Abstract
At the end of 2019, a new coronavirus disease, COVID‐19, emerged and quickly spread around the world. Severe acute respiratory syndrome Coronavirus 2 (SARS‐CoV‐2), the causative virus of this disease, belongs to the β‐coronavirus family, together with SARS and middle east respiratory syndrome, and has similar biological characteristics to these viruses. For obstetricians, the susceptibility and prognoses of pregnant women and the effects of the infection on the fetus have been the focus of attention; however, at present, the seriousness of the disease in pregnant women is not apparent, and COVID‐19 does not increase the rate of miscarriage, stillbirth, preterm labor or teratogenicity. Even so, carriers might transmit SARS‐CoV‐2 to pregnant women. Thus, we must keep in mind that all medical personnel must understand and maintain standard precautions in their clinical and laboratory practices.
Keywords: COVID‐19, pregnancy, SARS‐CoV‐2
Disaster strikes when we least expect it. (1929, Terada Torahiko)
It is easy to underestimate things or to be overly afraid of them, but it is difficult to justly evaluate them. (1935, Terada Torahiko)
Introduction: An Emerging Infection
On December 30, 2019, the Chinese Health Agency was made aware of “unexplained pneumonia” in Wuhan, Hubei Province, and identified its pathogen as a new coronavirus (2019‐nCoV). The genome of this virus was quickly sequenced, and genetic similarity to severe acute respiratory syndrome (SARS)‐CoV was noted. 1 , 2
Later, the WHO decided to name the emerging viral disease COVID‐19, and the name of the virus was designated Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) by the International Virus Naming Committee due to its high homology with SARS‐CoV. It spread from China to the whole world, and the WHO declared a pandemic on March 11. On April 8, the government of Japan declared a state of emergency for seven prefectures (Tokyo, Saitama, Chiba, Kanagawa, Osaka, Hyogo and Fukuoka), and the state of emergency was expanded throughout Japan on April 16. In a very short period of time, COVID‐19 had spread throughout the world, and as of May 16, a total of 4 660 611 patients and 309 710 deaths have been confirmed in 215 countries, areas or territories. 3 The number of new cases has already declined in China, where the epidemic first began. In regions of the Middle East and Europe, including Iran, Italy, Spain and France, where it had subsequently spread, the number of cases is also on the decline. Although the U.S. and UK are past their peaks on the infection curve, a large number of cases are still being reported. 4 Furthermore, although the disease spread to Japan early in the pandemic, the number of patients and deaths is still low. 5 Thus, it is unique and puzzling that no spike in the infection rate took place in Japan, even though the country did not implement strict urban blockades. To date, no one can adequately explain why the infection and mortality rates are low in Japan.
What is SARS‐CoV‐2?
Coronaviruses (CoVs) possess an enveloped, single, positive‐stranded RNA genome, which encodes four membrane proteins, namely, spike (S), envelope (E), membrane (M) and nucleocapsid (N) proteins 3–5. S proteins are essential for viral entry into host cells and determine viral pathogenicity. 6 In addition to the four types of coronaviruses that cause the common cold, two other types of coronaviruses were reported in the 21st century: SARS‐CoV, the pathogen underlying the SARS epidemic in 2003, and middle east respiratory syndrome (MERS)‐CoV, the pathogen underlying the MERS epidemic in 2012. SARS‐CoV‐2 has higher genetic homology with SARS‐CoV and MERS‐CoV than with the other four coronaviruses and has conserved sequences responsible for immunomodulation. To date, numerous coronaviruses have been identified in various wild and domestic animals. However, humans are not susceptible to these coronaviruses. From genetic analysis, SARS‐CoV‐2 developed zoonotic infectivity in animals such as bats and curlews toward human‐to‐human infection in a short period of time. This fact completely matches the theoretical prediction of most emerging human infections derived from zoonotic ones. 7
Genetic variants of SARS‐CoV‐2
Early reports from China have shown two major SARS‐CoV‐2 genome types (L and S). 8 Although the S type has been suggested to be the ancestral type, the most prevalent type in Wuhan was the L type. The evolutionarily older but less aggressive S types have relatively weak selection pressures, so their numbers have been decreased. 9 On the other hand, the S type spread to other cities in China as well as Japan during the urban blockade of Wuhan. However, after global spread, both the S and L types were detected in Japan. Phylogenetic analyses are being performed to obtain real‐time information on molecular epidemiology. On Apr 18, a molecular epidemiological report was published from Iceland, where viral subtypes could be determined in every patient. Based on this article, each country has a slightly different type of virus that is prevalent. East Asia has mainly the A and B types, the west coast of the United States has B1a types, Europe has mostly A2a types, and Iceland seems to have additional types, such as A2a3a, which branched off from Europe (all of these types appear to have originated in China). These data suggest that certain viral variants tend to favor certain ethnicities (i.e., viruses with certain variants are more prevalent in certain ethnicities). 10
Pathophysiology of COVID‐19
SARS‐CoV‐2 enters susceptible host cells via the Angiotensin Converting Enzyme (ACE)2 protein as its receptor. ACE2 is abundantly expressed in the type II alveolar epithelium of the lower respiratory tract but is also expressed in the upper respiratory tract, pharynx and gastrointestinal tract, especially the small intestine. 11 Viruses reach mucous membranes through droplets or via fingers that touch the surfaces of contaminated objects and infect susceptible cells. An ACE2‐independent pathway of infection via dipipetidyl peptidase 4(DPP4) and or glucose‐regulated protein 78(GPR78) also may exists in various cells including lymphocytes, which induces transient immunodeficiency; however, the details of this process are unknown. 12 Viremia is not frequently observed, but we cannot rule out blood‐borne dissemination of SARS‐CoV‐2 into various organs.
