Abstract
Background
Since its emergence in December 2019, COVID‐19 has spread to over 210 countries, with an estimated mortality rate of 3–4%. Little is understood about its effects during pregnancy.
Aims
To describe the current understanding of COVID‐19 illness in pregnant women, to describe obstetric outcomes and to identify gaps in the existing knowledge.
Methods
Medline Ovid, EMBASE, World Health Organization COVID‐19 research database and Cochrane COVID‐19 in pregnancy spreadsheet were accessed on 18/4, 18/5 and 23/5 2020. Articles were screened via Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. The following were excluded: reviews, opinion pieces, guidelines, articles pertaining solely to other viruses, single case reports.
Results
Sixty articles were included in this review. Some pregnant participants may have been included in multiple publications, as admission dates overlap for reports from the same hospital. However, a total of 1287 confirmed SARS‐CoV‐2 positive pregnant cases are reported. Where universal testing was undertaken, asymptomatic infection occurred in 43.5–92% of cases. In the cohort studies, severe and critical COVID‐19 illness rates approximated those of the non‐pregnant population. Eight maternal deaths, six neonatal deaths, seven stillbirths and five miscarriages were reported. Nineteen neonates were SARS‐CoV‐2 positive, confirmed by reverse transcription polymerase chain reaction of nasopharyngeal swabs. [Correction added on 2 September 2020, after first online publication: the number of neonates indicated in the preceding sentence has been corrected from ‘Thirteen’ to ‘Nineteen’.]
Conclusions
Where universal screening was conducted, SARS‐CoV‐2 infection in pregnancy was often asymptomatic. Severe and critical disease rates approximate those in the general population. Vertical transmission is possible; however, it is unclear whether SARS‐CoV‐2 positive neonates were infected in utero, intrapartum or postpartum. Future work should assess risks of congenital syndromes and adverse perinatal outcomes where infection occurs in early and mid‐pregnancy.
Keywords: COVID‐19, SARS‐CoV‐2, novel coronavirus, pregnancy
Introduction
In December 2019, the novel coronavirus SARS‐CoV‐2 first appeared in Wuhan, China, with both the virulence and transmissibility to infect on pandemic proportions. By 1 June 2020, there were more than 6 million infections worldwide and over 371 100 deaths. 1 These figures are increasing daily and crude global mortality is estimated at 3–4% by the World Health Organization. 2 Coronavirus disease 2019 (COVID‐19) characteristically presents with fever, cough and fatigue. A severe case is defined by progression to pneumonia with hypoxia, occurring in approximately 14% of infections. 3 In 5% of cases, this progresses to critical illness, with acute respiratory distress syndrome, septic shock or other systemic complications, and usually requires mechanical ventilation. 3
The Australian Government introduced strict physical distancing measures in mid‐March; since then the rate of new infections has been steadily declining. As of 1 June 2020, there were 7204 recorded cases and 103 deaths in Australia 4 – a mortality rate of 1.4%. While almost all Australian deaths have occurred in people aged 60 or older, the highest total number of infections exists in the 20–29‐year‐old age group 4 which overlaps with women of reproductive age. Clinical presentation in pregnancy, maternal outcomes and neonatal outcomes of COVID‐19 infection are yet to be thoroughly understood. Furthermore, the potential for SARS‐CoV‐2 vertical transmission is currently uncertain.
Other viral illnesses in pregnancy have shown disproportionately high rates of adverse maternal and perinatal outcomes. In 2010 a US study revealed that 5% of all deaths to H1N1 influenza occurred in pregnant patients, while 23% of infected pregnant women required intensive care unit (ICU) admission. 5 Seasonal influenza also poses an increased risk of preterm birth 6 and hospital admission, 7 particularly if contracted during the third trimester of pregnancy. 8 Severe acquired respiratory syndrome (SARS) exhibited a mortality rate of around 25% in pregnant women, with up to 50% of infected pregnant women requiring ICU admission. 9 In pregnancies complicated by Middle‐East respiratory syndrome (MERS), fetal death in utero occurred at a rate of 30% while 33% of ongoing pregnancies were delivered preterm. 10 There is no evidence of in utero transmission from either SARS or MERS. 9 , 10
The aims of this review are to: (i) describe what is known about COVID‐19 clinical disease in pregnant women; (ii) discuss obstetric outcomes; (iii) describe the risk of vertical transmission; (iv) use this data to highlight management issues in the pregnant population; and (5) identify gaps in the existing knowledge.
Materials and methods
The EMBASE and Medline Ovid databases were searched on 18 April, 18 May and 23 May 2020 using the keywords and Boolean terms ‘coronavirus OR COVID‐19 OR COVID 19 OR SARS‐CoV‐2 OR n19‐CoV’ and subject headings ‘pregnancy outcome’ and ‘pregnancy complications’. The full search strategy for both databases is listed in Data S1. A broad search of the World Health Organization ‘Global literature on coronavirus disease’ database was also conducted using the keywords ‘pregnancy’ and ‘pregnant’. These platforms collectively returned 911 papers of interest. No further searches were conducted after 23 May 2020.
After removal of duplicates, 412 articles remained (Fig. 1). Papers were included if they specifically referred to pregnancy and COVID‐19. Exclusion criteria were as follows: review articles, opinion pieces or guidelines, articles pertaining solely to MERS‐CoV, SARS‐CoV or other viruses, non‐peer‐reviewed papers and case reports of a single patient. In addition to the database search described, the Cochrane Library 11 was examined for reports of COVID‐19 in pregnancy that had not been identified in the original search, revealing a further eight articles. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19
Figure 1.
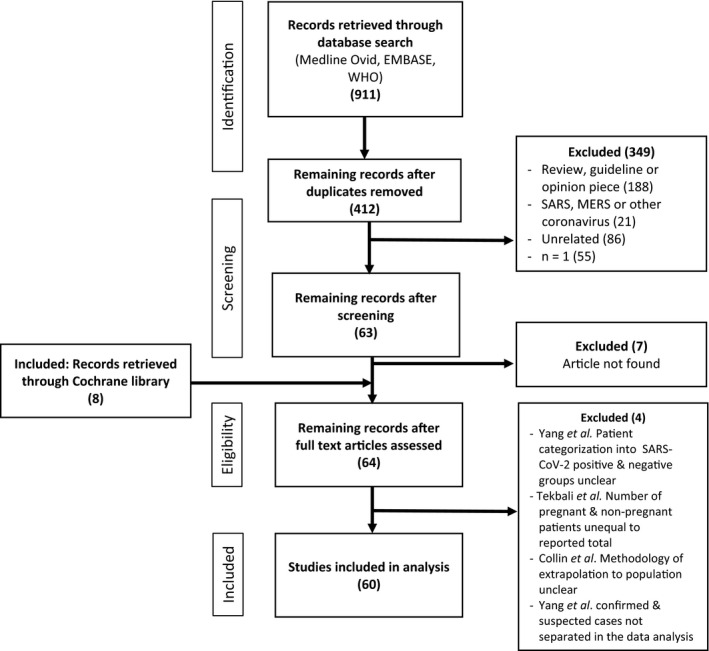
Research article identification and selection.
All selected publications were either case series or cohort studies. These cohorts included cases and either non‐infected pregnant controls or infected non‐pregnant controls.
Results
In total, 60 studies were identified. Details of included studies are listed in Table S1. The majority of papers arose from either Wuhan, China or the United States of America (US or USA), with five from Italy, three from the United Kingdom, one from Portugal and one from Iran also included. Of the Chinese studies, six were conducted at Renmin Hospital, Wuhan, 20 , 21 , 22 , 23 , 24 , 25 five at Tongji Hospital, Wuhan, 14 , 26 , 27 , 28 , 29 three at Union Hospital, Wuhan, 30 , 31 , 32 four at Maternal and Child Health Hospital, Wuhan 33 , 34 , 35 , 36 and four at Zhongnan Hospital, Wuhan. 13 , 37 , 38 , 39 Of the US studies, four were conducted at the New York Presbyterian Hospital System. 12 , 15 , 40 , 41 Admission dates overlap for participants reported in studies from the same hospital, hence it is unclear whether the same pregnant individuals have been included in multiple publications.
Overall, these 60 studies included a total of 3830 participants: 1287 SARS‐CoV‐2 positive pregnant women (confirmed by reverse transcription polymerase chain reaction (rtPCR)), 139 pregnant women who were assigned a clinical diagnosis of infection either based on computed tomography (CT) or symptomatology but rtPCR negative, 2004 negative pregnant controls and 400 SARS‐CoV‐2 rtPCR positive non‐pregnant controls. The number of participants in each study varied from two to 635. Details of these studies are listed in Table S1, including identification of patients who were confirmed by rtPCR to have SARS‐CoV‐2, as well as those suspected or negative. Unless otherwise specified, all results in this review pertain to rtPCR confirmed SARS‐CoV‐2 cases.
Clinical characteristics
The clinical symptoms among pregnant women with rtPCR confirmed SARS‐CoV‐2 infection are presented in Table 1. In symptomatic patients, fever was the most common sign, occurring in 10–100% of cases both at admission and postpartum. Only one study compared clinical symptoms between COVID‐19 positive pregnant and non‐pregnant groups: fever was less prevalent in pregnant than non‐pregnant patients (44% vs 100%, P < 0.05), while there was no significant difference in cough, dyspnoea or fatigue incidence. 34 Six studies universally tested all pregnant women at presentation with nasopharyngeal swab: Sutton et al. 12 , Gagliardi et al. 42 , Khalil et al. 43 , Vintzileos et al. 44 , Doria et al. 45 and Miller et al. 46 These papers reported asymptomatic infection in 43.5–92% of SARS‐CoV‐2 positive patients.
