Abstract
There is an urgent need to address the devastating pandemic, COVID‐19, caused by SARS‐CoV‐2. The efforts to understand the details of this disease in hope of providing effective treatments are commendable. It is clear now that the virus can cause far more damage in patients with comorbid conditions—particularly in those with respiratory, cardiovascular, or immune‐compromised system—than in patients without such comorbidities. Drug use can further exacerbate the condition. In this regard, the ill effects of smoking are amply documented, and no doubt can be a confounding factor in COVID‐19 progression. Although conflicting hypotheses on the potential role of nicotine in COVID‐19 pathology have recently been offered, we believe that nicotine itself, through its interaction with the nicotinic cholinergic system, as well as ACE2, may not only be of use in a variety of neuropsychiatric and neurodegenerative diseases, but may also be of potential use in COVID‐19. Thus, on one hand, while we strongly support smoking cessation as a means of harm reduction associated with COVID‐19, on the other hand, we support a potential therapeutic role for nicotine, nicotinic agonists, or positive allosteric modulators of nicotinic cholinergic receptors in COVID‐19, owing to their varied effects including mood regulation, anti‐inflammatory, and purported interference with SARS‐CoV‐2 entry and/or replication.
Keywords: ACE2, allosteric modulators, COVID‐19, inflammation, nAChR, nicotine, SARS‐CoV‐2, smoking
The COVID‐19 pandemic continues to exact a toll throughout the world. Individuals with underlying conditions (e.g., heart disease, lung disease, immunosuppression) are particularly susceptible to SARS‐CoV‐2. Smokers are also at a higher risk of succumbing to COVID‐19. However, the pharmacological properties of nicotine and the involvement of the nicotinic cholinergic system in immune regulation, neuroprotection, and suppression of inflammation suggest therapeutic potential for nicotinic agonists and/or modulators in COVID‐19.
Abbreviations
- ACh
acetylcholine
- ACE2
angiotensin‐converting enzyme 2
- ANG‐(1–7)
angiotensin 1‐7
- ARDS
acute respiratory distress syndrome
- AUD
alcohol use disorder
- CCL2
chemokine L2
- CNS
central nervous system
- COVID‐19
coronavirus disease of 2019
- IL
interleukin
- nAChRs
nicotinic cholinergic receptors
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SUD
substance use disorder
Introduction
The worldwide toll exacted by a single‐stranded RNA virus, the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), leading to the coronavirus disease of 2019 (COVID‐19), is a wake‐up call to better understand the role of viruses and infectious elements and to come up with an improved strategy for collective responses to pandemics.
It is quite evident that COVID‐19 pathologies discriminately involve patients with underlying diseases such as hypertension, cardiovascular and cerebrovascular diseases, diabetes, hepatitis B infections, chronic obstructive pulmonary disease, chronic kidney diseases, malignancy, and immunodeficiency vs. those without such conditions. Expectedly, having two or more comorbidities further escalates the risks of succumbing to COVID‐19 [1, 2, 3].
COVID‐19 could also be hitting hard some populations with substance use disorders (SUDs) owing to the fact that people who use tobacco or marijuana or those with opioid or methamphetamine use disorders may have compromised respiratory and pulmonary health [4]. Similarly, alcohol use disorder (AUD), by impairing the immune system and increasing susceptibility to respiratory illnesses, could contribute to greater mortality risk when combined with COVID‐19 [5]. There is, however, considerable controversy regarding the interaction of the plant alkaloid nicotine with COVID‐19.
Nicotinic cholinergic receptors
Nicotinic cholinergic receptors (nAChRs), target of nicotine, belong to ionotropic class of receptors, which act by directly regulating the opening of a cation channel in the neuronal membrane [6, 7, 8]. Various subtypes of these receptors with distinct anatomical, physiological, and pharmacological characteristics have been identified [6]. Although nAChRs are present at the neuromuscular junction, autonomic ganglia, and the central nervous system, the subunit structures of these receptors are different from each other [9]. Considerable information on interaction between neuronal nicotinic receptors, consisting mainly of alph4‐beta2 or homomeric alpha7 subunits and other neurotransmitter systems, is now available. Moreover, specific responses of these receptors to nicotine and their involvement in cognitive functions have been investigated [10, 11]. Indeed, therapeutic potentials for selective nicotinic receptor agonists in various neuropsychiatric and neurodegenerative disorders have been suggested. These include Parkinson's disease and Alzheimer's disease, schizophrenia, depression, pain, and smoking cessation [12, 13, 14, 15]. The potential role of nicotinic receptors in high‐order cognitive processing has been recently reviewed [16].