In contrast to children and young individuals who are less susceptible to COVID‐19, older adults and those with complications such as diabetes mellitus and hypertension have the highest mortality rates. 13 Although the majority of infected individuals are asymptomatic or have only mild symptoms, they might spread virus via cough, saliva and stool. After periods of incubation of up to 14 days, symptoms including fever, cough and malaise might appear. The asymptomatic carrier state is important because they also spread virus into the air and surroundings.
In severe COVID‐19 cases, cytokine storms are thought to be associated with systemic tissue destruction and subsequent poor prognoses. 14 However, whether cytokine storms are more prevalent in the older adults than young people is unknown, in contrast to the novel influenza A of 2009, which evoked cytokine storms in younger people. 15 Fortunately, there are no reports of pregnant women being prone to cytokine storms caused by COVID‐19.
An autopsy report of a 85‐year‐old Chinese male patient showed diffuse alveolar injury and pulmonary edema with infiltration of multiple lymphocyte types into the interstitial area.
Infected cells showed remarkable degeneration associated with nuclear enlargement, amphibole granules, multinucleation or syncytium formation. The increase in peripheral CCR4+ CCR6+ Th17 cells and perforin‐ and granzyme‐containing CD8+ T cells suggests that over activation of cytotoxic T cells might be involved in tissue destruction.
One of the most characteristic features is rapid exacerbation in some cases. Possible causes of rapid progression from relatively mild cold‐like symptoms to acute respiratory failure are presumed to be microthrombi in the lungs. 17 Morphologically, SARS‐CoV‐2 has been reported to proliferate in the vascular endothelium, 18 which can be a rationale for the effectiveness of anticoagulation therapy.
COVID‐19 and pregnancy
Two major concerns of pregnant individuals with COVID‐19 are their prognoses and the possibility of vertical transmission and subsequent miscarriage, malformations, fetal growth restriction and/or stillbirth. The worldwide common misunderstanding is that “pregnancy is an immunosuppressed condition, and pregnant subjects become susceptible to infectious disorders”. This is not true because pregnant subjects reject allograft transplants and produce an effective immune response against infectious pathogens. As reported by our group, 19 and others, in contrast to organ transplantation, which requires constant immunosuppression, a successful pregnancy requires a robust, dynamic and responsive immune system. In general, pregnant mothers become tolerant to fetoplacental semi‐allografts but maintain an adequate immune response to pathogens. Enhanced induction of interferon (IFN)‐β is crucial to protecting the fetus against viral infections at the maternal–fetal interface; thus, placental viral infections might threaten fetal survival even if they do not enter fetal tissues. One immunological concern is the suppressive activity of SARS‐CoV‐2 on IFN responses for its escape from the immune system. 20 However, fortunately, clinical data suggest no deleterious outcomes of pregnant women who are infected with COVID‐19 during pregnancy compared with those infected with SARS‐CoV or MERS. 21 The first case report from Wuhan described nine cases of pregnant women diagnosed with COVID‐19 in late pregnancy. 21 Their clinical courses and disease severities were not different from those of nonpregnant women, and no cases of intrauterine infection of their fetuses were observed. In another report from Wuhan, of 13 pregnant women who developed COVID‐19 during pregnancy, one woman delivered a dead fetus at 34 weeks of gestation, but the cause of fetal death was speculated to be severe maternal pneumonia and multiple organ failure rather than viral infection of the fetus. 