Table 1.
Maternal symptoms.
| Study† | No. rtPCR SARS‐CoV‐2 positive pregnant patients | Gestation at infection (weeks+days) | Asymptomatic (%) | Fever (%) | Cough (%) | Dyspnoea (%) | Chest tightness or pain (%) | Myalgia (%) | Headache (%) | Diarrhoea (%) | Fatigue (%) | Other – % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breslin et al. 15 | 7 | 26+0–37+5 | 28.5 | 28.5 | 43 | 28.5 | 43 | 28.5 | ||||
| Breslin et al. 40 | 43 | 32+4–38+6 | 33 | 48 | 65 | 24 | 17 | 38 | 28 | |||
| Sutton et al. 12 | 33 | 3rd trimester | 88 |
12 (admission) 10 (postpartum) |
||||||||
| Baergen et al. 41 | 20 | 32+2–40+4 | 80 | 10 | 10 | |||||||
| Chen et al. 22 | 17 |
n = 14 >37 n = 3 <37 |
24 | 24 | 6 | 6 | 6 | |||||
| Fan et al. 21 | 2 | 36+0 & 37+0 | 100 | Nasal congestion ‐ 100 | ||||||||
| Zhang et al. 20 | 16 | 35+5–41+0 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Khan et al. 23 | 17 | 35+0–41+0 | 18 | 35 | 12 | 18 | Nasal congestion ‐ 12 | |||||
| Khan et al. 24 | 3 |
n = 1 34+6 n = 1 39+1 n = 1 38+2 |
67 | 100 | 33 | |||||||
| Lei et al. 25 | 9 |
2nd tri: 56% 3rd tri: 44% |
100 | 78 | 44 | 33 | 44 | 33 | 56 | 44 | ||
| Chen et al. 27 | 5 | 38+0–41+0 | 100 (admission) | 100 (postpartum) | 40 | |||||||
| Yu et al. 26 | 7 | 37+0–40+2 | 86 | 14 | 14 | 14 | ||||||
| Zeng et al. 14 | 33 |
3 recorded: n = 1 40+0 n = 1 40+4 n = 1 31+2 |
24 (admission) 15 (postpartum) |
30 | ||||||||
| Liu et al. 28 | 10 | 35+2–41+2 | 70 | 40 | 10 | |||||||
| Yu et al. 29 | 2 | ‐ | 50 | 50 | 50 | 50 | ||||||
| Li et al. 33 | 16 | 33+6–40+0 |
25 (admission) 50 (postpartum) |
0 | 0 | |||||||
| Liu et al. 34 | 16 (confirmed) (Pregnant) | 22–40+5 |
100 non‐preg vs 44 preg (P < 0.05) 31 (postpartum) |
64 non‐preg 38 preg |
0 non‐preg 13 preg |
14 non‐preg 19 preg |
||||||
| Cao et al. 35 | 10 | 33+6–40+5 |
20 (admission) 50 (postpartum) |
10 | 10 | 10 | ||||||
| Wu et al. 36 | 6 | 36+4–40+3 | 100 (admission) | 33 (postpartum) | ||||||||
| Liu et al. 30 | 15 | 12+0–38+0 | 87 | 60 | 20 | 27 | ||||||
| Chen et al. 31 | 3 | 3rd trimester | 100 | |||||||||
| Xu et al. 32 | 5 | 34+4–38+6 | 100 | |||||||||
| Zeng et al. 13 | 6 | 3rd trimester | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Chen et al. 37 | 9 | 36+0–39+4 | 78 | 44 | 33 |
Sore throat ‐ 22 Malaise ‐ 22 |
||||||
| Yang et al. 38 | 7 | 36+0–38+2 | 71 | 14 | 14 | Abdominal pain ‐ 14 | ||||||
| Liao et al. 39 | 10 | 36+2–40+4 | 50 | 30 | 10 | |||||||
| Zhu et al. 61 | 9 | 31+0–39+0 | 78 | 40 | ||||||||
| Chen et al. 81 | 84 |
1st tri: 18.6% 2nd tri: 17.8% 3rd tri: 63.6% |
5 | 78 | 73 | 10 | 24 | 7.7 | 6.4 | 9 | 22 |
Nausea ‐ 5 Sore throat ‐ 2.6 Nasal congestion ‐ 2.6 |
| Yan et al. 52 | 65 | 5+0–41+2 | 9 | 69 | 43 | 17 | 8 | 1.5 | 20 | Sore throat ‐ 15 | ||
| Huang et al. 18 | 8 | 30+0–39+3 | 62.5 | 87.5 | 37.5 | 37.5 | 37.5 |
Sputum ‐ 25 Rhinorrhoea – 37.5 |
||||
| Liu et al. 51 | 13 |
n = 2 <28+0 n = 11 >28+0 |
77 | 23 | ||||||||
| Chen et al. 82 | 4 | 37+2–39+0 | 75 | 50 | 50 | 50 | ||||||
| Wu et al. 83 | 23 | 8+0–40+0 | 65 | 17 | 26 | Nasal congestion ‐ 4 | ||||||
| Juusela et al. 49 | 7 (only 2 described in detail) | 33+6 & 39+2 | 100 |
Tachycardia 100 Tachypnoea 100 |
||||||||
| Li et al. 84 | 4 (originally positive, negative at time of writing) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| CDC 85 | 143 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Blitz et al. 86 | 82 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Buonsenso et al. 54 | 7 (only 2 described in detail) | 34+4 & 37+3 | 50 | 100 | 50 | |||||||
| Chen et al. 87 | 3 |
n = 1 6 n = 1 25 n = 1 35 |
100 | 33 | 33 | 33 | ||||||
| Ferrazzi et al. 59 | 42 |
n = 30 >37 n = 7 34–37 n = 4 ≤34 |
48 (admission) 14 (postpartum) |
43 | 19 | 17 | 5 | |||||
| Gagliardi et al. 42 | 3 | Women admitted for delivery | 67 | Anosmia – 33 | ||||||||
| Govind et al. 56 | 9 | 27+0–39+0 | 44 | 89 | 44 | 56 | 67 |
Sore throat – 44 Anosmia – 78 |
||||
| Hantoushzadeh et al. 48 | 9 | 24+0–38+3 | 100 | 100 | 67 | 44 | ||||||
| Hirshberg et al. 53 | 5 | 25+2 –31+2 | 100 | 80 | 80 | 20 | 20 | |||||
| Khalil et al. 43 | 9 | ‐ | 89 | 11 | 11 | |||||||
| Penfield et al. 57 | 32 | 26+5–41+0 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Pierce‐Williams et al. 47 | 64 | 29 ± 6 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Qiancheng et al. 88 | 28 | Median 38+0 | 18 | 25 | 7 | 3.6 | Abdominal pain – 18 | |||||
| Vintzileos et al. 44 | 32 | ‐ | 66 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| An et al. 16 | 3 |
n = 1 38+2 n = 1 39+4 n = 1 38+5 |
100 | 67 | 67 | 33 | 33 | 33 | ||||
| Hu et al. 17 | 7 | 37+2–41+2 | ‐ | 57 | 14 | ‐ | ‐ | ‐ | ‐ | |||
| Sun et al. 19 | 3 |
n = 1 30 + 5 n = 1 36 n = 1 37 |
100 | 100 | 33 | |||||||
| Cooke et al. 58 | 2 | 28+4 & 28+6 | 100 | 100 | 50 | 50 | Vomiting – 50 | |||||
| Doria et al. 45 | 12 | 37+0–41+0 | 92 | |||||||||
| Lokken et al. 60 | 46 |
n = 23 3rd tri n = 20 2nd tri n = 3 1st tri |
51 | 70 | 44 | 30 | 33 | 7 | 28 |
Anosmia – 30 Sore throat – 28 Nasal congestion – 49 |
||
| Savasi et al. 50 | 77 |
n = 50 3rd tri n = 13 2nd tri n = 4 1st tri |
16 | 54 | 66 | 25 | ||||||
| London et al. 62 | 68 |
n = 65 3rd tri n = 1 25+0 n = 1 26+0 n = 1 17+0 |
32.4 | 26 | 43 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Miller et al. 46 | 23 | ‐ | 43.5 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Qadri & Mariona 63 | 16 | 22+0–40+3 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Patane et al. 55 | 2 described in detail |
n = 1 37+6 n = 1 35+1 |
100 | 100 |
Unless stated otherwise, all symptoms occurred at presentation. While some studies described suspected positive patients based on chest computed tomography or clinical symptoms, only reverse transcription polymerase chain reaction (rtPCR) positive patients have been included in this table unless otherwise specified. If a parameter was not discussed, this is indicated with a hyphen (‐).