nAChRs and inflammation
It is also of relevance that acetylcholine (ACh), both centrally and peripherally, acts not only through the nicotinic, but also through the muscarinic receptors, which are G protein‐coupled receptors and act via the second messenger system. Our emphasis here is solely on the ionotropic nicotinic receptors that act directly on channel opening. It is now believed that there exists a cholinergic anti‐inflammatory pathway that acts through nicotinic acetylcholine receptors [17, 18]. Thus, activation of these receptors (particularly α7 nAChR) can suppress production of pro‐inflammatory cytokines as these receptors are abundantly expressed in variety of immune cells including B cells, T cells, and macrophages [19]. Indeed, nicotine has been shown to inhibit pro‐inflammatory cytokines such as tumor necrosis factor‐α (TNF‐α), interleukin‐1 (IL‐1), and IL‐6 without affecting the anti‐inflammatory cytokines such as IL‐10 [20, 21]. Interestingly, several animal models associated with elevated levels of pro‐inflammatory cytokines such as sepsis, ischemia–reperfusion, and pancreatitis show improvement by stimulation of the vagus nerve, which is believed to be mediated via activation of α7 nAChRs on macrophages [22]. Moreover, mice deficient in α7 nAChRs exhibit increased endotoxin‐induced TNF‐α production, which do not respond to electrical vagal stimulation [22]. Thus, it may be concluded that nicotine—via α7 nAChR stimulation—might lead to inhibition of cytokine storm associated with COVID‐19 described further below. Importantly, nicotine use has been advocated in diseases associated with immune dysregulation (autoimmune diseases) or various inflammatory diseases such as multiple sclerosis, type 1 diabetes, rheumatoid arthritis, and inflammatory bowel diseases [23, 24, 25].
Smoking, nicotine, and COVID‐19
Substantial evidence indicates adverse effects of smoking on COVID‐19 including predisposition to the virus infection, severity of progression, and mortality [1, 26, 27, 28, 29]. However, there is a meta‐analysis report that concludes that active smoking is not associated with severity of coronavirus disease [30], although this contention has been challenged [26]. Still, some studies claim to have found a lower incidence of hospitalized COVID‐19 patients among smokers [31, 32].
However, the evidence accumulated up to now suggests that although smokers might be less hospitalized, their health risk increases as soon as they are hospitalized [33]. Thus, it seems prudent to indicate that smoking is a risk factor for COVID‐19 given the overwhelming epidemiological evidence cited above. However, the relationship of the stimulant in cigarettes, nicotine, with COVID‐19 is far from clear. Thus, some investigators argue that because of known nicotinic receptor interactions with angiotensin‐converting enzyme 2 (ACE2), a channel for SARS‐CoV‐2 entry into cells, nicotine exposure can increase the risk for COVID‐19 damage, including neuroinfection [34]. Moreover, it was reported that exposure to nicotine causes epithelial cells to increase ACE2 levels, whereas gene silencing of α7 nAChR appears to significantly dampen this response [35], leading to the suggestion that perhaps α7 nAChR‐selective antagonists, by altering ACE2 expression, may prevent SARS‐CoV‐2 entry into the airway epithelium [36]. These contentions, however, stand in stark contrast with potential beneficial effects of nicotine or nicotinic agonists in a variety of diseases [13, 37], including COVID‐19 [18, 19, 38].
