To the Editor,
Convergent arguments suggest that innate immunity related to neutrophils, and in particular neutrophil extracellular traps (NETs), could play a key role in response to SARS‐CoV‐2 infection. 1 The neutrophil‐to‐lymphocyte ratio is a predictor of severity. 2 Neutrophils are involved in the innate response to pulmonary viral infections. 3 The production of NETs (NETosis) is increased by inflammatory mediators and activates macrophages in pulmonary alveoli. 3 , 4 Despite these arguments, no direct evidence of the spread of NETs into circulation has been produced in the early symptomatic phase of COVID‐19 and other viral infections.
We assayed myeloperoxidase‐DNA (MPO‐DNA) and histone‐DNA complexes, two serum markers of circulating NETs, and blood cell counts in 60 consecutive ambulatory subjects attending a screening center for COVID‐19 RT‐PCR examination of nasopharyngeal swab samples. 5 The study was approved by the Institutional Ethical Committee. All subjects were symptomatic with at least two very recent symptoms among fever, dry cough, and dyspnea since <1 week and most had recent contact with infected cases. They were compared to matched asymptomatic controls recruited several months before the epidemy. The sera were used after completion of biochemical testing ordered by the clinician. The remaining samples were stored in the same conditions among groups, at 20°C in the 24 hours following blood withdrawal. Histone‐DNA and MPO‐DNA complexes were measured in serum by the Cell Death Detection ELISA Kit (Roche Diagnostics, Sigma Aldrich), using a biotinylated antibody against histones H1, H2A, H2B, H3, and H4 or MPO and a soluble peroxidase‐labeled anti‐DNA monoclonal antibody, respectively, as described previously. 6 Values were reported in 450 nm absorbance units (AU). Blood cell counts were determined in all subjects. Routine biochemical markers, including hemoglobin, bicarbonate, potassium, sodium, chloride, serum total proteins, C‐reactive protein, urea nitrogen, creatinine, bilirubin, conjugated bilirubin, aspartate aminotransferase, alanine aminotransferase, lactico‐dehydrogenase, gamma‐glutamyl transpeptidase, alkaline phosphatase, and creatine kinase, were assayed in a Cobas 8000 Analyzer (Roche Diagnostics).
RT‐PCR was positive in 34 subjects (56.6%). The mean age (±SD) in positive vs negative groups was 42 ± 17 vs 37 ± 15 (P = .3), and sex ratio (M/F) was 0.88 vs 0.73 (P = .4), respectively. We observed a higher level of MPO‐DNA complexes in symptomatic subjects with positive vs negative RT‐PCR (1.31 ± 0.18 vs 0.89 ± 0.21 AU, P < .001, Figure 1). The levels of MPO‐DNA complexes were 3.1‐fold and 2.1‐fold higher in the two groups, compared to asymptomatic control subjects recruited before the epidemy (0.42 ± 0.08 AU), respectively (P < .001). Eleven symptomatic PCR‐negative subjects had MPO‐DNA levels higher than the 0.95 AU upper limit reported in negative symptomatic subjects. We also observed a clear increase of histone‐DNA complex level in symptomatic subjects compared to controls (0.93 ± 0.25 vs 0.05 ± 0.01, P < .001) but no difference between positive and negative cases (0.94 ± 0.29 vs 0.92 ± 0.20, P = .9). We reported significant associations of MPO‐DNA and histone‐DNA complexes with markers of blood cell counts predicting severity, including neutrophil, lymphocyte, and platelet counts, and neutrophil‐to‐lymphocyte ratio (Figure 2) and no correlation with RT‐PCR. 2 MPO‐DNA was negatively correlated with lymphocytes and platelets, while histone‐DNA was positively correlated with neutrophils and neutrophil‐to‐lymphocyte ratio. The subjects with positive vs negative RT‐PCR had decreased blood counts of leucocytes, platelets, neutrophils, and lymphocytes and increased sodium and bicarbonate. We reported no difference in other routine biochemical markers between the two symptomatic groups (Table S1) and no correlation of these biochemical parameters with blood levels of MPO‐DNA and histone‐DNA complexes.
FIGURE 1.