22 Another report showed that 3 of 33 pregnant women who developed COVID‐19 during pregnancy in Wuhan showed evidence of intrauterine infection by cord blood PCR test. 23 Two of three suspected intrauterine infections were asymptomatic, but one neonate who was delivered at 31 weeks of gestation by emergency cesarean section due to maternal pneumonia had complications including severe pneumonia and sepsis, though the neonate was ultimately rescued. 23 Another report from Wuhan showed a relatively high incidence of intrauterine infection of up to 33% (two of six cases of late cesarean section) by cord blood IgM test. 24 On the other hand, no cases of intrauterine infection were observed from 106 deliveries at 25 hospitals in various cities in China from January to March, although 8% of 116 cases had serious pneumonia. 25 In every case, the mothers and neonates survived without serious sequelae. Only one woman had early spontaneous miscarriage, but no causal relationship was identified. 25 This discrepancy might reflect the difficulty of serum IgG and IgM testing because most studies employed less sensitive immunoblot detection systems. A systematic review by Zaigham 26 evaluated the risk of serious maternal outcomes in 2.4% (3 of 108 cases) and 1.8% of neonates (one neonatal death and one intrauterine fetal death). Although the total death rate in Italy is higher than that in China, the prognoses of pregnant women in Italy are almost comparable to those in China. 27 Ferrazzi et al. reviewed 42 pregnant women who were diagnosed with COVID‐19 among 7000 deliveries from February to April in Lombardy. There were two preterm births. Seven of the 42 pregnant patients became seriously ill and were admitted to the ICU, and all recovered quickly. No fetal or neonatal death was observed. However, a case report from Texas presented severe neonatal pneumonia caused by intrauterine COVID‐19 infection, 28 and there have been other tragedies. For example, a case of intrauterine fetal death at 31 weeks of gestation and maternal death due to severe pneumonia was reported in Iran. 29 Yan et al. reviewed 116 cases and concluded that COVID‐19 did not increase the risk of spontaneous preterm birth before 37 weeks of gestation. 25 Among 43 COVID‐19 PCR‐positive women in New York, 86% had mild disease, 9.3% had severe disease, and 4.7% had severe disease. 30 Another study reported that cases of COVID‐19 pneumonia during pregnancy were milder and that patients had a better recovery. 31 However, a very recent multicenter study from Iran reported the deaths of 7 of 9 pregnant women who contracted COVID‐19 in the late second and third trimesters. 32 The death rates can be influenced by local factors, including medical accessibility, economic and political conditions and social hygiene. We need to collect and analyze possible risk factors for maternal and neonatal outcomes to reduce the mortality rates of both mothers and infants.
The experience in the US has been more complex; the initial cases followed similar prognosis as those reported in Wuhan; unfortunately the US has been experienced a significant higher number of cases, including pregnant women. The most recent report from 12 US institutions evaluated 64 pregnant women hospitalized with COVID‐19, 69% had severe and 31% critical disease. Some of these patients had pre‐existing comorbidities including 25% with pulmonary condition and cardiac disease. Gestational age at symptom onset was at a mean of 29 weeks and hospital admission at 30 weeks. Eighty eight percent of pregnant women with critical COVID‐19 who delivered during their disease course were delivered preterm, 94% of them via cesarean. In all 75% of critically ill woman delivered preterm. There were no reports of still births or neonatal deaths or cases of vertical transmission. 33
Placental infection with COVID‐19 may be the critical factor that may lead to pregnancy complications. Although few, new reports have shown the presence of SARS‐CoV‐2 within the placenta correlating with pregnancy complications such as miscarriage and pre‐eclampsia. 34 Hosier et al reported a case of second trimester pregnancy with symptomatic COVID‐19 complicated by pre‐eclampsia and placental abruption. When they analyze the placenta they found the presence of SARS‐CoV‐2 localized predominantly to syncytiotrophoblast cells at the maternal‐fetal interface of the placenta. 35
