In the present study, majority of patients were male, and most were young and middle‐aged, which were similar to those initially infected in Wuhan. 4 , 6 In addition, fever and cough were also the most common symptoms at the onset, and comorbidities were reported in few patients. 4 , 6 , 20 Although more than 50% of patients had five lobes affected on thoracic CT scan, laboratory indices, except hs‐CRP, of most patients were in normal range. Compare with those initially infected in Wuhan, 4 , 6 , 20 symptoms of patients from Tianmen city are relatively mild, similar to those outside Hubei province. 16 , 19 , 21 One important reason might be timely hospitalization of patients with COVID‐19 in Tianmen city. However, in the early stage of the epidemic in Wuhan, patients with mild‐to‐moderate illness were self‐isolated and observed at home due to the shortage of hospital beds before the establishment of Fangcang shelter hospitals. 22
Compare with those in Wuhan, more patients in our cohort received antiviral agents, and less received antibiotics, corticosteroid, immunoglobulin and IMV, suggesting that the patients with COVID‐19 in Tianmen city were relatively mild. 4 , 6 , 20 However, in our study, the median duration of viral shedding was 20.0 days, and the shortest and longest observed duration of viral shedding were 9 and 39 days, respectively, similar to that in a cohort from Wuhan, 6 and longer than a cohort with more critical patients from Hangzhou and Shenzhen. 21 Therefore, it is worth noting that mild disease does not mean shorter duration of viral shedding.
Corticosteroid was independently associated with prolonged viral shedding of SARS‐CoV‐2 in the present study. It has been reported that corticosteroid therapy, not only high‐dose corticosteroid treatment, is independently related to prolonged viral RNA shedding in patients with avian influenza A (H3N2 and H7N9), 23 , 24 MERS 25 and SARS. 26 The mechanism might be related to the impaired T‐cell immunity. 27 However, in a recent retrospective study, corticosteroid was not found to be an independent risk factor of prolonged viral shedding of SARS‐CoV‐2. 21 The reason might be the differences in inclusion criteria, illness severity and variables included in the univariate and multivariate analysis. The usage of corticosteroid is always controversial in patients with respiratory infection. On the one hand, evidence suggested that corticosteroid might reduce the risk of death in patients with COVID‐19 who developed acute respiratory distress syndrome (ARDS) during hospitalization, 4 but on the other hand, corticosteroid was associated with prolonged viral shedding in mild patients, leading to prolonged hospitalization and increased consumption of medical resources.
In the present study, oseltamivir was another factor associated with prolonged viral shedding of SARS‐CoV‐2. On the contrary, arbidol was independently related to shorter duration of viral shedding. To date, no antiviral agent has yet been proved effective by randomized controlled trial (RCT). Arbidol was the most frequently used antiviral regimen in the present study. A retrospective study of 33 patients infected with SARS‐CoV‐2 revealed that arbidol, combined with lopinavir/ritonavir, was more effective than lopinavir/ritonavir alone in the elimination of virus. 28 Thus, it is of great importance to evaluate the efficacy of arbidol in clinical trials. Oseltamivir, a kind of neuraminidase inhibitor (NAI) effective in avian influenza A, should not be recommended to patients with COVID‐19 due to lack of neuraminidase of SARS‐CoV‐2. 29
Delayed hospital admission was independently related to prolonged viral shedding in patients with COVID‐19 in our study. The same result was observed in a recent study. 21 Similarly, in previous study of Avian Influenza A (H7N9) virus infection, delayed NAI treatment was independently associated with prolonged viral shedding. 23 Despite the lack of effective treatment proved by RCT, timely and standardized treatment of patients with COVID‐19, rather than self‐isolation and observation at home, plays an important role in the management of COVID‐19.
Our study has several limitations. First, due to its retrospective nature, not all laboratory tests were done in all patients on admission, including LDH, CK, CK‐MB and prothrombin time. Therefore, whether they had a decisive role in prolonged viral shedding could not be evaluated. Second, shedding of SARS‐CoV‐2 RNA in throat‐swab is not exactly the same as viral shedding, since recent research indicated extended duration of viral shedding in feces. 30 Third, patients with COVID‐19 in our study were relatively mild compare with those in Wuhan. We could not evaluate whether prolonged viral shedding is related to increased mortality. However, in the epidemic of this disease in Tianmen city, all symptomatic patients with COVID‐19 were timely treated, and no patients were self‐isolated or observed outside hospital due to limited medical resources. Thus, we believe our patients is representative for those treated in areas with sufficient medical resources.