Settings have been highlighted to show potential overlap in case ascertainment 
Maternal and neonatal outcomes
Maternal, obstetric and neonatal outcomes of rtPCR confirmed SARS‐CoV‐2 positive pregnant patients are listed in Table 2. Of the 13 cohort studies that reported on disease severity, severe COVID‐19 illness (pneumonia requiring oxygen support or non‐invasive ventilation) occurred in 0–18% of patients. Critical disease (defined as progression to acute respiratory distress syndrome, sepsis or acute organ dysfunction) was reported in 0–5% of cases. The exception to this was Pierce‐Williams et al. 47 where severe and critical COVID‐19 were, respectively, described in 69% and 31% of cases. A total of 111 severe and 40 critical cases were reported across the 13 cohort studies.
Table 2.
Pregnancy and neonatal outcomes.
| Study† | No. rtPCR SARS‐CoV‐2 positive pregnant patients‡ | No. participants diagnosed at <37 weeks gestation | Adverse maternal outcomes (of infection)§ | Adverse pregnancy outcomes | Neonatal outcomes (% of live deliveries) | Delivery method (% of live deliveries) | Breastfeeding |
|---|---|---|---|---|---|---|---|
| Breslin et al. 15 |
7 D – 2 |
n = 5 (71%) | Critical disease (n = 2, 28.6%) | ‐ | ‐ | CS (100%) – OI | ‐ |
| Breslin et al. 40 |
43 D – 18 |
‐ |
Severe disease (n = 3, 15%) Critical disease (n = 2, 4.7%) ICU admission for respiratory failure, septic shock, multiorgan failure (1 discharged & 1 still admitted at time of publication) |
Preterm birth (n = 1, 5.6%) due to preterm labour |
NICU admissions (n = 3, 15%)
|
CS (44%) – OI VD (55%) |
Yes |
| Sutton et al. 12 | 33 Delivery not discussed | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Baergen et al. 41 |
20 D – 21 (1 × twins) |
n = 4 (20%) | Severe disease (n = 1, 5%) |
|
VD (75%) CS (25%) – OI |
‐ | |
| Chen et al. 22 | 17 | n = 3 (18%) | ‐ | Preterm birth (18%) reason unclear | ‐ |
Elective CS (82%) Emergency CS (18%) OI |
‐ |
| Fan et al. 21 | 2 | n = 1 (50%) | ‐ | Preterm birth (n = 1, 50%) due to persistent maternal fever 38.5°C |
Fever, abdominal distension day 4 (n = 1, 50%) Pneumonia (n = 1, 50%) |
CS (100%) – C19 | Not permitted |
| Zhang et al. 20 |
16 D – 10 |
‐ | ‐ | No difference between pregnant & non‐pregnant group in PROM, meconium stained liquor, GDM, fetal distress, preterm birth | Pneumonia (n = 3) | CS (100%) – C19 | ‐ |
| Khan et al. 23 | 17 | ‐ | ‐ | Preterm birth (n = 3, 18%) reason unclear |
n = 2 SARS‐CoV‐2 positive neonates (at birth) Pneumonia (n = 5, 29%)
|
CS (100%) – C19 | ‐ |
| Khan et al. 24 | 3 | n = 1 (33%) | ‐ | Preterm birth (n = 1, 33%) reason unclear | ‐ | VD (100%) | ‐ |
| Lei et al. 25 |
9 D – 4 |
‐ |
Severe disease (n = 1, 11%) Critical disease (n = 1, 11%) |
|
‐ |
CS (75%) reason unclear VD (25%) |
‐ |
| Chen et al. 27 | 5 | ‐ | ‐ | Fetal distress (n = 1, 20%) | ‐ |
|
Not permitted |
| Yu et al. 26 | 7 | ‐ | ‐ | ‐ |
n = 1 SARS‐CoV‐2 positive neonate (36 h postpartum) mild pneumonia |
CS (100%) C19 | ‐ |
| Zeng et al. 14 | 33 | n = 1 (3%) | ‐ |
|
n = 3 SARS‐CoV‐2 positive neonates (2 days postpartum)
|
CS (79%) reason unclear VD (21%) |
‐ |
| Liu et al. 28 | 10 (confirmed) | ‐ | ‐ |
CS (95%) reason unclear VD (5%) |
‐ | ||
| Yu et al. 29 | 2 | n = 2 (100%) | ‐ | ‐ | ‐ | ‐ | ‐ |
| Li et al. 33 |
16 (confirmed) |
‐ | ‐ |
|
No significant differences in Apgar scores or gestational age at birth between confirmed cases & control (P> 0.05) |
CS (87.5%) – C19 VD (12.5%) |
‐ |
| Liu et al. 34 | 16 (confirmed) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Cao et al. 35 |
10 D – 11 (1 x twins) |
n = 3 (30%) |
Preterm birth (n = 3, 30%) for OI Fetal distress (n = 2, 20%) |
‐ |
VD (20%) Emergency CS (20%) OI – fetal distress Elective CS (60%) |
‐ | |
| Wu et al. 36 | 6 (confirmed) | n = 1 (17%) |
|
‐ |
CS (83%) – OI VD (17%) |
||
| Liu et al. 30 |
15 D – 11 |
‐ | ‐ | ‐ | ‐ |
CS (90%) C19 VD (10%) |
‐ |
| Chen et al. 31 | 3 | ‐ | ‐ | Preterm birth (n = 1, 33%) due to fetal distress | NICU admission of preterm neonate (n = 1, 33%) | CS (100%) reason unclear | ‐ |
| Xu et al. 32 | 5 | n = 2 (40%) | Preterm birth (n = 2, 40%) due to worsening C19 | ‐ |
VD (20%) CS (80%) n = 3 C19, n = 1 OI |
Not permitted | |
| Zeng et al. 13 | 6 | ‐ | ‐ | ‐ | ‐ | CS (100%) C19 | ‐ |
| Chen et al. 37 | 9 |
n = 4 (44%) |
‐ |
|
CS (100%) n = 1 C19, n = 8 OI (PROM, pre‐eclampsia, Hx CS) | ‐ | |
| Yang et al. 38 | 7 | n = 4 (57%) | ‐ | Preterm birth (n = 4, 57%) reason unclear |
Mild RDS in preterm neonates (n = 2, 28.5%) |
Elective CS (71.4%) C19 Emergency CS (28.6%) – OI pre‐eclampsia |
‐ |
| Liao et al. 39 | 10 | n = 1 (10%) | ‐ | No significant difference in PROM or preterm birth groups (10% SARS‐CoV‐2 positive vs 9.4% negative group, P> 0.05) | ‐ | VD (100%) | Not permitted for 14 days postpartum |
| Zhu et al. 61 |
9 D – 10 (1 x twins) |
‐ | ‐ |
|
Dyspnoea (n = 6, 60%) n = 1 neonatal death Postnatal day 9 due to multiorgan failure and DIC |
CS (80%) reason unclear VD (20%) |
‐ |
| Chen et al. 81 | 84 (confirmed) | ‐ |
Severe disease (n = 9, 8%) Critical disease (n = 1, 0.8%) |
|
‐ |
VD (7%) CS (93%) n = 38 C19, n = 24 OI |
‐ |
| Yan et al. 52 |
65 (confirmed) D – 50 |
n = 34 (52%) |
Severe disease (n = 6, 9.2%) Critical disease (n = 2, 3%) |
|
Severe neonatal asphyxia & neonatal death (n = 1, 2%) NICU admission (n = 17, 34%) |
CS (88%) n = 19 C19, n = 25 OI VD (12%) |
‐ |
| Huang et al. 18 |
8 D – 7 (1 x twins) |
n = 5 (62.5%) |
Severe disease (n = 2, 25%) Critical disease (n = 1, 12.5%) |
|
Severe neonatal asphyxia (n = 4, 56%) Neonatal death (n = 2, 28.6%): 1 at 18 days postpartum due to severe pneumonia, 1 at birth (reason unclear) NICU admission of preterm neonates (n = 3, 43%) |
Elective CS (33%) Emergency CS (50%) OI VD (17%) |
‐ |
| Liu et al. 51 |
13 D – 10 |
‐ | Critical disease (n = 1, 8%) |
|
‐ |
Elective CS (50%) Emergency CS (50%) OI |
‐ |
| Chen et al. 82 | 4 | ‐ | Severe disease (n = 1, 25%) | Decreased fetal movements (25%) | TTN (n = 1, 25%) |
CS (75%) – C19 VD (25%) |
Not permitted |
| Wu et al. 83 |
23 D – 21 (1 x twins) T – 3 |
n = 6 (26%) | ‐ | ‐ | ‐ |
CS (90%) reason unclear VD (10%) |
‐ |
| Juusela et al. 49 | 2 described in detail | n = 1 (50%) |
Cardiac arrest (n = 1, 50%) SVT (n = 1, 50%) |
|
‐ | CS (100%) – C19 | ‐ |
| Li et al. 84 | 4 (originally positive, negative at time of writing) | ‐ | Resolution of symptoms and discharged home, 100% still pregnant | ‐ | ‐ | ‐ | ‐ |
| CDC 85 | 143 | ‐ |
31 (22%) general admission 4 (3%) ICU Outcomes unclear |
‐ | ‐ | ‐ | ‐ |
| Blitz et al. 86 | 82 | ‐ | Severe disease (n = 8, 10%) | ‐ | ‐ | ‐ | ‐ |
| Buonsenso et al. 54 |
7 D – 2 |
n = 6 (86%) |
Spontaneous abortion at 8+0 (n = 1, 14%) Preterm birth (n = 1, 14%) reason unclear |
Elective CS (50%) Emergency CS (50%) OI |
Formula in hospital, breastfeeding with mask worn at home | ||
| Chen et al. 87 |
3 D – 1 T – 1 |
n = 2 (67%) | Fetal distress (n = 1, 33%) |
Emergency CS (100%) OI – fetal distress |
Formula in hospital, breastfeeding at home | ||
| Ferrazzi et al. 59 | 42 | n = 11 (26%) |
Severe disease (n = 7, 16%) ‐ n = 4 (9.5%) ICU admission, details unclear |
Preterm birth (n = 11, 26%) due to worsening maternal C19 symptoms (n = 2), spontaneous labour (n = 9) |
n = 3 SARS‐CoV‐2 positive neonates (Day 1 postpartum) Apgar < 7 @ 5 min (n = 2, 5%) Preterm neonates (<34 weeks) NICU admission (n = 3, 7%) n = 1 SARS‐CoV‐2 positive |
