Abstract
To report the clinical characteristics and potential risk factors of patients with coronavirus disease 2019 (COVID‐19) in Wuhan Stadium Cabin Hospital in Hubei Province. A total of 571 patients of COVID‐19 treated in the Wuhan Stadium Cabin Hospital were selected for analysis, univariable and multivariable logistic regression methods were used to explore the risk factors associated with disease aggravation. The main clinical symptoms of moderate COVID‐19 were fever, cough and dyspnea, hypertension, diabetes, and coronary heart diseases were the main comorbidities both in transferred and stable patients. Twenty‐six patients (4.55%) of mild and moderate patients had disease aggravation, and most of which occurred between 36 and 48 hours after admission. Multiple regression analysis showed increasing odds of disease aggravation associated with former smoker history, diabetes, dyspnea, consolidation, and interstitial abnormalities of computed tomography scanning, lymphopenia and elevated of C‐reactive protein, the time points of transferred patients mainly between 36 and 48 hours (65.38%), and the average hospital stay for stable patients was 15 days.It could help clinicians to identify patients with poor prognosis at an early stage, and provide early warning role for timely intervention.
Keywords: clinical characteristics, coronavirus disease‐19 (COVID‐19), logistic regression, pneumonia
Highlights
The main clinical symptoms of moderate COVID‐19 were fever, cough and dyspnea.
Hypertension, diabetes and coronary heart diseases were the main comorbidities both in transferred and stable patients.
4.55% of mild and moderate patients had disease aggravation, and most of which occurred between 36 hours to 48 hours after admission.
Increasing odds of disease aggravation associated with former smoker history, diabetes, dyspnea, consolidation and interstitial abnormalities of CT scanning, lymphopenia and elevated of CRP.
1. INTRODUCTION
Since December of 2019, a series of coronavirus pneumonia (coronavirus disease 2019 [COVID‐19]) break out in Wuhan City, Hubei Province and other parts of China. Recently it has been identified as a type of beta coronavirus. 1 , 2 Up to 24 March 2020, total 81 773 cases have been confirmed in China, including 67 801 cases in Hubei Province, 50 006 cases in Wuhan City, accumulative 60 323 cured cases in Hubei Province (43 214 cases in Wuhan), and cumulative 3160 deaths in Hubei Province (2524 cases in Wuhan). Other countries include 59 138 cases in Italy, 31 573 cases in the United States, 28 572 cases in Spain, 24 774 cases in Germany, 23 049 cases in Iran, 15 821 cases in France and so on. Considering the highly prevalence and infection, COVID‐19 has been classified as a Class B infectious disease according to the Law of the People's Republic of China on the prevention and control of infectious diseases and managed as Class A infectious disease. 3 At the same time, The World Health Organization (WHO) has recently declared COVID‐19 as a public health emergency of international concern, 4 which indicating this COVID‐19 poses a huge threat to global health.
To solve the problem of difficult hospitalization of the patients, By the end of February, the Chinese government totally opened 14 Cabin Hospitals to treat patients confirmed with mild or moderate of COVID‐19. With the effective intervention measures and isolation plans formulated by the government in an efficient manner, most patients received timely diagnosis and treatment.
Since the outbreak of COVID‐19, lots of basic, clinical and epidemiological studies have been reported successively, 5 , 8 and the severity of some cases of COVID‐19 mimicked that of severe acute respiratory syndrome coronavirus (SARS‐CoV), 6 , 9 however, there is no special study on the clinical characteristics of patients and potential risk factors for disease aggravation in Cabin hospital. As a centralized admission hospital for treating COVID‐19, we have treated 571 patients were diagnosed of COVID‐19. This study intends to summarize and analyze the clinical characteristics of the above‐mentioned patients, and explore the risk factors for disease aggravation, and hope to help clinicians to identify patients with poor prognosis at an early stage, and provide early warning function for timely intervention.
2. METHODS
2.1. Study design and participants
This was single‐center, retrospective cohort study, and the general information, epidemiological history, incidence, clinical manifestations, laboratory parameters, imaging examinations and treatment of patients confirmed COVID‐19 pneumonia were admitted in Wuhan Stadium Cabin Hospital, the study was approved by the Ethics Committee of Wuhan Stadium Cabin Hospital, and the written informed consent was waived because of the retrospective nature of the study and belongs to emergency medical service.