Part of these discrepancies might stem from the way of interpreting studies; for example, some of the conclusions on the impact of smoking on COVID‐19 are inferred, rather than directly analyzed [1, 31]. Another possible reason for the discrepancies might lie in the attempt to predict the final outcome of a drug based on its presumptive mode of action [38, 39]. Interaction of nicotine with ACE2 appears to be far more complicated to allow a definite prediction of the entry of the SARS‐CoV‐2 into the epithelium. This is due to the fact that not only our full understanding of the role of ACE2 in general, and in COVID‐19 in particular, is incomplete, but also our knowledge of interaction between nicotine and ACE2 is far from clear [38, 39, 40, 41]. Thus, although it is believed that SARS‐CoV‐2 uses ACE2 as a receptor for cell entry, involvement of other receptors cannot be totally ruled out [38, 41, 42, 43, 44]. Moreover, SARS‐CoV‐2 has been reported to either downregulate [45, 46, 47] or upregulate ACE2 [28, 36, 48]. In this regard, it is important to note that ACE2 polymorphism, which could influence both the susceptibility of people to SARS‐CoV‐2 infection and the outcome of the COVID‐19, was recently described in human populations [40, 45]. Importantly, it has been hypothesized that increased ACE2 levels may actually be beneficial rather than harmful, particularly in patients with lung injury as it has been shown to have potent vasodilatory, anti‐inflammatory, and antioxidant properties [49, 50, 51]. Interestingly, children and younger adults, who have milder COVID‐19 symptoms, have higher ACE2 levels compared to older people [52].
Smoking, nicotine, and ACE2
On one hand, it is proposed that smoking or nicotine may upregulate the detrimental angiotensin‐converting enzyme (ACE) but downregulate the compensatory ACE2/ANG‐(1–7) receptor axis and hence contribute to cardiovascular and pulmonary diseases [53, 54, 55]. On the other hand, nicotine, via activation of α7 nAChR exposure, may actually increase ACE2 levels in the epithelial cells [36]. This latter effect, consistent with the observations of several other investigators [55, 56], would jive with the hypothesis proposed by Vaduganathan et al [51] and elaborated above and would support potential beneficial effects of nicotine in countering COVID‐19. It must be emphasized that the action of nicotine per se should be distinguished from thousands of other chemicals in tobacco or even e‐cigarettes [57] that are known to be harmful to the body. Indeed, smoking is also a significant risk factor for mortality due to infections of various respiratory viruses including seasonal influenza as well as potential progression of COVID‐19 [1, 27, 28, 29, 33]. However, nicotine or nicotinic agonists, as discussed above, may have significant therapeutic potentials including COVID‐19 [37, 38, 51, 58].
nAChRs and cytokine storm
The postulated mechanism for the efficacy of nicotine in infectious diseases in general, and in COVID‐19 in particular, includes restoring and re‐activating the cholinergic anti‐inflammatory pathway that can suppress the cytokine storm, also referred to as macrophage activation syndrome [18, 59]. Cytokine storm, a dangerous hyperinflammatory state associated with elevated levels of several pro‐inflammatory cytokines, such as IL‐1β, IL‐2, IL‐6, IL‐17, IL‐8, TNF‐α, and chemokine L2 (CCL2), has been observed in COVID‐19 patients and appears to be the hallmark of severe cases [60, 61, 62]. For this reason, treatment with anti‐IL‐6 anti‐TNF‐α medications have been proposed and clinical trials are already underway [63, 64]. However, restoring and re‐activating the cholinergic anti‐inflammatory pathway may be far more beneficial than administering inhibitors of a single cytokine [31, 38, 58]. Animal studies also show that nicotine, by reducing leukocyte infiltration and pro‐inflammatory mediators in bronchoalveolar lavage fluid, has protective effects against lipopolysaccharide‐induced acute respiratory distress syndrome (ARDS) [65, 66]. Thus, as recently advocated by González‐Rubio and colleagues, use of nicotine or nicotinic agonists may also be effective in ameliorating cytokine storm by inhibiting several cytokines simultaneously [18].
COVID‐19—nAChRs—CNS
It is of interest to note that SARS‐CoV‐2 may enter the central nervous system (CNS) through the bloodstream, via the olfactory nerve across the cribriform plate, by disrupting the blood–brain barrier (BBB) or infecting the peripheral nerves [43, 67, 68, 69], where it may cause ACE2 downregulation and a local inflammatory response referred to as neuroinvasion, a common feature of coronaviruses [70]. Indeed, COVID‐19 has been shown to be associated with the CNS and neurological effects (e.g., dizziness, headache, confusion, agitation, anxiety, depression, strokes, seizures, and loss of smell and taste) [71, 72]. A recent report indicates that neurological complications may be manifested in more than half of hospitalized COVID‐19 patients [73].