Elective CS (43%) n = 10 C19, n = 8 OI VD (57%) |
Yes |
| Gagliardi et al. 42 | 3 | ‐ | ‐ | ‐ | |||
| Govind et al. 56 | 9 | n = 2 (22%) | Critical disease (n = 1, 11%) | Preterm birth (n = 2, 22%) due to worsening C19 |
n = 1 SARS‐CoV‐2 positive neonate
|
Emergency CS (33%) n = 2 C19, n = 1 OI Elective CS (67%) – OI VD (11%) |
Not permitted |
| Hantoushzadeh et al. 48 |
9 D – 6 (1 x twins) FDIU – 5 (1 x twins) |
n = 8, (89%) |
100% critical disease n = 7 maternal death (78%) n = 1 critical at time of writing (11%) n = 1 recovered (11%) |
FDIU (n = 5) n = 2 mothers mechanically ventilated patient in spont. labour, 3 fetuses n = 1 acute hypoxaemia, 1 fetus n = 1 PPROM, 1 fetus Preterm birth (n = 3) due to severe C19 n = 1 28+0 (twins) n = 1 30+5 n = 1 33+6 |
Neonatal death (n = 2, twins) Postnatal day 3 due to complications of prematurity |
CS (100% livebirths) C19 VD n = 1 FDIU CS n = 1 FDIU n = 3 remained in utero at time of writing |
‐ |
| Hirshberg et al. 53 |
5 D – 3 |
n = 5 (100%) |
100% critical disease n = 1 critical at time of writing n = 4 recovered |
Preterm birth (n = 3, 60%) due to worsening C19 |
Apgar 2 & 4 @ 1 & 5 mins (n = 1, 33%) Preterm 31+3 |
Emergency CS (100%) C19 | ‐ |
| Khalil et al. 43 | 9 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Penfield et al. 57 | 11 described in detail | n = 2 (18%) |
Severe disease (n = 2, 18%) Critical disease (n = 3, 27%) |
Preterm birth (n = 2, 18%) due to worsening C19 | ‐ |
CS (36%) reason unclear VD (64%) |
‐ |
| Pierce‐Williams et al. 47 |
64 D – 33 (1 x twins) |
‐ |
Severe disease (n = 44, 69%) Critical disease (n = 20, 31%) n = 1 cardiac arrest |
Preterm birth (n = 29, 88%). due to critical C19 (n = 15), unclear (n = 14) |
n = 1 SARS‐CoV‐2 positive neonate (day 2 postpartum) NICU admission (n = 21, 64%) reasons unclear |
CS (75%) – C19 VD (25%) |
‐ |
| Qiancheng et al. 88 |
28 D – 23 (1 x twins) T – 4 |
‐ | No significant difference in disease severity pregnant vs non‐pregnant patients (7% pregnant vs 2% non‐pregnant, P> 0.05) | Preterm birth (n = 1, 4.3%) due to spont. labour |
CS (77%) reason unclear VD (23%) |
‐ | |
| Vintzileos et al. 44 |
32 D – 29 |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| An et al. 16 | 3 | Severe disease (n = 3, 100%) | ‐ | ‐ |
CS (100%) n = 1 OI, n = 2 unclear |
‐ | |
| Hu et al. 17 | 7 | PROM (n = 1, 14%) | n = 1 SARS‐CoV‐2 positive neonate (day 1 postpartum) |
Elective CS (86%) C19 VD (14%) spont. labour |
Not permitted | ||
| Sun et al. 19 | 3 | n = 2 (67%) | Critical disease & maternal death (n = 1, 33%) | Preterm birth (n = 2, 67%) reason unclear |
n = 1 SARS‐CoV‐2 positive neonate (day 6 postpartum) NICU admission of preterm neonates (n = 2, 67%) |
Emergency CS (100%) reason unclear | ‐ |
| Cooke et al. 58 | 2 | n = 2 (100%) | Critical disease (n = 2, 100%) | Preterm birth at 28+5 & 29+1 (n = 2, 100%) due to C19 |
n = 1 spontaneous bowel perforation day 1 postpartum – resection & good recovery n = 1 Apgar 1 & 3 @ 1 & 5 min, good recovery |
CS (100%) C19 | ‐ |
| Doria et al. 45 |
12 D – 11 (1 x twins) |
n = 2 (17%) |
VD (40%) CS (60%) reason unclear |
‐ | |||
| Lokken et al. 60 |
46 D – 7 FDIU – 1 |
unclear | Severe disease (n = 6, 13%) |
Preterm birth (n = 1, 12.5%) due to C19 FDIU at 38+5 (n = 1, 12.5%) reason unclear |
VD (62.5%) CS (37.5%) n = 1 OI, n = 2 C19 |
‐ | |
| Savasi et al. 50 |
77 D – 57 |
Unclear |
Severe disease (n = 14, 18%) Critical disease (n = 4, 5%) n = 3 mechanical ventilation, n = 1 ECMO |
Preterm birth (n = 12, 21%) due to C19 (n = 11), spont. labour (n = 1) |
n = 4 SARS‐CoV‐2 positive neonates n = 3 day 1 postpartum n = 1 day 7 postpartum NICU admission (n = 9, 16%) reason unclear |
VD (61%) CS (39%) |
Yes |
| London et al. 62 |
68 D – 55 M ‐ 1 |
Unclear |
Severe disease symptomatic vs asymptomatic at presentation (26.1% symptomatic vs 0% asymptomatic, P < 0.05) Severe disease (n = 11, 18%) Critical disease (n = 1, 1.5%) |
Preterm birth significantly associated with symptomatic presentation (27.3% symptomatic vs 0% asymptomatic, P < 0.05) Preterm due to C19 (n = 8), decreased fetal movements (n = 1) Miscarriage at 17+0 (n = 1, 1.5%) reason unclear |
‐ |
VD (60%) CS (40%) reason unclear |
‐ |
| Miller et al. 46 | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Qadri & Mariona 63 | 16 | Unclear | Severe disease (n = 2, 16.5%) | Preterm birth (n = 1, 6%) due to cervical incompetence | Outcome of preterm neonate unclear |
VD (66%) CS (44%) – OI |
Yes with facemask & hand washing |
| Patane et al. 55 | 2 described in detail | n = 1, 50% | Preterm birth (n = 1, 50%) due to non‐reassuring fetal status |
n = 2 SARS‐CoV‐2 positive neonates n = 1 positive at birth n = 1 day 7 postpartum NICU admission of preterm neonate (n = 1, 50%) |
VD (50%) CS (50%) – OI |
Yes with facemask & hand washing |
Abbreviations: ARDS, acute respiratory distress syndrome; C19, for COVID‐19 symptoms; CS, caesarean section; ECMO, extracorporeal membrane oxygenation; FDIU, fetal death in utero; GGO, ground glass opacity; NNP swab, neonatal nasopharyngeal swab; OI, obstetric indication; PROM, premature rupture of membranes; RDS, respiratory distress syndrome; SOB, shortness of breath; SVT, supraventricular tachycardia; TTN, transient tachypnoea of the newborn; VD, vaginal delivery.

D, live deliveries (where # is unequal to # pregnant patients); T, terminations; M, miscarriage. Unless otherwise specified: All neonates are born to SARS‐CoV‐2 positive mothers confirmed by rtPCR; % maternal outcomes based on # positive pregnant women, # neonatal outcomes based on # live deliveries. If a parameter was not discussed, this is indicated with a hyphen (‐). If the outcome was not identified, the cell is blank.
WHO guidelines for categorisation of COVID‐19 disease severity. Severe disease: fever or suspected respiratory infection, plus one of the following: respiratory rate> 30 breaths/min; severe respiratory distress; or SpO2 ≤ 93% on room air. Critical disease: patients with acute respiratory distress syndrome (ARDS), sepsis or acute organ dysfunction, usually requires mechanical ventilation
Extrapulmonary complications occurred in some severe and critical patients, including cardiac impairment, renal failure and coagulopathy. 18 , 40 , 47 , 48 , 49 , 50 , 51 Cardiomyopathy with global hypokinesis and moderately reduced ejection fraction on echocardiogram was reported in two patients. 49 Both women underwent emergency caesarean section delivery, and each experienced postpartum complications of cardiac arrest and supraventricular tachycardia, respectively. Another woman also experienced cardiac arrest; this followed extubation after one week of mechanical ventilation. 47 At the time of publication, both women with cardiac arrest remained ventilated in ICU, while the woman with supraventricular tachycardia was still in hospital but stable. There were eight maternal deaths reported secondary to critical COVID‐19. 19 , 48 Seven of the maternal deaths occurred in a single country (Iran) during the surge in cases in March 2020; of note three of the seven deaths involved twin pregnancies. 48 The other critical cases were not revisited in further publications, so the ultimate outcomes of these patients are unclear.