In conclusion, compare with those severe patients from Wuhan, symptoms of patients from Tianmen city, Hubei province, are relatively mild. In these patients, corticosteroid, oseltamivir and delayed admission are independent risk factors for prolonged viral shedding in patients with COVID‐19. On the contrary, arbidol is independently related with shortened duration of viral shedding. Treatment for patients with COVID‐19 should be started as early as possible, and RCTs on arbidol should be conducted to demonstrate its effectiveness. Although proved effective in patients who developed ARDS during hospitalization, corticosteroid should be initiated with caution, especially in mild patients with COVID‐19.
Conflict of interests
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Author Contribution
This study was designed by all authors. Treatment protocol for these patients were made by Fuying Hu, Gang Yin and Youping Chen. Detection of SARS‐CoV‐2 RNA was conducted by Jiangqin Song. Data collection was completed by Maosong Ye and Jie Liu. Statistical analysis was conducted by Cuicui Chen, Yuanlin Song, Xinjun Tang and Yong Zhang. The manuscript was written by Xinjun Tang and revised by Yong Zhang.
Ethics
The present study was approved by the ethics committee of the First People’s Hospital of Tianmen and was granteda waiver of inform consent (No.2020001).
Acknowledgments
We thank all the health‐care workers from Tianmen city for their efforts in combating the COVID‐19 pandemic.
This work was supported by the National Natural Science Foundation of China (grant number 81800077), Shanghai Municipal Key Clinical Specialty (grant number shslczdzk02201) and Shanghai Top‐Priority Clinical Key Disciplines Construction Project (grant number 2017ZZ02013).
Hu F, Yin G, Chen Y, et al. Corticosteroid, oseltamivir and delayed admission are independent risk factors for prolonged viral shedding in patients with Coronavirus Disease 2019. Clin Respir J. 2020;14:1067–1075. 10.1111/crj.13243
Contributor Information
Xinjun Tang, Email: tangxj0531@126.com.
Yong Zhang, Email: zhang.yong@zs-hospital.sh.cn.
References
- 1. World Health Organization . Coronavirus Disease (COVID‐19) Outbreak. https://www.who.int. Accessed March 11, 2020. [Google Scholar]
- 2. World Health Organization. Coronavirus disease (COVID‐2019) situation reports . https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports. Accessed July 21, 2020.
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Assiri A, McGeer A, Perl TM, et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi KW, Chau TN, Tsang O, et al. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med. 2003;139(9):715‐723. [DOI] [PubMed] [Google Scholar]
- 10. Hong KH, Choi JP, Hong SH, et al. Predictors of mortality in Middle East respiratory syndrome (MERS). Thorax. 2018;73(3):286‐289. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Clinical Management Of Severe Acute Respiratory Infection When Novel Coronavirus NCoV Infection Is Suspected Interim Guidance. https://www.who.int/publications‐detail/clinical‐management‐of‐severe‐acute‐respiratory‐infection‐when‐novel‐coronavirus‐(ncov)‐infection‐is‐suspected. Accessed March 13, 2020.
- 12. National Health Commission of the People's Republic of China. Chinese management guideline for COVID‐19 (version 5.0) . http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440/files/7260301a393845fc87fcf6dd52965ecb.pdf. Accessed May 3, 2020.
- 13. Dong XC, Li JM, Bai JY, et al. Epidemiological characteristics of confirmed COVID‐19 cases in Tianjin. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(5):638‐642. [DOI] [PubMed] [Google Scholar]
- 14. Liu W, Tao ZW, Lei W, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133(9):1032‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tian S, Hu N, Lou J, et al. Characteristics of COVID‐19 infection in Beijing. J Infect. 2020;80(4):401‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID‐19): a multi‐center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80(4):388‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of COVID‐19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020;ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with Covid‐19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu K, Chen Y, Yuan J, et al. Factors associated with prolonged viral RNA shedding in patients with COVID‐19. Clin Infect Dis. 2020;ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen S, Zhang Z, Yang J, et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395(10232):1305‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y, Guo Q, Yan Z, et al. Factors associated with prolonged viral shedding in patients with avian influenza A(H7N9) virus infection. J Infect Dis. 2018;217(11):1708‐1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arabi YM, Mandourah Y, Al‐Hameed F, et al. Corticosteroid therapy for critically Ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757‐767. [DOI] [PubMed] [Google Scholar]
- 26. Lee N, Allen CK, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS‐associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bender BS, Croghan T, Zhang L, Small PJ. Transgenic mice lacking class I major histocompatibility complex‐restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175(4):1143‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect. 2020;81(1):e1‐e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li H, Wang YM, Xu JY, Cao B. Potential antiviral therapeutics for 2019 Novel Coronavirus. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(3):170‐172. [DOI] [PubMed] [Google Scholar]
- 30. Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS‐CoV‐2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]