The established anti‐inflammatory effects of nicotine, together with its capability to improve olfactory impairment in a mouse model of Parkinson’s disease [74], provide further credence to its potential usefulness in COVID‐19, as loss of smell is also a common symptom of COVID‐19 [75]. Moreover, nicotine might counter some of the neurological effects of COVID‐19, not only because of its anti‐inflammatory, but also because of its neuroprotectant and mood improvement properties [13, 14, 15]. Indeed, it has been proposed that COVID‐19 may be a disease of the cholinergic nicotinic system [76]. Hence, nicotine administration could be added on top of antiviral or other therapeutic options for COVID‐19. This application is very feasible as various pharmaceutical nicotine products such as nicotine patch, gum, nasal spray, and inhaler are already available and FDA‐approved for smoking cessation. Additionally, while some cytokine inhibitors are associated with elevated risk of opportunistic infections [77], no such effect has been attributed to pure nicotine [78]. In this regard, it is noteworthy that the mode of nicotine administration has to be carefully considered as the pharmacokinetic and pharmacodynamic of different nicotinic preparation can be significantly different [79]. Recently, it has also been proposed that positive allosteric modulators of the α7 nAChRs may be used to boost nicotine’s effects in countering excessive inflammation caused by SARS‐CoV‐2 [80]. Interestingly, ivermectin, a positive allosteric modulator of α7 nAChR [81], has been shown to inhibit the replication of SARS‐CoV‐2 in cells in vitro [82]. Hence, it may be suggested that a combination of nicotine and ivermectin might be a reasonable pharmacotherapy in COVID‐19. In the same vein, it should be noted that cotinine, a metabolite of nicotine, but without its addictive properties, has been shown to have similar anti‐inflammatory effects as nicotine and hence cotinine or selective nAChR agonists or even varenicline, a partial alpha4‐beta2 agonist used for smoking cessation may be potential substitutes for nicotine [83, 84].
COVID‐19—nAChRs—coagulation
Finally, another prominent feature of COVID‐19 is coagulopathy that results in thromboembolic complications, which are associated with higher mortality rate [85, 86]. Interestingly, platelets express functional α7 nAChRs, deficiency of which may increase platelet activity and increase the chance of clot formation [87]. Since acetylcholine may act as an endogenous inhibitor of platelet activation [88], it would be of significant interest to examine whether nicotine or selective nAChR agonists may also affect COVID‐19‐induced coagulopathy. In this regard, it is of interest to note that whereas extracts of electronic cigarettes can enhance platelet adhesion potential toward fibrinogen, pure nicotine may actually inhibit platelet function [89].
In summary, detrimental effects of smoking on general health and aggravation of viral infections should be a serious consideration for quitting smoking. On the other hand, nicotine, nicotinic receptor agonists, or positive modulators of these receptors may be of therapeutic potential in a variety of diseases including countering at least some of the harms of COVID‐19 (Fig. 1).
Fig. 1.
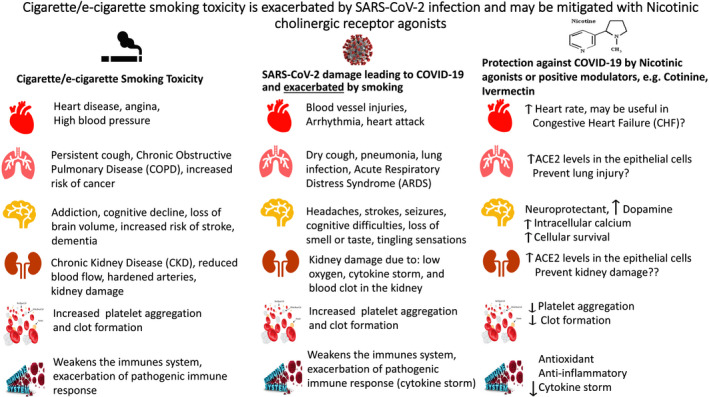
Detrimental effects of cigarette/e‐cigarette smoking and exacerbation of these effects with SARS‐CoV‐2 infection in columns 1 and 2, respectively. Column 3 depicts potential therapeutic intervention by nicotinic agonists or positive allosteric modulators in COVID‐19.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
YT, BG, MA, and RLC conceived and wrote the paper. YT and BG provided the figure.
Acknowledgement
The study was supported by NIH/NIAAA R03AA022479 (YT) and NIH/NIEHS R01ES10563 and R01ES07331 (MA).