Of the 22 studies that identified and reported outcomes for women diagnosed with COVID‐19 at less than 37 weeks gestation, preterm birth was reported in 10% to 100% of cases (Table 2). With the exception of Yan et al. 52 , Hirshberg et al. 53 and Buonsenso et al., 54 all SARS‐CoV‐2 positive women in these studies who were diagnosed at less than 37 weeks were also delivered preterm. Preterm delivery occurred due to: an obstetric indication (preterm labour, preterm prelabour rupture of membranes, fetal distress), 36 , 37 , 54 , 55 worsening COVID‐19, 14 , 21 , 32 , 48 , 49 , 53 , 56 , 57 , 58 for no reported indication 19 , 22 , 24 , 38 , 39 , 52 , 59 or a combination of these causes. 18 , 41 Three studies compared the rates of preterm birth between rtPCR confirmed SARS‐CoV‐2 positive and negative pregnant women. Both Zhang et al. 20 and Liao et al. 39 identified no significant difference between groups (Zhang et al. 20 6% SARS‐CoV‐2 positive vs 13% control; Liao et al. 39 10% SARS‐CoV‐2 positive vs 9.4% control, P > 0.05). Conversely, Li et al. 33 noted an increased incidence of preterm birth in the SARS‐CoV‐2 positive group (23.5% vs 5% control, P < 0.05). Caesarean section was performed for over 40% of deliveries in all but five studies. 24 , 39 , 50 , 57 , 60
Nineteen neonates were SARS‐CoV‐2 positive, based on rtPCR of nasopharyngeal swabs. Three of these were febrile, 15 were asymptomatic and one was born at 31+2 weeks; he developed disseminated intravascular coagulopathy (DIC) but was recovering at the time of publication. There were no reported deaths of SARS‐CoV‐2 positive neonates. Six neonates had Apgar scores of less than seven at either one or five minutes postpartum. Five of those were preterm while one was term and SARS‐CoV‐2 positive. Six deaths of SARS‐CoV‐2 negative neonates were reported, due to: multiple organ failure and DIC (n = 1), 61 preterm birth (n = 2), 48 severe neonatal asphyxia (n = 1), 52 pneumonia (visualised on chest X‐ray) (n = 1) 18 and for unknown reasons (n = 1). 18 There were seven reported stillbirths: two for unclear reasons, 51 , 60 three following spontaneous labour in mechanically ventilated patients, 48 one due to acute maternal hypoxaemia necessitating delivery 48 and one due to preterm rupture of membranes. 48 There were five spontaneous miscarriages. Two occurred at 8+0 weeks 54 and 17+0 weeks, 62 while gestation was not reported for the other three. 48
The possibility of vertical transmission was assessed with rtPCR of neonatal nasopharyngeal secretions, placenta, cord blood, amniotic fluid and breastmilk (Table 3). Nineteen of a total 655 neonatal nasopharyngeal swabs were SARS‐CoV‐2 positive by rtPCR across ten studies. Four placenta samples and one cord blood sample were positive; however, neonatal nasopharygneal swabs in these cases were negative. 54 , 57 Anti‐SARS‐CoV‐2 immunoglobulin M (IgM) and IgG antibodies were elevated in the serum of three neonates. 13
Table 3.
Perinatal testing for maternal to fetal transmission
| Study† | No. neonates | Neonatal nasopharyngeal swab rtPCR | Cord blood rtPCR | Amniotic fluid rtPCR | Placenta rtPCR | Breast milk rtPCR | Neonatal serum‡ | Other |
|---|---|---|---|---|---|---|---|---|
| Breslin et al. 15 | 2 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Breslin et al. 40 | 18 |
Negative (n = 17, 94%) Indeterminate (n = 1, 6%) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Sutton et al. 12 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Baergen et al. 41 | 21 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Chen et al. 22 | 17 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Fan et al. 21 | 2 | Negative | Negative | Negative | Negative | Negative | ‐ | ‐ |
| Zhang et al. 20 | 10 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Khan et al. 23 | 17 |
Positive (n = 2, 12%) |
Negative | ‐ | ‐ | ‐ | ‐ | ‐ |
| Khan et al. 24 | 3 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Lei et al. 25 | 4 | Negative | Negative | Negative | ‐ | Negative | ‐ | Vaginal secretions: negative |
| Chen et al. 27 | 5 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Yu et al. 26 | 7 |
Positive (n = 1, 14%) |
Negative | ‐ | Negative | ‐ | ‐ | ‐ |
| Zeng et al. 14 | 33 |
Positive (n = 3, 9%) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Liu et al. 28 | 10 | Negative | Negative | Negative | ‐ | Negative | ‐ | Neonatal gastric fluid, urine & faeces: negative |
| Yu et al. 29 | ‐ |
Negative (n = 2, 100%) – sampled via amniocentesis |
||||||
| Li et al. 33 | 16 (confirmed) | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Liu et al. 34 | 6 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Cao et al. 35 | 11 (only 4 neonates tested) | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Wu et al. 36 | 6 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Liu et al. 30 | 11 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Chen et al. 31 | 3 | Negative | ‐ | ‐ | Negative | ‐ | ‐ | ‐ |
| Xu et al. 32 | 5 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Zeng et al. 13 | 6 | Negative | ‐ | ‐ | ‐ | ‐ | Elevated IgG & IgM (n = 2, 33%) | ‐ |
| Chen et al. 37 | 9 | Negative | Negative | Negative | ‐ | Negative | ‐ | ‐ |
| Yang et al. 38 | 7 | Negative | Negative | Negative | ‐ | ‐ | ‐ | ‐ |
| Liao et al. 39 | 10 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Zhu et al. 61 | 10 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Chen et al. 81 |
8 neonates 3 mothers (breastmilk) |
Negative | ‐ | ‐ | ‐ | Negative | ‐ | ‐ |
| Yan et al. 52 | 86 – unclear if from confirmed or suspected SARS‐CoV‐2 + patients | Negative (n = 86) |
Negative (n = 10) |
Negative (n = 10) |
‐ |
Negative (n = 12) |
‐ | Vaginal secretions – negative (n = 6) |
| Huang et al. 18 |
7 (1 x twins) |
Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Liu et al. 51 | 10 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Chen et al. 82 | 4 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Wu et al. 83 | 20 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Juusela et al. 49 | 2 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Li et al. 84 | 4 (originally positive, negative at time of writing) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| CDC 85 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Blitz et al. 86 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Buonsenso et al. 54 | 2 | Negative |
Positive (n = 1, 50%) |
Negative |
Positive (n = 1, 50%) |
Negative |
SARS‐CoV‐2 IgG ‘slightly positive’ (n = 1, 50%) |
|
| Chen et al. 87 | 3 | Negative | Negative | Negative | Negative | Negative | ‐ | ‐ |
| Ferrazzi et al. 59 | 42 |
Positive (n = 3, 7%) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Gagliardi et al. 42 | 3 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Govind et al. 56 | 9 |
Positive (n = 1, 11%) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Hantoushzadeh et al. 48 |
6 (1 x twins) |
Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Hirshberg et al. 53 | 3 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Khalil et al. 43 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Penfield et al. 57 | 11 described in detail |
Positive (n = 3, 27%) |
||||||
| Pierce‐Williams et al. 47 | 33 |
Positive (n = 1, 3%) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Qiancheng et al. 88 | 23 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Vintzileos et al. 44 | 29 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| An et al. 16 | 3 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Hu et al. 17 | 7 |
Positive (n = 1, 14%) |
‐ | Negative | ‐ | ‐ | Negative | Urine & faeces – negative |
| Sun et al. 19 | 3 |
Positive (n = 1, 33%) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Cooke et al. 58 | 2 | Negative | ||||||
| Doria et al. 45 |
11 (1 x twins) |
Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Lokken et al. 60 | 7 ‐ only 1 tested | ‐ | ‐ | ‐ | Negative (n = 1) Post mortem of FDIU | ‐ | ‐ | ‐ |
| Savasi et al. 50 | 57 |
Positive (n = 4, 7%) |
‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| London et al. 62 | 55 | Negative | ||||||
| Miller et al. 46 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Qadri & Mariona 63 | 16 | Negative | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Patane et al. 55 | 2 described in detail | Positive (n = 2) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
All neonates are born to SARS‐CoV‐2 positive mothers confirmed by reverse transcription polymerase chain reaction (rtPCR). Mothers with suspected SARS‐CoV‐2 and their neonates have not been included. If a parameter was not tested, this is indicated with a hyphen (‐).
 .
.