The diagnosis of COVID‐19 is based on the WHO interim guidance 10 and new coronavirus pneumonia diagnosis and treatment program (6th ed.) (in Chinese), 11 only laboratory‐confirmed positive cases were included in this analysis through Real‐time fluorescence quantitative polymerase chain reaction assay of pharyngeal swab specimens. 5
2.2. Diagnostic criteria
2.2.1. Epidemiological history
-
(1)
Travel history or residence history of Wuhan and surrounding areas or other communities with case reports within 14 days before the onset of illness;
-
(2)
History of contact with new coronavirus infected persons (positive nucleic acid test) within 14 days before onset;
-
(3)
Have been exposed to patients with fever or respiratory symptoms from Wuhan and surrounding areas, or from communities with case reports;
-
(4)
Cluster of diseases.
2.2.2. Clinical manifestations
-
(1)
Fever and /or respiratory symptoms;
-
(2)
Imaging characteristics of pneumonia: multiple small patchy shadows and interstitial changes in the early stage, subsequently developed into bilateral ground‐glass opacity, infiltrates in both lungs. In severe cases, pulmonary consolidation may occur with little pleural effusion;
-
(3)
Total number of white blood cells in the early stage of disease was normal or decreased, and the lymphocyte count was decreased.
2.3. Clinical classifications
According to the guideline of New coronavirus pneumonia diagnosis and treatment program, the confirmed patients were divided into the following types: (1) Mild: the clinical symptoms were light, and there was no sign of pneumonia on imaging; (2) moderate: with fever, respiratory tract and other symptoms, imaging suggests pneumonia; (3) severe: coincide with any of the following: (a) respiratory distress, respiration rate (RR) ≥ 30 times /min; (b) the oxygen saturation ≤93% in the resting state; (c) PaO2/FiO2 ≤ 300mm Hg (1mm Hg = 0.133 kPa); (4) Critically ill: coincide with any of the following: (a) respiratory failure occurs and mechanical ventilation is required; (b) shock; (c) the patient develops other organ failure and needs ICU monitoring and treatment. The mild and moderate types were collected as subjects in our study.
2.4. Real‐time reverse transcription polymerase chain reaction assay for novel coronavirus
Reverse transcription polymerase chain reaction assays were performed in accordance with the protocol established by the WHO. 12 Throat swab samples were collected for extracting 2019‐nCoV (novel coronavirus) RNA from patients suspected of having 2019 nCoV infection. After collection, the throat swabs were placed in to a collection tube with150 μL of virus preservation solution, and total RNA was extracted within 2 hours using the respiratory sample RNA isolation kit (Zhongzhi, Wuhan, China). The nucleic acid testing was performed by a third institution recognized by Chinese Center for Disease Control and Prevention, and details regarding laboratory confirmation processes are provided in the Supplementary Appendix (Table S1).
2.5. Statistical analysis
Continuous variables with normal distribution were expressed as mean ± standard deviation; non‐normal continuous variables were reported as median (interquartile range [IQR]). For P value, the Mann‐Whitney U test were used for analyzing the normally distributed continuous variables, and the Kruskal‐Wallis test for non‐normally distributed data. The categorical variables were presented as percentage and analyzed by the χ 2 test or the Wilcoxon rank‐sum test. All statistical analyses were performed with Stata version 14.2 for Mac (StataCorp, College Station, TX), and P value less than .05 was considered statistically significant. Multiple logistic regression models were used to identify factors that influenced outcomes and presented as odds ratios (ORs) and 95% confidence intervals (CIs). The differences in baseline characteristics among nonanemic and anemic patients entered into the multivariate regression. Results were reported follow that all associations as ORs in logistic regression models, with their corresponding 95% CIs.