References
- 1. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM et al. (2020) Comorbidity and its impacts on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J 55, 2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan MN, Crawford JM, McGinn T & Davidson KW. (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA 323, 2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang B, Li R, Lu Z & Huang Y (2020) Does comorbidity increase the risk of patients with COVID‐19: evidence from meta‐analysis. Aging (Albany NY) 12, 6049–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Institute of Drug Abuse (2020) COVID‐19: Potential implications for individuals with substance use disorders. Available at: https://www.drugabuse.gov/related‐topics/covid‐19‐resources.
- 5. National Institute of Alcohol Abuse and Alcoholism (2020) Alcohol poses different challenges during the COVID‐19 pandemic. NIAAA Director’s Blog, Available at: https://www.niaaa.nih.gov/directors‐blog. [Google Scholar]
- 6. Dani JA (2015) Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int Rev Neurobiol 124, 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Changeux JP (2018) The nicotinic acetylcholine receptor: a typical 'allosteric machine'. Philos Trans R Soc Lond B Biol Sci 373, 20170174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papke RL & Lindstrom JM (2020) Nicotinic acetylcholine receptors: conventional and unconventional ligands and signaling. Neuropharmacology 168, 108021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalamida D, Poulas K, Avramopoulou V et al. (2007) Muscle and neuronal nicotinic acetylcholine receptors. Structure, function and pathogenicity. FEBS J 274, 3799–3845. [DOI] [PubMed] [Google Scholar]
- 10. Valentine G & Sofuoglu M (2008) Cognitive effects of nicotine: recent progress. Curr Neuropharmacol 16, 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Azimi M, Oemisch M & Womelsdorf T (2020) Dissociation of nicotinic α7 and α4/β2 sub‐receptor agonists for enhancing learning and attentional filtering in nonhuman primates. Psychopharmacology 237, 997–1010. [DOI] [PubMed] [Google Scholar]
- 12. Bagdas D, Gurun MS, Flood P, Papke RL & Damaj MI (2018) New insights on focus on α7 nAChRs. Curr Neuropharmacol 16, 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tizabi Y, Getachew B, Csoka AB, Manaye KF & Copeland RL (2019) Novel targets for parkinsonism‐depression comorbidity. Prog Mol Biol Transl Sci 167, 1–24. [DOI] [PubMed] [Google Scholar]
- 14. Gandelman JA, Newhouse P & Taylor WD (2018) Nicotine and networks: potential for enhancement of mood and cognition in late‐life depression. Neurosci Biobehav Rev 84, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conti AA, Tolomeo S, Steele JD & Baldacchino AM (2020) Severity of negative mood and anxiety symptoms occurring during acute abstinence from tobacco: a systematic review and meta‐analysis. Neurosci Biobehav Rev 115, 48–63. [DOI] [PubMed] [Google Scholar]
- 16. Koukouli F & Changeux JP (2020) Do nicotinic receptors modulate high‐order cognitive processing? Trends Neurosci S0166–2236 (20), 30125–9. [DOI] [PubMed] [Google Scholar]
- 17. Tracey KJ (2002) The inflammatory reflex. Nature 420, 853–859. [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez‐Rubio J, Navarro‐Lopez C, Lopez‐Najera E et al. (2020) Cytokine release syndrome (CRS) and nicotine in COVID‐19 patients: trying to calm the storm. Front Immunol 11, 1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khani MA, SalehiRad M, Darbeheshti S & Motaghinejad M (2020) Survival of COVID‐19 patients requires precise immune regulation: the hypothetical immunoprotective role of nicotinic agonists. Med Hypotheses 143, 109871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li Q, Zhou XD, Kolosov VP & Perelman JM (2011) Nicotine reduces TNF‐α expression through a α7 nAChR/MyD88/NF‐ĸB pathway in HBE16 airway epithelial cells. Cell Physiol Biochem 27, 605–612. [DOI] [PubMed] [Google Scholar]
- 21. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S et al. (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388. [DOI] [PubMed] [Google Scholar]
- 22. Ulloa L (2005) The vagus nerve and the nicotinic anti‐inflammatory pathway. Nat Rev Drug Discov 4, 673–684. [DOI] [PubMed] [Google Scholar]
- 23. Lunney PC & Leong RW (2012) Review article: ulcerative colitis, smoking and nicotine therapy. Aliment Pharmacol Ther 36, 997–1008. [DOI] [PubMed] [Google Scholar]