All samples tested by rtPCR unless otherwise specified.FDIU, fetal death in utero
SARS‐CoV‐2 was not identified in any of the 45 breastmilk samples tested. Breastfeeding with facemask and hand washing before neonatal contact was permitted in four studies. No neonate tested positive for SARS‐CoV‐2 by nasopharyngeal swab rtPCR in Breslin et al. 40 and Qadri and Mariona, 63 while one neonate in Patane et al. 55 was SARS‐CoV‐2 positive at birth and hence permitted to breastfeed. One neonate in Savasi et al. 50 tested positive at day seven after breastfeeding and rooming in. Breastfeeding was not permitted in seven studies, while no mention of feeding method was made in the remaining papers.
Discussion
Our comprehensive review of published case series and cohort studies describing SARS‐CoV‐2 infection in pregnancy has demonstrated a wide variation in symptoms and presentation. While severe illness and adverse maternal outcomes have been described, they appear to approximate rates seen in the non‐pregnant population. Prevalence of preterm birth was elevated in numerous studies; however, those with the highest rates tended to have the smallest participant numbers. Vertical transmission appears possible; however, the occurrence of in utero transmission remains unclear.
In the six studies to universally test all pregnant women for SARS‐CoV‐2 infection regardless of symptoms, rates of asymptomatic infection were high (43.5–92%). Two earlier large systematic reviews of non‐pregnant individuals identified fever in 87.3–88.7% and cough in 57.6–58.1% of SARS‐CoV‐2 rtPCR positive patients, indicating that symptomatic presentation is highly typical in the wider population. 64 , 65 Pregnant women require frequent contact with the medical system and the differences observed may represent different approaches to testing. The universal screening approach utilised by the six studies may have resulted in earlier detection of positive pregnant patients before symptom onset, whereas the non‐pregnant population is currently more likely to present only once symptoms emerge. It is also possible that the higher detection rate of asymptomatic pregnant women compared to the general population may be related to age, given that the true prevalence of infection in the broader 20–40‐year‐old population is unknown due to the absence of large‐scale screening measures and the current reliance on symptomatology for testing. Widespread screening in unselected populations will be required to resolve this disparity. A significantly reduced fever incidence in pregnancy compared to non‐pregnant patients was observed by Liu et al. 34 This suggests that some symptoms and signs of SARS‐CoV‐2 infection may be modified by pregnancy; however, it must be acknowledged that only 16 pregnant and 14 non‐pregnant rtPCR positive patients were compared.
Unlike for SARS, MERS or influenza, pregnancy does not appear to be associated with an elevated risk of serious maternal COVID‐19 illness compared with the non‐pregnant population. In almost all cohort studies describing disease severity, rates of severe and critical disease approximated those of the general population (14% severe and 5% critical as reported by the World Health Organization 3 ). It was therefore noteworthy that Pierce‐Williams et al. 47 observed 69% severe and 31% critical disease; however, all study participants were hospital inpatients admitted for COVID‐19, hence selection bias may explain these findings. Ferrazzi et al. 59 noted an ICU admission rate of 9.5%; however, it was unclear what the admission criteria for ICU were and whether the women had severe or critical COVID‐19 disease. For clarity, future work must transparently describe the exact nature of clinical features, patient management and adverse outcomes. Maternal mortality may occur in critical cases of COVID‐19. Given that full outcome data for most of the critical patients were not confirmed, the true impact of SARS‐CoV‐2 infection on maternal mortality compared to the general population cannot be reliably ascertained at present.
A broad range of extrapulmonary complications of COVID‐19 have been reported in non‐pregnant patients, including acute cardiac injury and acute kidney injury. 64 , 65 Cardiomyopathy was described in two cases in pregnancy. A study of non‐pregnant patients with COVID‐19 found an elevated risk of cardiomyopathy in those with hypertension, diabetes mellitus or cardiovascular disease. 66 These are the comorbidities most frequently associated with severe and critical COVID‐19. 67 Pregnant patients with cardiometabolic conditions may require additional monitoring if SARS‐CoV‐2 infection occurs during pregnancy.
Prior to the SARS‐CoV‐2 pandemic, a cohort study comparing pregnant women with and without pneumonia reported that preterm birth, low birthweight and fetal growth restriction were significantly more prevalent in women with pneumonia. 68 In the current review, there was a wide variation in the incidence of preterm birth, which contrasts with the 5–18% global incidence in the general pregnant population. 69 The true rate of preterm birth related to SARS‐CoV‐2 infection cannot be determined from studies in this review; small sample size, lack of widespread population screening or matched control groups, insufficient information on the numbers of women diagnosed preterm, and a lack of clear explanation for the causes of preterm birth render it difficult to draw meaningful conclusions. The impact of antenatal infection with COVID‐19 on neonatal outcomes is similarly unclear, and longer‐term follow‐up of infants born to mothers with COVID‐19 is required to assess whether there are any long‐term implications.
Caesarean section was commonly performed for patients with COVID‐19. As such, the incidence of caesarean section in most studies ranged from 40% to 100% – greater than the Chinese and US averages of 37% 70 and 32%, 71 respectively. Caesarean section was undertaken for obstetric indications or due to uncertainty about maternal to child transmission via vaginal delivery with active infection. While only two studies described 100% vaginal delivery, it is reassuring that no neonates in these cases were SARS‐CoV‐2 positive. Both the International Federation of Gynecology and Obstetrics (FIGO) and the Royal College of Obstetricians and Gynaecologists (RCOG) state that mode of delivery should not be influenced by presence of COVID‐19 (unless urgent delivery is indicated due to severe respiratory compromise). 72 , 73 These recommendations arose after many of the cases reported in this review had delivered.
Nineteen neonates were diagnosed with COVID‐19, raising the possibility of vertical transmission. This contrasts with SARS, where no evidence of vertical transmission exists. 74 , 75 The timing of sampling and contact with the infected mother may be pivotal to ascertaining when this transmission occurred. Only Khan et al. 23 and Patane et al. 55 took nasopharyngeal swabs immediately following delivery to identify possible in utero transmission (caesarean and vaginal delivery, respectively). All others with SARS‐CoV‐2 positive neonates took samples between 24 hours and six days postpartum, so it is possible that infection occurred either prior to, during or after birth. Zeng et al. 13 identified elevated serum anti‐SARS‐CoV‐2 IgM in two neonates. Given that IgM antibodies are too large to cross the placenta and serum was sampled immediately postpartum, this again suggests the possibility of in utero transmission, although further work in this area is needed given the small numbers to date. In both of these cases the nasopharyngeal swabs were negative. The significance of three positive placenta samples in Penfield et al. 57 and one positive placenta and cord blood sample in Buonsenso et al. 54 is uncertain given that neonates were SARS‐CoV‐2 negative.
The lack of SARS‐CoV‐2 detection in breastmilk suggests that the virus may not be excreted via this route; however, results of only 45 samples have been reported. Breastfeeding also requires significant maternal‐neonatal contact, which poses risks of contact and droplet transmission. The Centers for Disease Control and Prevention, FIGO and the Royal Australian and New Zealand College of Obstetricians and Gynaecologists recommend that breastfeeding be encouraged for mild and moderate infections, with the application of a surgical mask and careful hand washing before contact. 72 , 76 , 77 In severe infection, expression of breastmilk should be considered where possible. Breastfeeding from birth with the use of surgical mask and hand washing was permitted in four studies. 40 , 50 , 55 , 63 The absence of neonatal COVID‐19 in Breslin et al. 40 and Qadri and Mariona 63 supports the guidelines for breastfeeding. The significance of one neonate testing positive for SARS‐CoV‐2 after rooming in and breastfeeding is uncertain. 50
Our review has some limitations. As per our inclusion criteria even very small case series were included which may result in some clinical features and outcomes to appear amplified. There was substantial heterogeneity between the included studies, particularly in the study type (case series, cohorts or universal screening), clinical management and reporting of outcomes. This may engender considerable bias in the proportions of features identified and limits the comparison between studies. However, our broader inclusion criteria meant that a large number of countries were represented, allowing superior characterisation of the international experience. A meta‐analysis was not possible due to the possibility of individual cases being reported in multiple studies from the same institution. For clarity and enhanced accuracy of datasets, future papers should clearly identify participants who are included in previous publications.
A prospective and comprehensive collection of case details is required to address the currently limited data and to improve management of pregnant patients. It is promising that there are global efforts to collect prospectively harmonised data regarding maternal, perinatal and neonatal outcomes, to better inform clinicians and appropriately manage pregnant patients. These include the US PRIORITY study (ClinicalTrials.gov ID NCT04323839) which has recruited 790 women as of 1 June 2020, 78 the UK Obstetric Surveillance System (UKOSS) study which will soon publish a report of 427 COVID‐19 affected pregnancies 79 and the Australian‐based CHOPAN registry (ANZ Clinical Trials registry ID ACTRN12620000449932). 80
No study has yet assessed the effect of COVID‐19 in the first trimester on the developing fetus. This data is unlikely to be available until after June 2020 given this novel virus only emerged in December 2019. Given the paucity of data pertaining to the consequences of early and mid‐pregnancy infection, FIGO has recommended monthly ultrasound scans for fetal morphology and growth. 72 It will be of future interest to assess whether infection during this stage of pregnancy predisposes to an increased rate of birth defects or other adverse paediatric and maternal outcomes.