3. RESULTS
In our study, 571 cases confirmed with COVID‐19 in Wuhan Stadium Cabin Hospital were involved (Figure 1), all of these cases had no epidemic history of contacting with seafood market of South China directly. Among them, 293 were females (51.56%), 278 were males (48.70%), the median age is 50 years (IQR, 38.0‐59.0), 26 patients (4.55%) were transferred to a designated specialized hospital for further treatment within 72 hours of admission due to the aggravation of the disease. The time points of transferred patients mainly between 36 and 48 hours (65.38%) (Figure 2). There were 14 cases (53.85%) of transferred patients with a history of smoking, and 122 cases (22.39%) in stable patients. Hypertension, diabetes, and coronary heart diseases were the main comorbidities both in transferred patients (26.92%, 15.38%, 15.38%) and in stable patients (9.51%, 2.38%, 1.65%), The main clinical symptoms were fever, cough, and dyspnea, which accounted for 15 (57.69%), 20 (76.90%), and 16 (61.54%) cases in transferred patients, and 284 (51.92%), 382 (70.09%), and 89 (16.27%) cases in stable patients. Computed tomography (CT) scanning showed consolidation and interstitial abnormalities were obviously seen in transferred patients (38.46%, 61.54%) than that in stable patients(5.32%, 8.25%) (Table 1). The other symptoms included fatigue, muscle aches, chest tightness, headache, diarrhea, etc. There was no significant difference between the transferred and stable patients.
Figure 1.
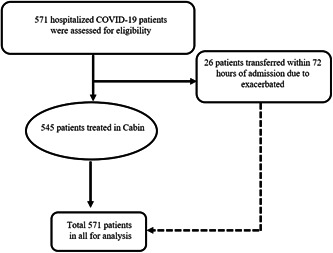
Flow diagram of clinical trial in the study. COVID‐19, coronavirus disease 2019
Figure 2.
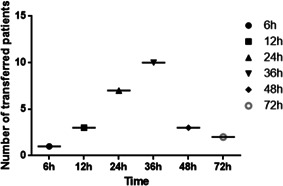
Number of transferred patients at different timepoints
Table 1.
Clinical characteristics of 571 patients infected with COVID‐19
| Characteristics | Total patients (N = 571) | Transferred patients (N = 26) | Stable patients (N = 545) | P value |
|---|---|---|---|---|
| Age, y | ||||
| Median (IQR) | 50.0 (38.0‐59.0) | 50.0 (31.0‐60.25) | 50.0 (38‐58.5) | .711 |
| Sex: 0.845 | ||||
| Female | 293/571 (51.30%) | 12/26 (46.20%) | 281/545 (51.60%) | |
| Male | 278/571 (48.70%) | 14/26 (53.80%) | 264/545 (48.40%) | |
| Smoking history | ||||
| Former smoker | 56/571 (9.80%) | 10/26 (38.46%) | 46/545 (8.44%) | .0001*** |
| Current smoker | 80/571 (14.0%) | 4/26 (15.38%) | 76/545 (13.94%) | .979 |
| Comorbidities | ||||
| Hypertension | 55/571 (9.63%) | 7/26 (26.92%) | 52/54 5(9.51%) | .0234* |
| Diabetes | 17/571 (2.98%) | 4/26 (15.38%) | 13/545 (2.38%) | .0007*** |
| Coronary heart disease | 12/571 (2.10%) | 3/26 (15.38%) | 9/545 (1.65%) | .002** |
| Chronic kidney disease | 1/571 (0.18%) | 0 | 1/545 (0.18%) | .976 |
| Gout | 2/57 1 (0.35%) | 0 | 2/545 (0.37%) | .953 |
| Signs and symptoms on admission | ||||
| Fever ≥37.3°C | 299/571 (52.36%) | 15/26 (57.69%) | 284/545 (51.92%) | .856 |
| Cough | 402/571 (70.40%) | 20/26 (76.9%) | 382/545 (70.09%) | .757 |
| Fatigue, muscle aches | 95/571 (16.63%) | 8/26 (30.77%) | 77/545 (14.77%) | .077 |
| Dyspnea | 105/571 (18.39%) | 16/26 (61.54%) | 89/545 (16.27%) | <.0001*** |
| Headache | 55/571 (9.63%) | 3/26 (11.54%) | 52 /545 (9.51%) | .945 |
| Sore throat | 28/571 (4.90%) | 2/26 (7.69%) | 26/545 (4.75%) | .797 |
| Nasal congestion | 17/571 (2.98%) | 2/26 (7.69%) | 15/545 (2.75%) | .351 |