Vertical transmission
While early studies showed no evidence of vertical transmission of SARS‐CoV‐2 from mother‐to‐child in late pregnancy, 21 recent reports have shown possible in utero transmission. 24 , 36
Possible cases of postpartum infections emphasize the importance of adequate physical separation between mothers and neonates. 37 The presence of cord blood IgM is direct evidence of vertical transmission. One study reported a case in which anti‐SARS‐CoV‐2 IgM antibody was present at 2 h after birth. 36 The importance of the proper definition of vertical transmission is critical for understanding the risks associated with fetal development and maternal mortality. Although a few studies have shown the presence of the virus at the placenta, these findings cannot be considered as vertical transmission. We define vertical transmission when the virus is able to reach fetal organs and they can be detected in fetal organs. Presence of the virus at the placenta cannot be considered as vertical transmission. The function of the placenta as an immunological barrier includes the fact that can be infected but prevents the crossing of the virus from the maternal side into the fetal side. 38
Diagnosis of COVID‐19
Currently, there are four methods used to diagnose COVID‐19: (i) Detection of viral RNA, (ii) Detection of specific antibodies in the blood, and (iii) Detection of ground‐glass opacities in the lungs by CT scanning, (iv) Detection of viral antigens. To detect viral nucleic acids, real‐time PCR or loop‐mediated isothermal amplification (LAMP) are the most common and sensitive methods for clinical specimens, but both methods are time‐consuming, require extensive effort and have a high false‐negative rate. Blood antibodies are not currently recommended due to reliability issues, such as the fact that it takes more than 1 week for antibody conversion even after infection; additionally, IgM antibodies are not detected in the early stages. 39 The sensitivity and specificity of CT are good, but CT is not recommended for pregnant women due to radiation exposure; furthermore, CT cannot distinguish COVID‐19 from other viral pneumonias. Very recently, detection of viral antigens by the Immunoblot with saliva have been reported as a novel method. However, its sensitivity is still low compared to PCR and needs to be improved to make it as a routine examination. 40
Treatment of COVID‐19 in pregnant patients
At this time, there is no specific medicine or effective vaccine for COVID‐19. Mechanisms of SARS‐CoV‐2 replication and the sites of expected antiviral medicines are shown in Figure 1. The current therapeutics that are expected to be effective and undergoing clinical trials are listed in Table 1. A cocktail of protease inhibitors (Kaletra [lopinavir/ritonavir]) was tested in the first clinical trials in China. Lopinavir and ritonavir were originally used against HIV‐1 and are effective. Based on their efficacy against MERS in vitro and in animal models, they have been expected to be effective against COVID‐19; however, they were not effective against COVID‐19 in 199 patients with severe disease in China. A randomized trial showed no significant difference in either clinical improvement or 28‐day mortality. 41
Figure 1.
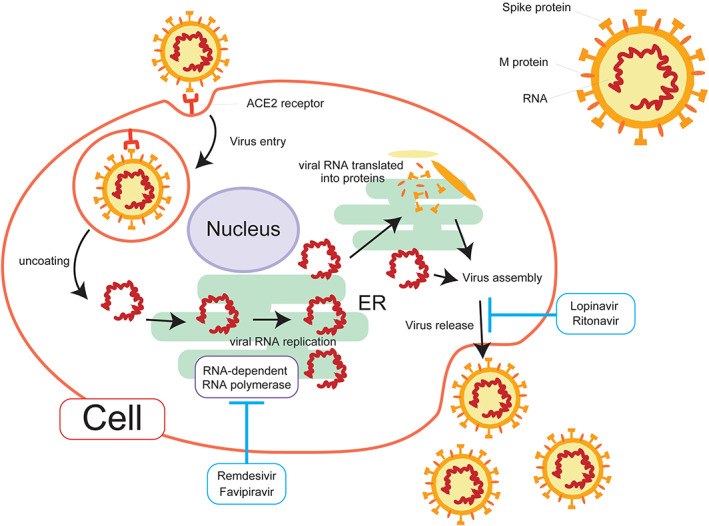
Replication of Severe acute respiratory syndrome Coronavirus 2 (SARS‐COV‐2) in a competent cell. The envelope spoke glycoprotein of SARS‐CoV‐2 binds its cellular receptor angiotensin‐converting enzyme 2 (ACE2). After membrane fusion, the viral RNA genome is released into the cytoplasm. The RNA is uncoated to tlans‐locate into endoplasmic reticulum. The viral genome is then replicated by RNA dependent RNA polymerase. Remdesivir and Fabipiravor inhibit this process. Viral proteins are independently synthesized from RNA genome and assembled in cytoplasm, Virions are then released. Ritonavir+Lopinavir, proteinase inhibitors act this stage.
Table 1.