In conclusion, this review has collated the published case series and cohort studies involving pregnant women with SARS‐CoV‐2 infection up until May 23, 2020. This data has demonstrated maternal mortality rates comparable with those in the non‐pregnant population. Rates of preterm birth and caesarean delivery appear high, but it is unclear how much this relates to the clinical course of the disease and how much is iatrogenic. Uncommon but serious complications of infection for both mother and neonate are recognised. Vertical transmission is possible; however, timing of infection (in utero, intrapartum or postpartum) and rate of transmission are unclear. A number of questions remain unanswered, and further systematic reviews will be required as more data is accumulated in this rapidly evolving pandemic.
Supporting information
Data S1. Database search strategy.
Table S1. Details of included studies.
Conflict of interest: The authors report no conflicts of interest.
References
- 1. who.int [homepage on the internet]. Coronavirus disease 2019 (COVID‐19) Situation report ‐ 133. Geneva: WHO. [Accessed 02 June 2020.] Available from URL: https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200601‐covid‐19‐sitrep‐133.pdf?sfvrsn=9a56f2ac_4 [Google Scholar]
- 2. who.int [homepage on the internet]. Coronavirus disease 2019 (COVID‐19) Situation report – 46. Geneva: WHO; [Accessed 9 Apr 2020.] Available from URL: https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200306‐sitrep‐46‐covid‐19.pdf?sfvrsn=96b04adf_2] [Google Scholar]
- 3. who.int [homepage on the internet] . Geneva: World Health Organisation Interim guidance: Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected. v1.2 [Cited 21 April 2020]. Available from URL: https://www.who.int/publications‐detail/clinical‐management‐of‐severe‐acute‐respiratory‐infection‐when‐novel‐coronavirus‐(ncov)‐infection‐is‐suspected
- 4. health.gov.au [homepage on the internet] . Canberra, Australia. Coronavirus (COVID‐19) current situation and case numbers [Cited 2 June 2020]. Available from URL: https://www.health.gov.au/news/health‐alerts/novel‐coronavirus‐2019‐ncov‐health‐alert/coronavirus‐covid‐19‐current‐situation‐and‐case‐numbers
- 5. Siston AM, Rasmussen SA, Honein MA et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010; 303: 1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anonymous . ACOG Committee Opinion No. 753: assessment and treatment of pregnant women with suspected or confirmed influenza. Obstet Gynecol 2018; 132: e169–e173. [DOI] [PubMed] [Google Scholar]
- 7. Mertz D, Geraci J, Winkup J et al. Pregnancy as a risk factor for severe outcomes from influenza virus infection: A systematic review and meta‐analysis of observational studies. Vaccine 2017; 35: 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. RANZCOG . ranzcog.edu.au [homepage on the internet]. Influenza Vaccination During Pregnancy (and in women planning pregnancy): (C‐Obs 45), 2017. [Accessed 22 Apr 2020.] Available from URL: https://ranzcog.edu.au/RANZCOG_SITE/media/RANZCOG‐MEDIA/Women%27s%20Health/Statement%20and%20guidelines/Clinical‐Obstetrics/Influenza‐vaccination‐in‐pregnancy‐(C‐Obs‐45)‐Review‐March‐2017.pdf?ext=.pdf [Google Scholar]
- 9. Wong S, Chow K, Leung T et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 2004; 191: 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alfaraj SH, Al‐Tawfiq JA, Memish ZA. Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect 2019; 52: 501–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. cgf.cochrane.org [homepage on the internet]. COVID‐19 (coronavirus disease) ‐ Fertility and Pregnancy. London, UK. [Accessed 19 May 2020.] Available from URL: https://cgf.cochrane.org/news/covid‐19‐coronavirus‐disease‐fertility‐and‐pregnancy [Google Scholar]
- 12. Sutton D, Fuchs K, D’Alton M, Goffman D. Universal screening for SARS‐CoV‐2 in women admitted for delivery. N Engl J Med 2020; 382: 2163–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeng H, Xu C, Fan J et al. Antibodies in infants born to mothers with COVID‐19 pneumonia. JAMA 2020; 323: 1848‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeng L, Xia S, Yuan W et al. Neonatal early‐onset infection With SARS‐CoV‐2 in 33 neonates born to mothers with COVID‐19 in Wuhan, China. JAMA Pediatr 2020. 10.1001/jamapediatrics.2020.0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Breslin N, Baptiste C, Miller R et al. COVID‐19 in pregnancy: early lessons. Am J Obstet Gynecol MFM 2020; 2: 100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. An P, Wood BJ, Li W et al. Postpartum exacerbation of antenatal COVID‐19 pneumonia in 3 women. Can Med Assoc J 2020; 192. 10.1503/cmaj.200553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu X, Gao J, Luo X et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vertical transmission in neonates born to mothers with coronavirus disease 2019 (COVID‐19) pneumonia. Obstet Gynecol 2020; 136: 65–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang W, Zhao Z, He Z et al. Unfavorable outcomes in pregnant patients with COVID‐19 outside Wuhan, China. J Infect 2020. 10.1016/j.jinf.2020.05.014. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun M, Xu G, Yang Y et al. Evidence of mother‐to‐newborn infection with COVID‐19. Br J Anaesth 2020; 20: 30281–30286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang L, Jiang Y, Wei M et al. Analysis of the pregnancy outcomes in pregnant women with COVID‐19 in Hubei Province. Chin J Obstet Gynecol 2020; 55: E009. [DOI] [PubMed] [Google Scholar]
- 21. Fan C, Lei D, Fang C et al. Perinatal transmission of COVID‐19 associated SARS‐CoV‐2: should we worry? Clin Infect Dis 2020. 10.1093/cid/ciaa226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen R, Zhang Y, Huang L et al. Safety and efficacy of different anesthetic regimens for parturients with COVID‐19 undergoing Cesarean delivery: a case series of 17 patients. Can J Anaesth 2020; 67: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khan S, Jun L, Nawsherwan et al. Association of COVID‐19 infection with pregnancy outcomes in healthcare workers and general women. Clin Microbiol Infect 2020; 26: 788–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khan S, Peng L, Siddique R et al. Impact of COVID‐19 infection on pregnancy outcomes and the risk of maternal‐to‐neonatal intrapartum transmission of COVID‐19 during natural birth. Infect Control Hosp Epidemiol 2020; 41: 748–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lei D, Wang C, Li C et al. Clinical characteristics of COVID‐19 in pregnancy: analysis of nine cases. Chin J Perinat Med 2020; 23: 159–165. [Google Scholar]
- 26. Yu N, Li W, Kang Q et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID‐19 in Wuhan, China: a retrospective, single‐centre, descriptive study. Lancet Infect Dis 2020; 20: 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen S, Liao E, Cao D et al. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol 2020: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu W, Wang J, Li W et al. Clinical characteristics of 19 neonates born to mothers with COVID‐19. Front Med. 2020; 14: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu N, Li W, Kang Q et al. No SARS‐CoV‐2 detected in amniotic fluid in mid‐pregnancy. Lancet Infect Dis 2020; 22: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu D, Li L, Wu X et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID‐19) pneumonia: a preliminary analysis. Am J Roentgenol 2020; 1–6. [DOI] [PubMed] [Google Scholar]
- 31. Chen S, Huang B, Luo DJ et al. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases. Chin J Pathol 2020; 49: E005. [DOI] [PubMed] [Google Scholar]
- 32. Xu L, Yang Q, Shi H et al. Clinical presentations and outcomes of SARS‐CoV‐2 infected pneumonia in pregnant women and health status of their neonates. Sci Bull 2020. 10.1016/j.scib.2020.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li N, Han L, Peng M et al. Maternal and neonatal outcomes of pregnant women with COVID‐19 pneumonia: a case‐control study. Clin Infect Dis 2020. 10.1093/cid/ciaa352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu H, Liu F, Li J et al. Clinical and CT imaging features of the COVID‐19 pneumonia: focus on pregnant women and children. J Infect 2020; 11: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cao D, Yin H, Chen J et al. Clinical analysis of ten pregnant women with COVID‐19 in Wuhan, China: a retrospective study. Int J Infect Dis 2020; 95: 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu C, Yang W, Wu X et al. Manifestation and laboratory characteristics of SARS‐CoV‐2 infection in pregnant women. Virol Sin 2020;1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen H, Guo J, Wang C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020; 395: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang P, Wang X, Liu P et al. Clinical characteristics and risk assessment of newborns born to mothers with COVID‐19. J Clin Virol 2020; 127: 104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liao J, He X, Gong Q et al. Analysis of vaginal delivery outcomes among pregnant women in Wuhan, China during the COVID‐19 pandemic. Int J Gynaecol Obstet 2020; 150: 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Breslin N, Baptiste C, Gyamfi‐Bannerman C et al. COVID‐19 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals . Am J Obstet Gynecol MFM 2020; 2: 100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baergen RN, Heller DS. Placental pathology in Covid‐19 positive mothers: preliminary findings. Pediatr Dev Pathol 2020; 23: 177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gagliardi L, Danieli R, Suriano G et al. Universal SARS‐CoV‐2 testing of pregnant women admitted for delivery in two Italian regions. Am J Obstet Gynecol 2020; 11: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khalil A, Hill R, Ladhani S et al. SARS‐CoV‐2 in pregnancy: symptomatic pregnant women are only the tip of the iceberg. Am J Obstet gynecol 2020. 10.1016/j.ajog.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vintzileos WS, Muscat J, Hoffmann E et al. Screening all pregnant women admitted to Labor and Delivery for the virus responsible for COVID‐19. Am J Obstet Gynecol 2020. 10.1016/j.ajog.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doria M, Peixinho C, Laranjo M et al. Covid‐19 during pregnancy: a case series from an universally tested population from the north of Portugal. Eur J Obstet Gynecol Reprod Biol 2020; 15: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miller ES, Grobman WA, Sakowicz A et al. Clinical Implications of universal severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) testing in pregnancy. Obstet Gynecol 2020; 19: 19. [DOI] [PubMed] [Google Scholar]