| Chest tightness | 100/571 (17.51%) | 5/26 (19.23%) | 95/545 (17.37%) | .973 |
| Diarrhea | 51/571 (8.93%) | 2/26 (7.69%) | 49/545 (8.96%) | .975 |
| Nausea or vomiting | 21/571 (3.68%) | 2/26 (7.69%) | 19/545 (3.49%) | .538 |
| Abnormalities on chest CT | ||||
| Ground‐glass opacity | 277/571 (48.51%) | 8/26 (30.76%) | 276/545 (50.64%) | .141 |
| Normal density shadow | 108/571 (18.91) | 0 | 108/545 (19.82%) | .007** |
| Consolidation | 39/571 (6.83%) | 10/26 (38.46%) | 29/545 (5.32%) | <.0001*** |
| Nodular lesion | 93/571 (16.29%) | 6/26 (23.07%) | 87/545 (15.96%) | .631 |
| Interstitial abnormalities | 61/571 (10.68%) | 16/26 (61.54%) | 45/545 (8.25%) | <.0001*** |
| Treatments | ||||
| Antibiotic treatment | 571/571 (100%) | 26/26 (100%) | 545/545 (100%) | |
| Antiviral treatment | 571/571 (100%) | 26/26 (100%) | 545/545 (100%) | |
| Traditional Chinese medicine | 571/571 (100%) | 26/26 (100%) | 545/545 (100%) | |
| Hormone therapy | 26/571 (4.55%) | 26/26 (100%) | 0 | <.0001*** |
| Intranasal oxygen inhalation | 38/571 (6.65%) | 26/26 (100%) | 135/545 (24.77%) | .0002*** |
| Mechanical ventilation | Unknown | Unknown | 0 | |
| ECMO | Unknown | Unknown | 0 | |
| Clinical outcome | ||||
| Discharged | Unknown | Unknown | 545/545 (100%) | |
| Remained in hospital | Unknown | Unknown | 0 | |
| Died | Unknown | Unknown | 0 | |
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease‐19; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range.
P < .05.
P < .01.
P < .001.
Laboratory results showed the white blood cell count, neutrophils and lymphocytes were all lower in transferred patients than stable patients, whereas, lactate dehydrogenase (LDH) and C‐reactive protein (CRP) were both in significantly higher in transferred patients than stable patients. No significant difference of the other laboratory results between in the two groups patients (Table 2).
Table 2.
Laboratory tests of 571 patients infected with COVID‐19
| Variables | Total patients (N = 571) | Transferred patients (N = 26) | Stable patients (N = 545) | P value |
|---|---|---|---|---|
| Blood routine (IQR) | ||||
| WBC count (x109/L) | 5.55 (3.94, 8.11) | 4.47 (3.44, 5.83) | 5.65 (4.06, 8.13) | .007*** |
| NEU count (x109/L) | 4.17 (3.00, 5.87) | 3.50 (2.55, 4.48) | 4.21 (3.03, 5.95) | .030* |
| LYM count (x109/L) | 0.72 (0.42, 1.10) | 0.53 (0.41, 0.72) | 0.74 (0.43, 1.13) | .004*** |
| Hb, g/L | 134.0 (125.0, 144.0) | 142.0 (130.3, 145.0) | 134.0 (125.0, 143.5) | .142 |
| PLT count (x109/L) | 256.0 (226.0, 289.0) | 247.0 (224.8, 287.3) | 256.0 (226.0, 289.0) | .637 |
| Coagulation (IQR) | ||||
| PT, s | 11.20 (10.40, 11.60) | 11.20 (10.30, 11.53) | 11.20 (10.42, 11.60) | .716 |
| APTT, s | 34.22 (29.78, 36.50) | 31.69 (29.13, 34.79) | 34.44 (29.78, 36.76) | .133 |
| D‐D, ng/mL | 189.5 (144.5, 306.2) | 198.0 (148.0, 271.0) | 198.0 (148.0, 263.5) | .878 |
| Myocardial enzymes (IQR) | ||||
| CK, IU/L | 235.3 (178.2, 290.3) | 234.6 (170.1, 264.6) | 235.3 (178.2, 290.8) | .744 |
| LDH, U/L | 287.7 (210.5, 373.2) | 432.7 (411.1, 496.6) | 287.5 (205.0, 354.2) | <.0001** |
| CK‐MB, U/L | 19.23 (16.43, 22.45) | 19.71 (18.05, 22.68) | 19.22 (16.43, 22.45) | .345 |
| Biochemical indicators (IQR) | ||||
| ALT, IU/L | 34.56 (26.34, 42.34) | 36.94 (30.01, 43.76) | 34.56 (26.23, 42.34) | .345 |