Possible therapeutics for COVID‐19 during Pregnancy
| Description | Licensed for | Administration | Status of Use in Japan | Indication for pregnant patients | |
|---|---|---|---|---|---|
| Corticosteroids | Analogues of steroid hormones | Bronchial asthma etc. | Inhalted | Ongoing clinical trial | Yes |
| Hydroxy chloroquine | Malaria, Systemic lupus erythematosus | Malaria, SLE etc. | Oral or intravenous | Ongoing clinical trial | Yes |
| Ritonavir+Lopinavir(Kaletra) | Protease inhibitors | HIV/AIDS | Oral | Ongoing clinical trial | Yes |
| Favipiravir (Avigan) | RNA polymerase inhibitor | Influenza | Oral | Ongoing clinical trial |
No (teratogenous) |
| Convalescent plasma | Polyclonal human antibodies | Intravenous | Not obtainable in Japan | Yes | |
| Remdesivir | RNA polymerase inhibitor | Various RNA virus infections | Intravenous | Covered by National Health Insurance | Yes |
| Tocilizumab | Anti IL‐6 monoclonal Ab | Rheumatoid arthritis | Intravenous | Ongoing clinical trial | Yes |
Favipiravir, a selective inhibitor of viral RNA polymerase, was developed to treat novel influenza. Based on its broad spectrum antiviral efficacy against various RNA viruses, including Ebola, clinical trials are ongoing. A nonrandomized controlled trial from China reported shorter virus disappearance times. 42 Many case reports are available on the website of the Japan Society for Infective Diseases. 43 However, favipiravir is contraindicated in pregnant patients as well as men and women who wish to conceive due to its strong teratogenicity.
Remdesivir is an RNA‐dependent RNA polymerase inhibitor that is active against a wide range of RNA viruses. It was originally developed for the treatment of Ebola virus infection and shows good activity against COVID‐19 in vitro. Foreign and domestic clinical reports show its possible clinical importance, but an RCT of remdesivir was discontinued in China. 44 Although remdesivir may be effective against COVID‐19, serious side effects, including severe liver dysfunction, diarrhea, skin rash and renal dysfunction, are frequent.
Hydroxychloroquine, a derivative of the antimalaria drug chloroquine, is widely used in European and American countries. Clinical studies of hydroxychloroquine as a single agent or as an adjunctive therapy are underway. In an early nonrandomized controlled trial in France, hydroxychloroquine in combination with Azithromycin significantly reduced the viral road, 45 but other studies failed to show this effect. 46
Ciclesonide is an inhaled steroid, originally indicated for the treatment of bronchial asthma. In addition to its anti‐inflammatory effects, its specific antiviral activity has been reported. 47 In addition to many case reports, clinical trials are ongoing in Japan.
Tocilizumab, a humanized anti‐human IL‐6 receptor monoclonal antibody, has been used for rheumatoid arthritis and other collagen diseases. Its effectiveness against COVID‐19 has been reported in China. A single‐arm study showed of a higher survival rate for patients with severe pneumonia. 48
One important feature of COVID‐19 is its effect on the blood coagulation system. The Royal College of Obstetricians and Gynecologists (RCOG) advised clinicians that infection with COVID‐19 may increase the risk of venous thromboembolism and that this risk may be compounded by reduced mobility due to self‐separation. Thus, RCOG recommended that pregnant individuals hospitalized with COVID‐19 should receive prophylactic low‐molecular‐weight heparin to reduce the risk of pulmonary embolism. 49
Mode of delivery for COVID‐19 patients at term
Based on initial reports from China, every term or premature case with COVID‐19 with acute distress and/or severe maternal conditions was delivered by cesarean section. However, in an Italian study, among 42 deliveries, 24 (57%) women delivered vaginally, with three cases undergoing induction of labor for obstetric reasons, while elective cesarean section was performed in 18 (43%) cases: in 8 cases, the indication was unrelated to COVID‐19 infection, but in 10 cases, the indications were worsening dyspnea or other COVID‐19‐related symptoms. 50 A systematic review showed that overall, 90.1% of deliveries were cesarean sections. It is reasonable to choose cesarean section to reduce the opportunity for viral transmission from pregnant subjects with COVID‐19 to other patients and medical staff, but vaginal delivery must be chosen if the woman is multiparous and the uterine cervix has been fully open to complete the delivery sooner. Guidelines from various countries are controversial in terms of the choice of delivery mode. This is because it depends on the availability of medical assets as well as human resources.