- 47. Pierce‐Williams RAM, Burd J, Felder L et al. Clinical course of severe and critical COVID‐19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol MFM 2020: 100134. 10.1016/j.ajogmf.2020.100134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hantoushzadeh S, Shamshirsaz AA, Aleyasin A et al. Maternal death due to COVID‐19 disease. Am J Obstet Gynecol 2020; 223: 109.e1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Juusela A, Nazir M, Gimovsky M. Two cases of COVID‐19 related cardiomyopathy in pregnancy. Am J Obstet Gynecol MFM. 2020; 2: 100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Savasi VM, Parisi F, Patane L et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID‐19). Obstet Gynecol 2020; 19: 19. [DOI] [PubMed] [Google Scholar]
- 51. Liu Y, Chen H, Tang K, Guo Y. Clinical manifestations and outcome of SARS‐CoV‐2 infection during pregnancy. J Infect 2020. 10.1016/j.jinf.2020.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yan J, Guo J, Fan C et al. Coronavirus disease 2019 (COVID‐19) in pregnant women: a report based on 116 cases. Am J Obstet Gynecol 2020; 223: 111.e1–111.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hirshberg A, Kern‐Goldberger AR, Levine LD et al. Care of critically ill pregnant patients with COVID‐19: a case series. Am J Obstet Gynecol 2020. 10.1016/j.ajog.2020.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Buonsenso D, Costa S, Sanguinetti M et al. Neonatal late onset infection with severe acute respiratory syndrome coronavirus 2. Am J Perinatol 2020; 37: 869–872. 10.1055/s-0040-1710541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Patane L, Morotti D, Giunta MR et al. Vertical transmission of COVID‐ 19: SARS‐CoV‐2 RNA on the fetal side of the placenta in pregnancies with COVID‐19 positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020; 100145. 10.1016/j.ajogmf.2020.100145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Govind A, Essien S, Kartikeyan A et al. Re: Novel Coronavirus COVID‐19 in late pregnancy: outcomes of first nine cases in an inner city London hospital. Eur J Obstet Gynecol Reprod Biol 2020. 10.1016/j.ejogrb.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Penfield CA, Brubaker SG, Limaye MA et al. Detection of SARS‐COV‐2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM 2020; 100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cooke WR, Billett A, Gleeson S et al. SARS‐CoV‐2 infection in very preterm pregnancy: experiences from two cases. Eur J Obstet Gynecol Reprod Biol 2020; 15: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ferrazzi E, Frigerio L, Savasi V et al. Vaginal delivery in SARS‐CoV‐2 infected pregnant women in Northern Italy: a retrospective analysis. BJOG 2020. 10.1111/1471-0528.16278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lokken EM, Walker CL, Delaney S et al. Clinical characteristics of 46 pregnant women with a SARS‐CoV‐2 infection in Washington State. Am J Obstet Gynecol 2020. 10.1016/j.ajog.2020.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhu H, Wang L, Fang C et al. Clinical analysis of 10 neonates born to mothers with 2019‐nCoV pneumonia. Transl Pediatr 2020; 9: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. London V, McLaren R Jr, Atallah F et al. The Relationship between status at presentation and outcomes among pregnant women with COVID‐19. Am J Perinatol 2020; 19: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Qadri F, Mariona F. Pregnancy affected by SARS‐CoV‐2 infection: a flash report from Michigan. J Matern Fetal Neonatal Med 2020; 1–3. [DOI] [PubMed] [Google Scholar]
- 64. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutierrez‐Ocampo E et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis 2020; 34: 101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS‐CoV‐2: a systematic review and meta‐analysis. J Med Virol 2020. 10.1002/jmv.25822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guo T, Fan Y, Chen M et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020. 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Aboussouan LS, Mireles‐Cabodevila E. Sleep‐disordered breathing in neuromuscular disease: diagnostic and therapeutic challenges. Chest 2017; 152: 880–892. [DOI] [PubMed] [Google Scholar]
- 68. Chen YH, Keller J, Wang IT et al. Pneumonia and pregnancy outcomes: a nationwide population‐based study. Am J Obstet Gynecol 2012; 207: 288.e1–288.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. who.int [homepage on the internet]. Preterm Birth. Geneva: WHO, 2018. [Accessed 30 Apr 2020.] Available from URL https://www.who.int/news‐room/fact‐sheets/detail/preterm‐birth [Google Scholar]
- 70. Li H, Hellerstein S, Zhou Y et al. Trends in cesarean delivery rates in China, 2008–2018. JAMA 2020; 323: 89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. cdc.gov [homepage on the internet] . Georgia, USA. Cesarean Delivery Rate by State [Cited 30 April 2020]. Available from URL: https://www.cdc.gov/nchs/pressroom/sosmap/cesarean_births/cesareans.htm
- 72. Poon LC, Yang H, Kapur A et al. Global interim guidance on coronavirus disease 2019 (COVID‐19) during pregnancy and puerperium from FIGO and allied partners: Information for healthcare professionals. Int J Gynecol Obstet 2020; 149: 273–286. 10.1002/ijgo.13156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. RCOG RCoM, Royal College of Paediatrics and Child Health, Public Health England and Public Health Scotland . Coronavirus (COVID‐19) infection and pregnancy: information for healthcare professionals, 2020. Version 8 [updated Friday 17 April 2020.] Available from URL: https://www.rcog.org.uk/coronavirus‐pregnancy [Google Scholar]
- 74. Longman RE, Johnson TR. Viral respiratory disease in pregnancy. Curr Opin Obstet Gynecol 2007; 19: 120–125. [DOI] [PubMed] [Google Scholar]
- 75. Wong S, Chow K, Leung T et al. Pregnancy and perinatal outcomes of women with severe acute respiratory distress syndrome. Am J Obstet Gynecol 2004; 191: 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. RANZCOG . A Message for Pregnant Women and their Families, 2020. [Accessed 28 Apr 2020.] Available from URL: https://ranzcog.edu.au/statements‐guidelines/covid‐19‐statement/information‐for‐pregnant‐women [Google Scholar]
- 77. cdc.gov [homepage on the internet]. Coronavirus Disease (COVID‐19) and Breastfeeding, Atlanta, Georgia, USA. [Accessed 23 May 2020.] Available from URL: https://www.cdc.gov/breastfeeding/breastfeeding‐special‐circumstances/maternal‐or‐infant‐illnesses/covid‐19‐and‐breastfeeding.html [Google Scholar]
- 78. priority.ucsf.edu [homepage on the internet]. California: PRIORITY: Pregnancy Coronavirus Outcomes Registry. [Accessed 02 June 2020.] Available from URL: https://priority.ucsf.edu/ [Google Scholar]
- 79. Knight M, Bunch K, Vousden N et al. Characteristics and outcomes of pregnant women hospitalised with confirmed SARS‐CoV‐2 infection in the UK: a national cohort study using the UK Obstetric Surveillance System (UKOSS). BMJ 2020. 10.1101/2020.05.08.20089268 [DOI] [Google Scholar]
- 80. anzctr.org.au [homepage on the internet] . Prospective registry of maternal, perinatal and neonatal outcomes from pregnancies infected with SARS‐COV2 (COVID‐19). [Cited 5 May 2020]. Available from URL: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379572&isReview=true [Google Scholar]
- 81. Chen L, Li Q, Zheng D et al. Clinical characteristics of pregnant women with Covid‐19 in Wuhan, China. N Engl J Med 2020; 382: e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen Y, Peng H, Wang L et al. Infants born to mothers with a new coronavirus (COVID‐19). Front Pediatr 2020; 8: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wu X, Sun R, Chen J, Xie Y, Zhang S, Wang X. Radiological findings and clinical characteristics of pregnant women with COVID‐19 pneumonia. Int J Gynaecol Obstet 2020; 150: 58–63. 10.1002/ijgo.13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li L, Liu D, Yang L. Follow‐up information about the four pregnant patients with coronavirus disease (COVID‐19) pneumonia who were still in the hospital at the end of our study. AJR Am J Roentgenol 2020: W1–W2. [DOI] [PubMed] [Google Scholar]
- 85. CDC COVID‐19 Response Team . Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 – United States, February 12‐March 28, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Blitz MJ, Grünebaum A, Tekbali A et al. Intensive care unit admissions for pregnant and non‐pregnant women with COVID‐19. Am J Obstet Gynecol 2020. 10.1016/j.ajog.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chen X, Li Y, Wang J, Cai H, Cao H, Sheng J. [Pregnant women complicated with COVID‐19: a clinical analysis of 3 cases]. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020; 49: 240–244. (In Chinese.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Qiancheng X, Jian S, Lingling P et al. Coronavirus disease 2019 in pregnancy. Int J Infect Dis 2020; 95: 376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Database search strategy.
Table S1. Details of included studies.