- 24. Ko JK & Auyeung KK (2014) Inflammatory bowel disease: etiology, pathogenesis and current therapy. Curr Pharm Des 20, 1082–1096. [DOI] [PubMed] [Google Scholar]
- 25. Gomes JP, Watad A & Shoenfeld Y (2018) Nicotine and autoimmunity: the lotus' flower in tobacco. Pharmacol Res 128, 101–109. [DOI] [PubMed] [Google Scholar]
- 26. Gallus S, Lugo A & Gorini G. (2020) No double‐edged sword and no doubt about the relation between smoking and COVID‐19 severity. Europ J Int Med 6205, 33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamer M, Kivimäki M, Gale CR & Batty GD (2020) Lifestyle risk factors for cardiovascular disease in relation to COVID‐19 hospitalization: a community‐based cohort study of 387,109 adults in UK. medRxiv Preprint. 2020.05.09.20096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patanavanich R & Glantz SA. (2020) Smoking is associated with COVID‐19 progression: a meta‐analysis [published online ahead of print, 2020 May 13]. Nicot Tob Res, ntaa082. 10.1093/ntr/ntaa082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith JC, Sausville EL, Girish V et al. (2020) Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS‐CoV‐2 receptor ACE2 in the respiratory tract. Dev Cell 53, 514–529.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lippi G & Henry BM (2020) Active smoking is not associated with severity of coronavirus disease 2019 (COVID‐19). Eur J Intern Med 75, 107–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Farsalinos K, Barbouni A & Niaura R (2020) Systematic review of the prevalence of current smoking among hospitalized COVID‐19 patients in China: could nicotine be a therapeutic option? Intern Emerg Med 15, 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farsalinos K, Barbouni A, Poulas K, Polosa R, Caponnetto P & Niaura R (2020) Current smoking, former smoking, and adverse outcome among hospitalized COVID‐19 patients: a systematic review and meta‐analysis. Ther Adv Chronic Dis 11, 2040622320935765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farsalinos K, Angelopoulou A, Alexandris N & Poulas K (2020) COVID‐19 and the nicotinic cholinergic system. Eur Respir J 56, 2001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kabbani N & Olds JL (2020) Does COVID19 infect the brain? If so, smokers might be at a higher risk. Molec Pharm 97, 351–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Russo P, Bonassi S, Giacconi R, Malavolta M, Tomino C & Maggi F (2020) COVID‐19 and smoking. is nicotine the hidden link? Eur Respir J 55, 2001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leung JM, Yang CX, Tam A, Shaipanich T, Hackett TL, Singhera GK et al. (2020) ACE‐2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID‐19. Eur Respir J 55, 2000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tindle HA, Newhouse PA & Freiberg MS. (2020) Beyond Smoking Cessation: Investigating Medicinal Nicotine to Prevent and Treat COVID‐19 [published online ahead of print, 2020 May 8]. Nicot Tob Res, ntaa077. 10.1093/ntr/ntaa077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Changeux JP (2020) A nicotinic hypothesis for Covid‐19 with preventive and therapeutic implications. CR Biol 343, 1–7. [DOI] [PubMed] [Google Scholar]
- 39. Olds JL & Kabbani N (2020) Is nicotine exposure linked to cardiopulmonary vulnerability to COVID‐19 in the general population? [published online ahead of print, 2020 Mar 18]. FEBS J 287, 3651–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Devaux CA, Rolain JM, Colson P & Raoult D (2020) New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID‐19? Int J Antimicrob Agents 55, 105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dhillon P, Breuer M & Hirst N (2020) COVID‐19 breakthroughs: separating fact from fiction [published online ahead of print, 2020 Jun 5]. FEBS J 287, 3612–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shang J, Wan Y, Luo C et al. (2020) Cell entry mechanisms of SARS‐CoV‐2. Proc Natl Acad Sci U S A 117, 11727–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steardo L, Steardo L Jr, Zorec R & Verkhratsky A. (2020) Neuroinfection may potentially contribute to pathophysiology and clinical manifestations of COVID‐19. Acta Physiol (Oxf) e13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Farsalinos K, Eliopoulos E, Leonidas D, Papadopoulos G, Tzartos S & Poulas K (2020) Molecular modelling and docking experiments examining the interaction between SARS‐CoV‐2 spike glycoprotein and neuronal nicotinic acetylcholine receptors. Preprint, 2020050365. 10.20944/preprints202005.0365.v1 [DOI]