Advice for pregnant and expectant mothers and those who wish to become pregnant
Both obstetricians and general practitioners as well as health care professionals are requested to advise pregnant women on how to avoid SARS‐CoV‐2 infection and that if they do become infected, these women need not be afraid because we have a general understanding of the emerging infection and disease. First, pneumonia might be more severe in pregnant women, regardless of COVID‐19, because the enlarged uterus lifts the diaphragm and compresses the lungs, inhibiting ventilation and making the lungs more prone to congestion. Thus, pregnant women need to avoid any chance of infection as much as possible. To prevent SARS‐CoV‐2 infection, they are recommended to not leave their house unnecessarily, avoid crowds and wash their hands frequently. They can consult with their employer regarding the type of work they perform and their work environment. Although it is desirable to wear a mask when going out to prevent droplet infection, the WHO does not regard wearing a mask as being effective against preventing droplet infection in healthy people but a mask is effective only in preventing people with symptoms from spreading droplets around them. As the virus is excreted in the feces, women must be sure to wash their hands with soap after toilet use and before eating. Frequent hand washing and disinfection with alcohol after touching a touch panel, such as an ATM, in a public place or after touching a train strap or handrail is recommended.
If patients suspect that they have COVID‐19 and wish to be examined, they should first consult the Consultation Center for Returnees and Contact Persons to prevent the spread of infection. They are not recommended to visit medical institutions except for emergencies because infection among patients in some institutions could trigger local outbreaks. The best way to protect them is to ensure satisfactory communication with their primary care physicians by telephone or email for online treatment, prescriptions and appropriate advice. We have published guidelines for pregnant women on the website of the Japanese Society of Infectious Diseases in Obstetrics and Gynecology. 51
Estimated numbers of pregnant patients with COVID‐19
Putra et al. used mathematical epidemiology to predict the number of pregnant women infected with COVID‐19 in the United States. 52 Their projections suggest the likelihood of 860 475 infections, including in 16 601 pregnant women, in the U.S. Between March and December of this year, among pregnant women infected with SARS‐CoV‐2, 3308 might develop serious disease, 681 cases may develop critical disease, and 52 patients might die.
Although there is no need to panic because the medical system in the U.S. is capable of dealing with the disease and its treatment capacity, they recommend that citizens be careful to avoid contracting the disease. 52
The unique governmental policy in Japan has been effective in preventing explosive infection. As of May 17, there was no urban lockdown, only relatively moderate furlough control, cluster control and PCR testing that is limited to those who need it; however, the numbers of new patients and COVID‐19 deaths remain among the lowest in the world. Some argue that PCR‐based testing should be performed on the entire population, even if they are asymptomatic, but the financial cost, low positivity rate and limited medical resources and manpower make this approach impractical. The epidemic in Guangdong, far from Wuhan, occurred when the virus was imported from outside the city and was largely controlled by traffic blocks. Overall, out of approximately 1.6 million people tested by PCR, only 1300 were infected, or less than one in 1000 people in Guangdong. Thus, contact restrictions rather than increasing PCR‐based testing is more effective in preventing the spread of infection. 53
Conclusion
Human history has experienced numerous epidemics, including the plague of the 13‐14 th century; the Columbian exchange of measles, smallpox and syphilis in the 16th century; and cholera in the late 19th century. 54 The COVID‐19 pandemic is the first pandemic in 100 years, since the Spanish influenza pandemic of 1918. However, in the last 100 years, we have developed new weapons against infection based on microbiology, immunology and molecular biology. With these weapons, we can fight new enemies in the clinical arenas of obstetrics and infectious disease control with a speed and precision that are incomparable to those of the early 20th century. We believe that victory can be achieved by using human wisdom and abandoning conspiracy theories, such as biological weapons and the economic, ideological and religious positions of each country, to fight the true enemy.