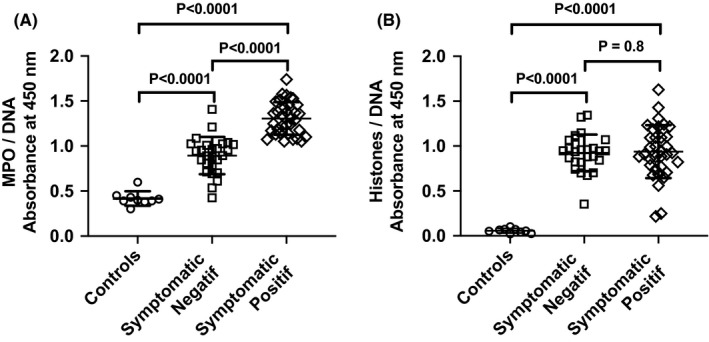
Measurement of myeloperoxidase‐DNA (MPO‐DNA) and histone‐DNA complexes as marker of neutrophil extracellular traps (NETs) in serum of symptomatic ambulatory cases with positive or negative RT‐PCR diagnosis of SARS‐CoV‐2 infection and 9 controls. Values were reported in 450 nm absorbance units. Bars represent mean ± SD. Data were compared by Mann‐Whitney test
FIGURE 2.
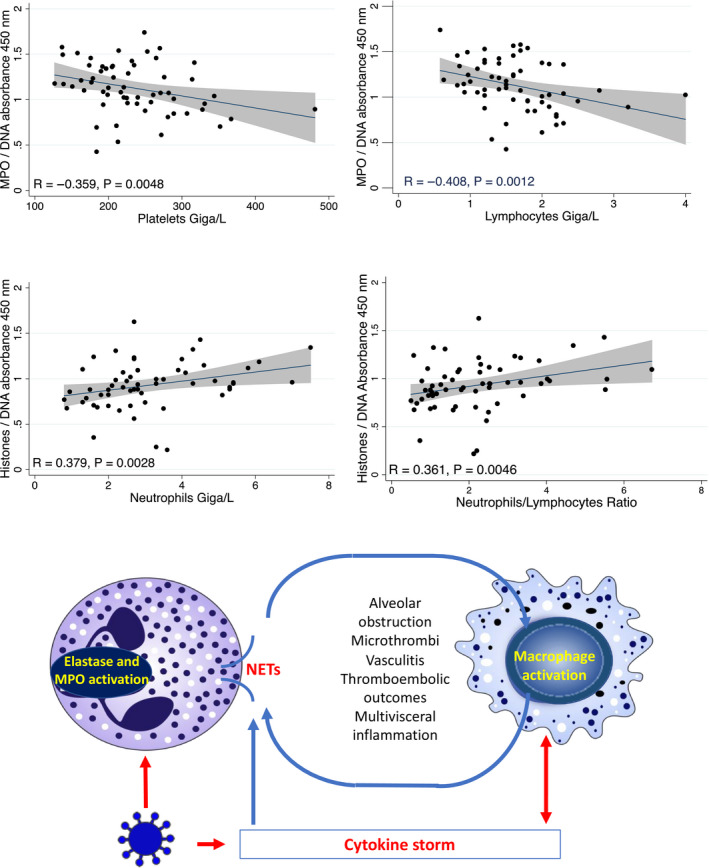
A, (Top): Associations of myeloperoxidase‐DNA (MPO‐DNA) and histone‐DNA complexes with blood counts of lymphocytes, neutrophils, and platelets. Correlations were assessed by Spearman rank correlation. The linear correlation and 95% confidence interval are represented by a line and a gray area, respectively. B, (Bottom): presumed role of NETs in SARS‐CoV‐2 according to pathological mechanisms reported in other viral pneumonias. Red arrows show associations documented in COVID‐19, while blues arrows show mechanisms evidenced in other viral lung infections
The increase of MPO‐DNA and histone‐DNA complexes in blood shows that the release of NETs is involved in the early host answer to COVID‐19. The release could result from neutrophil exposure to virus particles, cytokine production, and/or response to host damage‐associated molecular patterns (DAMPs) produced by infected cells, as reported in other pulmonary viral infections. 3 , 4 Our data also show that MPO‐DNA blood level is a sensitive marker of the early phase of COVID‐19. All positive patients had a level dramatically higher than that observed in controls. Our data suggest to further evaluate MPO‐DNA as a early marker, which could help to discriminate symptomatic patients with false‐negative RT‐PCR from those with negative RT‐PCR related to another viral lung infection, in addition to computed tomography. 7 We estimated the number of COVID‐19 cases with false‐negative RT‐PCR to 11 among the 60 symptomatic subjects, considering the 70%‐80% sensitivity of RT‐PCR usually reported at very early infection step. 7 This estimate is exactly the number of symptomatic negative cases who had a MPO‐DNA blood level similar to that reported in symptomatic positive cases. This hypothesis is likely since these symptomatic negative cases had recent contact with infected cases. The other symptomatic patients with negative RT‐PCR could have other viral infections that increase MPO‐DNA to a lesser extent than COVID‐19. 3 , 4 , 5 , 7 This could explain the intermediate increase of MPO‐DNA complexes in the symptomatic negative group, compared to controls and symptomatic positive subjects. Whether components of NETosis predict and/or contribute to evolution severity of COVID‐19 remains an open question. Low lymphocyte and platelet counts and high neutrophils and neutrophil‐to‐lymphocyte ratio are associated with the risk of severe COVID‐19, in a recent meta‐analysis of 15 studies. 2 The association of MPO‐DNA and histone‐DNA complexes with these blood cell count markers of severity (Figure 2) is in favor of this hypothesis. We suggest evaluating further whether the kinetics of blood level of NETs predict the evolution severity of COVID‐19 in a larger population. NETosis participate to cytokine storm and vascular and tissue damages, including pulmonary alveoli obstruction, microthrombi with vasculitis and thromboembolic manifestations in other viral pneumonias (Figure 2). 3 , 4 , 8 Very recently, a study reported 12 cases with MPO‐DNA levels higher than those of controls among 50 patients hospitalized for COVID‐19, and interestingly, 3 had major changes in oxygen requirement and concomitant increase of MPO‐DNA levels and no increase of citrullinated histone H3‐DNA. 9