- 45. Alifano M, Alifano P, Forgez P & Iannelli A (2020) Renin‐angiotensin system at the heart of COVID‐19 pandemic. Biochimie 174, 30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lanza K, Perez LG, Costa LB et al. (2020) Covid‐19: the renin‐angiotensin system imbalance hypothesis. Clin Sci (Lond) 134, 1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Verdecchia P, Cavallini C, Spanevello A & Angeli F (2020) The pivotal link between ACE2 deficiency and SARS‐CoV‐2 infection. Eur J Intern Med 76, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen L, Li X, Chen M, Feng Y & Xiong C (2020) The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS‐CoV‐2. Cardiovasc Res 116, 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alexandre J, Cracowski JL, Richard V & Bouhanick B (2020) 'Drugs, COVID‐19' working group of the French Society of pharmacology, therapeutics. renin‐angiotensin‐aldosterone system and COVID‐19 infection. Ann Endocrinol (Paris) 81, 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Danser AHJ, Epstein M & Batlle D (2020) Renin‐angiotensin system blockers and the COVID‐19 pandemic: at present there is no evidence to abandon renin‐angiotensin system blockers. Hypertension 75, 1382–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA & Solomon SD (2020) Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N. Engl J Med 382, 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ciaglia E, Vecchione C & Puca AA (2020) COVID‐19 infection and the predictive ACE2 soluble levels: the favourable protection of children and women. Front Pediatr 8, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ferrari MF, Raizada MK & Fior‐Chadi DR (2008) Differential regulation of the renin‐angiotensin system by nicotine in WKY and SHR glia. J Mol Neurosci 35, 151–160. [DOI] [PubMed] [Google Scholar]
- 54. Oakes JM, Fuchs RM, Gardner JD et al. (2018) Nicotine and the renin‐angiotensin system. Am J Physiol Regul Integr Comp Physiol 315, R895–R906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yue X, Basting TM, Flanagan TW et al. (2018) Nicotine downregulates the compensatory angiotensin‐converting enzyme 2/angiotensin type 2 receptor of the renin–angiotensin system. Ann Am Thorac Soc 15, S126–S127. [Google Scholar]
- 56. Cattaruzza MS, Zagà V, Gallus S, D'Argenio P & Gorini G (2020) Tobacco smoking and COVID‐19 pandemic: old and new issues. A summary of the evidence from the scientific literature. Acta Biomed 91, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ruszkiewicz JA, Zhang Z, Gonçalves FM, Tizabi Y, Zelikoff JT & Aschner M (2020) Neurotoxicity of e‐cigarettes. Food Chem Toxicol 138, 111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kloc M, Ghobrial RM & Kubiak JZ. (2020) How nicotine can inhibit cytokine storm in the lungs and prevent or lessen the severity of COVID‐19 infection?. Immunol Lett 224, 28–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shimabukuro‐Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M et al. (2018) Cytokine release syndrome. J ImmunoTherapy Cancer 6, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McGonagle D, Sharif K, O’Regan A & Bridgewood C (2020) The role of cytokines including Interleukin‐6 in COVID‐19 induced pneumonia and macrophage activation syndrome‐like disease. Autoimmun Rev 19, 102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS & Manson JJ (2020) HLH across speciality collaboration, UK COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Georgiev T (2020) Coronavirus disease 2019 (COVID‐19) and anti‐rheumatic drugs. Rheumatol Int 40, 825–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M et al. (2020) Trials of anti‐tumour necrosis factor therapy for COVID‐19 are urgently needed. Lancet 395, 1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ni YF, Tian F, Lu ZF et al. (2011) Protective effect of nicotine on lipopolysaccharide‐induced acute lung injury in mice. Respiration 81, 39–46. [DOI] [PubMed] [Google Scholar]
- 66. Mabley J, Gordon S & Pacher P (2011) Nicotine exerts an anti‐inflammatory effect in a murine model of acute lung injury. Inflammation 34, 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Manji H, Carr AS, Brownlee WJ & Lunn MP (2020) Neurology in the time of covid‐19. J Neurol Neurosurg Psych 91, 568–570. [DOI] [PubMed] [Google Scholar]
- 68. Baig AM, Khaleeq A, Ali U & Syeda H (2020) Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host‐virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11, 995–998. [DOI] [PubMed] [Google Scholar]