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1. Acter T, Uddin N, Das J, Akhter A, Choudhury TR, Kim S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) as coronavirus disease 2019 (COVID‐19) pandemic: A global health emergency. Sci Total Environ 2020; 730: 138996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riou J, Althaus CL. Pattern of early human‐to‐human transmission of Wuhan 2019 novel coronavirus (2019‐nCoV), December 2019 to 2020. Euro Surveill 2020; 25(4): 2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID‐19 Coronavirus Pandemic. Available from URL: https://www.worldometers.info/coronavirus/?utm_campaign=homeAdvegas1?%22%20%5Cl%22countries
- 4.Johns Hopkins University Coronavirus Resource Center. Available from URL: https://coronavirus.jhu.edu/
- 5.Ministry of health labor and welfare. About Coronavirus Disease 2019 (COVID‐19). Available from URL: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/newpage_00032.html
- 6. Magrone T, Magrone M, Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin‐converting enzyme 2 as a potential drug target—a perspective. Endocr Metab Immune Disord Drug Targets 2020. Published online: E‐Pub Apr 27. 10.2174/1871530320666200427112902. [DOI] [PubMed] [Google Scholar]
- 7. Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature 2007; 447: 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of SARS‐CoV‐2 genomes. Proc Natl Acad Sci U S A 2020; 117: 9241–9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiaolu Tang CW, Li X, Song Y et al. On the origin and continuing evolution of SARS‐CoV‐2. Natl Sci Rev 2020;7(6): 1012–1023. 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gudbjartsson DF, Helgason A, Jonsson H et al. Spread of SARS‐CoV‐2 in the Icelandic population. N Engl J Med 2020. Published online: E‐Pub Apr 14; 382: 2302–2315. 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamers MM, Beumer J, van der Vaart J et al. SARS‐CoV‐2 productively infects human gut enterocytes. Science 2020. (Jul 3); 369(6499): 50–54. 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gelman R, Bayatra A, Kessler A et al. Targeting SARS‐CoV‐2 Receptors as a means for reducing infectivity and improving antiviral and immune response: An algorithm‐based method for overcoming resistance to antiviral agents. Emerg Microbes Infect 2020. Dec; 9(1): 1397–1406. 10.1080/22221751.2020.1776161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun H, Ning R, Tao Y et al. Risk factors for mortality in 244 older adults with COVID‐19 in Wuhan, China: A retrospective study. J Am Geriatr Soc 2020. Published online: E‐Pub May 8; 68: E19–E23. 10.1111/jgs.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘cytokine Storm’ in COVID‐19. J Infect 2020; 80: 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Short KR, Kedzierska K, van de Sandt CE. Back to the future: Lessons learned from the 1918 influenza pandemic. Front Cell Infect Microbiol 2018; 8: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu QWR, Qu GQ, Wang YY et al. Gross examination report of a COVID‐19 death autopsy. Fa Yi Xue Za Zhi 2020; 36: 21–23. [DOI] [PubMed] [Google Scholar]
- 17. Mucha SR, Dugar S, McCrae K et al. Coagulopathy in COVID‐19. Cleve Clin J Med 2020. (Jun 10). 10.3949/ccjm.87a.ccc024. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 18. Varga Z, Flammer AJ, Steiger P et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020; 395: 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 2017; 17: 469–482. [DOI] [PubMed] [Google Scholar]
- 20. Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer‐Smadja N. Type 1 interferons as a potential treatment against COVID‐19. Antiviral Res 2020; 178: 104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen H, Guo J, Wang C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020; 395: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Y, Chen H, Tang K, Guo Y. Clinical manifestations and outcome of SARS‐CoV‐2 infection during pregnancy. J Infect 2020. (Mar 4). 10.1016/j.jinf.2020.02.028. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeng L, Xia S, Yuan W et al. Neonatal early‐onset infection with SARS‐CoV‐2 in 33 neonates born to mothers with COVID‐19 in Wuhan, China. JAMA Pediatr 2020. (Mar 26);174(7): 722–725. 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeng H, Xu C, Fan J et al. Antibodies in infants born to mothers with COVID‐19 pneumonia. JAMA 2020; 323(18): 1848–1849. 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yan J, Guo J, Fan C et al. Coronavirus disease 2019 (COVID‐19) in pregnant women: A report based on 116 cases. Am J Obstet Gynecol 2020; .223(1): 111.e1–111.e14. 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID‐19: A systematic review of 108 pregnancies. Acta Obstet Gynecol Scand 2020. Published online: E‐Pub Apr 7; 99: 823–829. 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferrazzi EM, Frigerio L, Cetin I et al. COVID‐19 obstetrics task force, Lombardy, Italy: Executive management summary and short report of outcome. Int J Gynaecol Obstet 2020. Published online: E‐Pub Apr 8; 149: 377–378. 10.1002/ijgo.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alzamora MC, Paredes T, Caceres D, Webb CM, Valdez LM, La Rosa M. Severe COVID‐19 during pregnancy and possible vertical transmission. Am J Perinatol 2020; 37(8): 861–865. 