| AST, IU/L | 32.12 (24.54, 36.12) | 31.30 (24.35, 34.32) | 32.12 (24.54, 36.43) | .530 |
| ALB, g/L | 44.57 (39.81, 48.32) | 43.90 (38.76, 47.57) | 44.57 (39.89, 48.32) | .2141 |
| Cr, µmol/L | 87.34 (76.45, 98.60) | 88.00 (76.31, 98.63) | 87.34 (76.45, 98.70) | .8198 |
| BUN, mmol/L | 6.54 (5.44, 7.45) | 6.43 (5.42, 7.50) | 6.54 (5.44, 7.45) | .7606 |
| Indicators of inflammation (IQR) | ||||
| CRP, mg/L | 39.32 (26.71, 67.32) | 60.73 (42.41, 77.43) | 38.33 (25.85, 67.23) | .002*** |
| PCT, ng/ML | 0.031(0.019, 0.043) | 0.027 (0.019, 0.043) | 0.031 (0.019, 0.043) | .5296 |
Abbreviations: ALB, Albumin; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, urea nitrogen; CK, creatine kinase; CK‐MB, creatine kinase‐MB; COVID‐19, coronavirus disease‐19; Cr, creatinine; CRP, C‐reactive protein; D‐D, DD dimers; Hb, hemoglobin; IQR, interquartile range; LDH, lactate dehydrogenase; LYM, lymphocytes; NEU, neutrophils; PCT, procalcitonin; PLT, platelets; PT, prothrombin time; WBC, white blood cells.
P < .05.
P < .01.
P < .001.
In univariable analysis, odds of disease aggravation were higher with diabetes or coronary heart disease or hypertension (Table 1). Former smoker history, lymphopenia, LDH, and CRP were also associated with disease aggravation (Tables 1 and 2).
We included 571 patients with complete data for all variables (26 transferred patients and 545 stable patients) in the multivariable logistic regression model. We found that former smoker history, diabetes, dyspnea, consolidation, and interstitial abnormalities of CT scanning, lymphopenia, and elevated of CRP were associated with increased odds of disease aggravation (Table 3).
Table 3.
Risk factors associated with transferred hospital
| Characteristics | Univariable analyses | Multiple variable regression | |
|---|---|---|---|
| P value | OR, 95%CI | P value | |
| Age, y, median (IQR) | .711 | ||
| Female | .845 | ||
| Male | |||
| Former smoker | .0001 | 14.53 (3.18, 66.20) | .001** |
| Current smoker | .979 | ||
| Hypertension | .0234 | 0.75 (0.12, 4.59) | .755 |
| Diabetes | .0007 | 22.64 (2.30, 223.022) | .0008* |
| Coronary heart disease | .002 | 6.75 (0.629, 72.61) | .115 |
| Chronic kidney disease | .976 | ||
| Gout | .953 | ||
| Fever ≥37.3°C | .856 | ||
| Cough | .757 | ||
| Fatigue, muscle aches | .077 | ||
| Dyspnea | <.0001 | 10.93 (2.95, 40.45) | <.0001* |
| Headache | .945 | ||
| Sore throat | .797 | ||
| Nasal congestion | .351 | ||
| Chest tightness | .973 | ||
| Diarrhea | .975 | ||
| Nausea or vomiting | .538 | ||
| Ground‐glass opacity | .141 | ||
| No abnormal density shadow | .007 | ||
| Consolidation | <.0001 | 15.52 (2.86, 84.24) | .001** |
| Nodular lesion | .631 | ||
| Interstitial abnormalities | <.0001 | 13.40 (4.05, 44.31) | <.0001* |
| Antibiotic treatment | |||
| Antiviral treatment | |||
| Traditional Chinese medicine | |||
| Lymphocyte counting | 0.89 (0.81, 0.97) | .008** | |
| Elevated LDH | 5.01 (1.00, 25.35) | .05 | |
| CRP (>40 mg/L) | <.0001 | 5.03 (1.41, 19.16) | .013*** |
| Hormone therapy | <.0001 | ||
| Intranasal oxygen inhalation | |||
| Mechanical ventilation | |||
| ECMO | |||
| Discharged | |||
| Remained in hospital | |||
| Died | |||
Abbreviations: CI, confidence interval; CRP, C‐reactive protein; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; LDH, lactate dehydrogenase; OR, odds ratio.