- 69. Alam SB, Willows S, Kulka M & Sandhu JK (2020) Severe acute respiratory syndrome coronavirus 2 may be an underappreciated pathogen of the central nervous system [published online ahead of print, 2020 Jul 15]. Eur J Neuro. 10.1111/ene.14442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Desforges M, Le Coupanec A & Dubeau P (2020) Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 12, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mao L, Jin H, Wang M et al. (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77, 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nalleballe K, Reddy Onteddu S, Sharma R et al. (2020) Spectrum of neuropsychiatric manifestations in COVID‐19. Brain Behav Immun 88, 71–74. 10.1016/j.bbi.2020.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Romero‐Sánchez CM, Díaz‐Maroto I, Fernández‐Díaz E et al. (2020) Neurologic manifestations in hospitalized patients with COVID‐19: The ALBACOVID registry. Neurology;10.1212/WNL.0000000000009937. [published online ahead of print, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yang J, Lv DJ, Li LX, Wang YL, Qi D, Chen J et al. (2019) Nicotine improved the olfactory impairment in MPTP‐induced mouse model of Parkinson's disease. Neurotoxicology 73, 175–182. [DOI] [PubMed] [Google Scholar]
- 75. Moein ST, Hashemian SMR, Mansourafshar B, Khorram‐Tousi A, Tabarsi P & Doty RL (2020) Reply to: Psychophysical olfactory testing in COVID‐19: is smell function really impaired in nearly all patients?. Int Forum Allergy Rhinol 10, 953–954. 10.1002/alr.22638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Farsalinos K, Niaura R, Le Houezec J, et al. (2020) Editorial: Nicotine and SARS‐CoV‐2: COVID‐19 may be a disease of the nicotinic cholinergic system. Toxicol Rep 7, 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rutherford AI, Subesinghe S, Hyrich KL & Galloway JB (2018) Serious infection across biologic‐treated patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 77, 905–910. [DOI] [PubMed] [Google Scholar]
- 78. Sørensen LT (2012) Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg 255, 1069–1079. [DOI] [PubMed] [Google Scholar]
- 79. Tizabi Y & Getachew B (2017) Nicotinic receptor intervention in Parkinson's disease: future directions. Clin Pharmacol Transl Med 1, 14–19. [PMC free article] [PubMed] [Google Scholar]
- 80. Manni L, Tieri P & Soligo M (2020) A Contribution to the hypothesis of nicotine challenge as therapeutics option for COVID‐19 patients. Qeios ID: UJX3KN
- 81. Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP et al. (1998) Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol 53, 283–294. [DOI] [PubMed] [Google Scholar]
- 82. Caly L, Druce JD, Catton MG, Jans DA & Wagstaff KM (2020) The FDA‐approved drug Ivermectin inhibits the replication of SARS‐CoV‐2 in vitro. Antiviral Res 178, 104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Echeverria V, Grizzell JA & Barreto GE (2016) Neuroinflammation: a therapeutic target of cotinine for the treatment of psychiatric disorders? Curr Pharm Des 22, 1324–1333. [DOI] [PubMed] [Google Scholar]
- 84. Jordan CJ & Xi ZX (2018) Discovery and development of varenicline for smoking cessation. Expert Opin Drug Discov 13, 671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cui S, Chen S, Li X, Liu S & Wang F (2020) Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 18, 1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tang N, Li D, Wang X & Sun Z (2020) Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18, 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kooijman S, Meurs I, van der Stoep M, Habets KL, Lammers B, Berbée JF et al. (2015) Hematopoietic α7 nicotinic acetylcholine receptor deficiency increases inflammation and platelet activation status but does not aggravate atherosclerosis. J Thromb Haemost 13, 126–135. [DOI] [PubMed] [Google Scholar]
- 88. Bennett JA, Ture SK, Schmidt RA, Mastrangelo MA, Cameron SJ, Terry LE et al. (2019) Acetylcholine inhibits platelet activation. J Pharmacol Exp Ther 369, 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hom S, Chen L, Wang T, Ghebrehiwet B, Yin W & Rubenstein DA (2016) Platelet activation, adhesion, inflammation, and aggregation potential are altered in the presence of electronic cigarette extracts of variable nicotine concentrations. Platelets 27, 694–702. [DOI] [PubMed] [Google Scholar]