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karami P, Naghavi M, Feyzi A et al. Mortality of a pregnant patient diagnosed with COVID‐19: A case report with clinical, radiological, and histopathological findings. Travel Med Infect Dis 2020. Published online: E‐Pub Apr 11: 101665. 10.1016/j.tmaid.2020.101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Breslin N, Baptiste C, Gyamfi‐Bannerman C et al. COVID‐19 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of new York City hospitals. Am J Obstet Gynecol MFM 2020. Published online: E‐Pub Apr 9; 2: 100118. 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu D, Li L, Wu X et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID‐19) pneumonia: A preliminary analysis. Am J Roentgenol 2020; 215(1): 127–132. 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 32. Hantoushzadeh S, Shamshirsaz AA, Aleyasin A et al. Maternal death due to COVID‐19 disease. Am J Obstet Gynecol 2020; 223(1): 109.e1–109.e16. 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pierce‐Williams RAM, Burd J, Felder L et al. Clinical course of severe and critical COVID‐19 in hospitalized pregnancies: A US cohort study. Am J Obstet Gynecol MFM 2020. (May 8): 100134. 10.1016/j.ajogmf.2020.100134. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baud D, Greub G, Favre G et al. Second‐trimester miscarriage in a pregnant woman with SARS‐CoV‐2 infection. JAMA 2020. Published online: E‐Pub Apr 30; 323: 2198. 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hillary Hosier SF, Morotti R, Deshmukh U et al. SARS‐CoV‐2 infection of the placenta. J Clin Invest 2020. 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dong L, Tian J, He S et al. Possible vertical transmission of SARS‐CoV‐2 from an infected mother to her newborn. JAMA 2020; 323(18): 1846–1848. 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qiao J. What are the risks of COVID‐19 infection in pregnant women? Lancet 2020; 395: 760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol 2015; 73: 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vogel G. First antibody surveys draw fire for quality, bias. Science 2020; 368: 350–351. [DOI] [PubMed] [Google Scholar]
- 40. Lippi G, Mattiuzzi C, Bovo C, Plebani M. Current laboratory diagnostics of coronavirus disease 2019 (COVID‐19). Acta Biomed 2020; 91: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cao B, Wang Y, Wen D et al. A trial of Lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med 2020; 382: 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cai Q, Yang M, Liu D et al. Experimental treatment with Favipiravir for COVID‐19: An open‐label control study. Engineering (Beijing) 2020. (Mar 18). 10.1016/j.eng.2020.03.007. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.COVID‐19 Case Reports (In Japanese). Available from URL: http://www.kansensho.or.jp/modules/topics/index.php?content_id=31. The Japanese Association for infectious diseases. Available from URL: http://www.kansensho.or.jp/
- 44. Li Z, Wang X, Cao D, Sun R, Li C, Li G. Rapid review for the anti‐coronavirus effect of remdesivir. Drug Discov Ther 2020; 14: 73–76. [DOI] [PubMed] [Google Scholar]
- 45. Gautret P, Lagier JC, Parola P et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: Results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents 2020. (Mar 20): 105949. 10.1016/j.ijantimicag.2020.105949. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46. Geleris J, Sun Y, Platt J et al. Observational study of hydroxychloroquine in hospitalized patients with Covid‐19. N Engl J Med 2020. Published online: E‐Pub May 7; 382: 2411–2418. 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matsuyama S, Kawase M, Naganori N et al. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. bioRxiv 2020. Published online: E‐Pub. 10.1101/2020.03.11.987016. [DOI] [Google Scholar]
- 48. Xu X, Han M, Li T et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020. Published online: E‐Pub Apr 29; 117: 10970–10975. 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49."Coronavirus (COVID‐19) infection and pregnancy Version 10 ". Royal College of Obstetricians & Gynaecologists. 4 Jun 2020. Retrieved 2020‐6‐10.
- 50. Ferrazzi E, Frigerio L, Savasi V et al. Vaginal delivery in SARS‐CoV‐2 infected pregnant women in Northern Italy: a retrospective analysis. BJOG 2020. Published online: E‐Pub Apr 27. 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Notice to Pregnant Women and Those Wishing to Become Pregnant. Japanese Society of Infectious Diseases in Obstetrics and Gynecology. Available from URL: http://jsidog.kenkyuukai.jp/information/information_detail.asp?id=102820
- 52. Putra M, Kesavan MM, Brackney K, Hackney DN, Roosa MKM. Forecasting the impact of coronavirus disease during delivery hospitalization: An aid for resources utilization. Am J Obstet Gynecol MFM 2020. Published online: E‐Pub Apr 25: 100127. 10.1016/j.ajogmf.2020.100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lu J, du Plessis L, Liu Z et al. Genomic epidemiology of SARS‐CoV‐2 in Guangdong Province, China. Cell 2020. Published online: E‐Pub Apr 28; 181: 997–1003.e9. 10.1016/j.cell.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aberth S. Plagues in World History (Exploring World History). Lanham, ND: Rowman & Littlefield Pub Inc, 2015. Published online: E‐Pub. [Google Scholar]