P < .05.
P < .01.
P < .001.
All patients were treated with a single antibiotic moxifloacin, antiviral therapy (oseltamivir/arbidol), and traditional Chinese medicine (Lian hua qing wen capsule). Twenty‐six patients (4.55%) were transferred to a designated specialized hospital for further treatment within 72 hours of admission due to the aggravation of the disease. In stable patients, 135 patients (24.77%) used intranasal oxygen inhalation, and no patient used intravenous drug and invasive ventilator.
Clinical outcomes of the transferred patients were unknown due to transferred to another hospital and lost connection, according to the discharge standard, up to 10 March, 545 cases (100%) of stable patients were discharged from the Cabin hospital, and no patient died. The average hospital stay for discharge patients was 15 days.
4. DISCUSSION
This study was a single‐center retrospective cohort study that reported the clinical characteristics of patients diagnosed with COVID‐19 and potential risk factors for disease aggravation in Wuhan Stadium Cabin Hospital. With the epidemic development of CVIOD‐19 pneumonia, the number of patients diagnosed as COVID‐19 in Wuhan has increased sharply since January. To solve the problem of difficult hospitalization of the patients, By the end of February, the Chinese government totally opened 14 Cabin Hospitals to treat patients confirmed with mild or moderate of COVID‐19. With the effective intervention measures and isolation plans formulated by the government in an efficient manner, most patients received timely diagnosis and treatment. Among the patients admitted to Wuhan Stadium Cabin Hospital, except for 26 patients who were exacerbated and transferred to the designated special hospital for further treatment, the remaining 545 patients showed mild and common symptoms, and improved significantly after symptomatic treatment.
In our study, we identified several risk factors for disease aggravation in cabin hospital with COVID‐19. Through univariable analysis, odds of disease aggravation were higher with diabetes or coronary heart disease or hypertension. Former smoker history, lymphopenia, LDH, and CRP were also associated with disease aggravation. In the multivariable logistic regression model, we found that former smoker history, diabetes, dyspnea, consolidation and interstitial abnormalities of CT scanning, lymphopenia and elevated of CRP were associated with increased odds of disease aggravation.
Earlier reports on SARS‐CoV‐2 and COVID‐19 suggested that they were similar to SARS and Middle East respiratory syndrome (MERS) in the early years, with most patients presented with fever, dry cough, fatigue, and dyspnea, 5 , 13 , 14 our study also found that fever dry cough and dyspnea accounted for 15 (57.69%), 20 (76.90%), and 16 (61.54%) cases in transferred patients, and 284 (51.92%), 382 (70.09%), and 89 (16.27%) cases in stable patients. However, in this study, the typical symptoms of upper respiratory tract infection, such as runny nose, sneezing, and sore throat are not common, which are different from the previously reported SARS and MERS related onset symptoms. 13 , 14
Older age has been reported as an important independent predictor of mortality not only in SARS and MERS, 15 , 16 but also in COVID‐19, 17 however, in our study, age was not a risk factor for disease aggravation, this may be related to the majority of mild and moderate patients admitted to the cabin hospital.
Although smoking history was not an independent risk factor for disease exacerbations in previous studies, however, in our multivariate regression analysis, former smoker history was associated with disease aggravation, which may have an impact on breathing, and further research is needed to determine whether smoking is an independent risk factor for assessing of disease prognosis.