In conclusion, our results on a consecutive ambulatory population of COVID‐19 cases suggest that blood level of MPO‐DNA complexes could be a useful biomarker of the early phase of SARS‐CoV‐2 infection. If further studies confirm that the dramatic production of NETs is a pathological mechanism of innate immunity involved in early step of SARS‐CoV‐2 infection, our data could open therapeutic perspectives by targeting NET production with inhibitors already tested in other lung infectious diseases.
CONFLICTS OF INTEREST
All authors have no conflict of interest to declare.
Funding information
This work was financed by FHU ARRIMAGE and the French PIA project “Lorraine Université d’Excellence,” reference ANR‐15‐IDEX‐04‐LUE.
Supporting information
Table S1
ACKNOWLEDGMENTS
This study was registered by the Institutional Review Board of Clinical Research (DRCI) of the University Hospital of Nancy (N° 2020PI087) and approved by the Ethical Committee.
REFERENCES
- 1. Barnes BJ, Adrover JM, Baxter‐Stoltzfus A, et al. Targeting potential drivers of COVID‐19: neutrophil extracellular traps. J Exp Med. 2020;217(6):e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeng F, Li L, Zeng J, et al. Can we predict the severity of COVID‐19 with a routine blood test? Pol Arch Intern Med. 2020;130(5):400‐406. 10.20452/pamw.15331 [DOI] [PubMed] [Google Scholar]
- 3. McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including Interleukin‐6 in COVID‐19 induced pneumonia and macrophage activation syndrome‐like disease. Autoimmun Rev. 2020;19(6):102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18:134‐147. [DOI] [PubMed] [Google Scholar]
- 5. Amrane S, Tissot‐Dupont H, Doudier B, et al. Rapid viral diagnosis and ambulatory management of suspected COVID‐19 cases presenting at the infectious diseases referral hospital in Marseille, France, ‐ January 31st to March 1st, 2020: a respiratory virus snapshot. Travel Med Infect Dis. 2020:101632. 10.1016/j.tmaid.2020.101632. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Popovic B, Zannad F, Louis H, et al. Endothelial‐driven increase in plasma thrombin generation characterising a new hypercoagulable phenotype in acute heart failure. Int J Cardiol. 2019;294:195‐201. [DOI] [PubMed] [Google Scholar]
- 7. Long C, Xu H, Shen Q, et al. Diagnosis of the coronavirus disease (COVID‐19): rRT‐PCR or CT? Eur J Radiol. 2020;126:108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cortjens B, de Boer OJ, de Jong R, et al. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J Pathol. 2016;238:401‐411. [DOI] [PubMed] [Google Scholar]
- 9. Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID‐19. JCI Insight. 2020;5(11):e138999. 10.1172/jci.insight.138999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