It is well known that diabetes is an independent risk factor for a variety of diseases, in previous studies, diabetes was considered as a risk factor for disease exacerbation of COVID‐19, 18 , 19 through our regression analysis, it was also found that patients with diabetes had higher rate of transferred, previous data suggested that ACE2 expression was increased in diabetes and treatment with ACE inhibitors and ARBs increased ACE2 expression, however, whether the specific mechanism of diabetes affecting prognosis of COVID‐19 through the expression of ACE2 still need further study.
Our study has some limitations. First, due to the retrospective study design, not all laboratory tests were done in all patients, which might be underestimated in predicting disease condition. Second, the results and prognosis of 26 patients were unknown due to transferred to a designated specialized hospital for further treatment, last but not least, interpretation of our findings might be limited by the sample size. However, by including the number of cases in cabin hospital, we believe our study population is representative disease condition of mild and moderate of COVID‐19.
In summary, we hope the potential risk factors of former smoker history, diabetes, dyspnea, consolidation and interstitial abnormalities of CT scanning, lymphopenia and elevated of CRP could help clinicians to identify patients with poor prognosis at an early stage, and provide early warning function for timely intervention.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
LS, NJ, and GF conceived and designed the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. XW, XC, and ML contributed to analysis the infection situation and write the report. LS, MW, KD, JW, XW, YC, and JY assisted in data collection, extraction and evaluation the eligibility of the original data. LS and ML analyzed the data. LS and GF interpreted the data and contributed to the writing of the final version of the manuscript. All authors agreed with the results and conclusions of this article.
ETHICS STATEMENT
This study was approved by the Institutional Review Board of Wuhan Stadium Cabin Hospital. All data were anonymized to comply with the provisions of personal data protection legislation. The written informed consent was waived because of the retrospective nature of the study and belongs to emergency medical service.
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors thank the authors of the primary studies for their timely and helpful responses to our information requests. This work was supported by grant of National Natural Science Foundation of China (81670013) and Key Research and Development Project of Jiangsu Province (BE2020616).
Shu L, Wang X, Li M, et al. Clinical characteristics of moderate COVID‐19 patients aggravation in Wuhan Stadium Cabin Hospital: A 571 cases of retrospective cohort study. J Med Virol. 2021;93:1133–1140. 10.1002/jmv.26414
Lei Shu, Xiaoyan Wang, Mingquan Li, and Xiaolin Chen contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020. [DOI] [PMC free article] [PubMed]
- 2. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63(3):457‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanne JP, Chest CT. Findings in 2019 novel coronavirus (2019‐nCoV) infections from Wuhan, China: key points for the radiologist. Radiology, 2020:200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Coronavirus disease (COVID‐19) outbreak. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020. [DOI] [PMC free article] [PubMed]
- 7. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92(4):418‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. https://www.who.int/internalpublications-detail/clinical-management-of-severe-acuterespiratoryinfection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed 11 January 2020.
- 11.National Health Commission of People's Republic of China. New coronavirus pneumonia diagnosis and treatment program (6th ed.) (in Chinese). http://www.nhc.gov.cn/xcs/zhengcwj/202002/3b09b894ac9b4204a79db5b8912d4440.shtml
- 12.World Health Organization. Coronavirus disease (COVID‐19) technical guidance: laboratory testing for 2019‐nCoV in humans. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance
- 13. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986‐1994. [DOI] [PubMed] [Google Scholar]
- 14. Assiri A, Al‐Tawfiq JA, Al‐Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi KW, Chau TN, Tsang O, et al. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med. 2003;139(9):715‐723. [DOI] [PubMed] [Google Scholar]
- 16. Hong KH, Choi JP, Hong SH, et al. Predictors of mortality in Middle East respiratory syndrome (MERS). Thorax. 2018;73(3):286‐289. [DOI] [PubMed] [Google Scholar]
- 17. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020. [DOI] [PMC free article] [PubMed]
- 18. Zhang J, Dong X, Cao Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730‐1741. [DOI] [PubMed] [Google Scholar]
- 19. Wang YC, Luo H, Liu S, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


