To the Editor,
Seroprevalence studies provide a more accurate picture of coronavirus disease 2019 (COVID‐19) than polymerase chain reaction (PCR)‐confirmed cases as antibodies can be detected in mild or asymptomatic cases who otherwise remain undiagnosed. Seroprevalence can also be used as an indicator of population immunity. The majority of seroprevalence studies to date have been carried out in developed countries. High levels of herd immunity were recently estimated to be needed to control the spread of COVID‐19 in different countries, including Malaysia. 1
The first COVID‐19 case in Malaysia was reported on 25 January 2020, and the main wave occurred between early March and mid‐April. With a national movement control order instituted on March 18, aggressive testing and public health measures, 8354 cases had been reported as of 30 June 2020, 2 or 0.03% of the population. As of June 6, most restrictions had been lifted as part of a phased recovery. We aimed to determine severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) seroprevalence in residual serum samples collected at a teaching hospital serving Kuala Lumpur and Selangor state, which together have reported 4483 cases (51.9% of national cases), or 0.05% of the combined population. 2
We retrieved 816 serum samples sent for diagnostic testing for nonrespiratory infections (mainly dengue) and archived at −20°C. These were divided into periods according to dates of collection: prepandemic (June‐August 2019, n = 228), main wave (29 January to 14 April 2020, n = 327), and postwave (15 April to 6 June 2020, n = 261). For each period, between 17 and 65 samples were included from every 10‐year age group (<10, 10‐19, 20‐29, 30‐39, 40‐49, 50‐59, 60‐69, and >70 years). The samples were from 368 females and 448 males.
Samples were first screened with an in‐house indirect enzyme‐linked immunosorbent assay (ELISA) detecting IgG to SARS‐CoV‐2 receptor binding domain (RBD), and shown to be 100% sensitive for samples collected from 14 days postonset of illness. 3 Screen‐seropositive samples were confirmed with a highly sensitive and specific (99.3%‐100%) surrogate viral neutralization test (sVNT; cPass, GenScript) based on total antibody‐mediated blockage of angiotensin‐converting enzyme 2 receptor‐RBD interaction, 4 , 5 which has received provisional authorization from the Singapore Health Sciences Authority. A two‐step testing process of screening with a highly sensitive assay and confirmation with a highly specific assay is useful for low‐prevalence settings where seropositives have a low predictive value. 5
These two assays were evaluated in our laboratory with the 228 (ELISA) or 26 (sVNT) prepandemic serum samples as negative controls and 35 samples collected from PCR‐confirmed patients with COVID‐19 at least 16 days post‐onset of illness. The sensitivity and specificity rates for the screening ELISA were 97.1% and 88.6%, respectively. For the confirmatory sVNT assay, sensitivity and specificity rates were 100%, after increasing the inhibition cutoff from 20% to 25%, as suggested by the manufacturer after assessing background reactivity in our setting. Our two‐step testing process thus utilized assays with 97% sensitivity (screening) and 100% specificity (confirmatory), exceeding the United States Food and Drug Administration‐recommended minimum sensitivity of 90% and specificity of 95% for serology tests with emergency use authorization. 6 Crude seroprevalence rates are reported with 95% exact binomial confidence intervals (CI) approximated with Poisson distribution.
A total of 46 (7.8%) main wave and postwave samples screened positive, of which three were confirmed by sVNT. Two were from the main wave (seroprevalence 0.6%; 95% CI, 0.07%‐2.2%) and one from the postwave period (0.4%, 95% CI, 0.01%‐2.1%) (Figure 1). Two were from males aged in their 20s with previous diagnoses of COVID‐19. The third was from a 65‐year‐old man with a 7‐day history consistent with COVID‐19, who was not tested for SARS‐CoV‐2. As rates for the main wave and postwave periods were similar, they were combined to give a crude seroprevalence rate of 0.5% (95% CI, 0.1%‐1.5%). Using 2019 age‐ and gender‐stratified population data for Kuala Lumpur and Selangor from the Department of Statistics, Malaysia (http://pqi.stats.gov.my/searchBI.php), a direct age‐standardized seroprevalence rate was calculated as 0.4% (95% CI, 0%‐0.93%).
Figure 1.
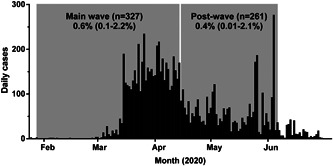
Epidemic curve of COVID‐19 in Malaysia in 2020 from the first reported case on 25 January to 30 June. The periods of serum sampling are shown (main wave, 29 January to 14 April 14; postwave, 15 April to 6 June) with crude seroprevalence rates (95% CI). CI, confidence interval; COVID‐19, coronavirus disease 2019
This study is potentially limited by bias arising from the use of residual inpatients serum. However, the residual serum can provide similar estimates of seroprevalence to cohort studies 7 and is a convenient option when preliminary data are needed during a lockdown. The rate may also be underestimated because antibodies may take 2 weeks to appear and may be undetectable in some mild or asymptomatic cases.
The age‐standardized seroprevalence of 0.4% for Kuala Lumpur and Selangor found in this study is higher than the period prevalence of confirmed cases of 0.05%. This is consistent with other seroprevalence studies revealing 6 to 24 times more COVID‐19 infections than are reported. 8 As this was a single‐center study, a more extensive national serosurvey is necessary to confirm our preliminary indication that Malaysia has experienced limited SARS‐CoV‐2 transmission to date. With little herd immunity, Malaysia remains highly susceptible to COVID‐19 as we emerge from lockdown. Continued vigilance in surveillance and public health measures are critical pending availability of an effective vaccine.
CONFLICT OF INTERESTS
CWT is a patent holder in cPass. The other authors declare no competing interests.
ETHICS STATEMENT
This study was approved by the University Malaya Medical Centre medical ethics committee (no. 2017116‐5794).
ACKNOWLEDGMENTS
We are grateful to Professor Lin‐Fa Wang, Duke‐NUS Medical School, Singapore, and GenScript Biotech for providing the sVNT testing kits.
REFERENCES
- 1. Kwok KO, Lai F, Wei WI, Wong S, Tang J, Tang J. Herd immunity ‐ estimating the level required to halt the COVID‐19 epidemics in affected countries. J Infect. 2020;80(6):e32‐e33. 10.1016/j.jinf.2020.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ministry of Health Malaysia . 2020. Press statement from the Director‐General of Health, 30 June 2020: updates on the coronavirus disease 2019 (COVID‐19) situation in Malaysia. https://kpkesihatan.com/2020/06/30/kenyataan-akhbar-kpk-30-jun-2020-situasi-semasa-jangkitan-penyakit-coronavirus-2019-covid-19-di-malaysia/. Accessed August 8, 2020.
- 3. Chia WN, Tan CW, Foo R, et al. Serological differentiation between COVID‐19 and SARS infections. Emerg Microbes Infect. 2020;9(1):1497‐1505. 10.1080/22221751.2020.1780951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan CW, Chia WN, Qin X, et al. A SARS‐CoV‐2 surrogate virus neutralization test (sVNT) based on antibody‐mediated blockage of ACE2‐spike (RBD) protein‐protein interaction [published online ahead of print July 23, 2020]. Nat Biotechnol. 2020. 10.1038/s41587-020-0631-z [DOI] [PubMed] [Google Scholar]
- 5. Bond K, Nicholson S, Lim SM, et al. Evaluation of serological tests for SARS‐CoV‐2: implications for serology testing in a low‐prevalence setting [published online ahead of print August 06, 2020]. J Infect Dis. 2020. 10.1093/infdis/jiaa467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. US Food & Drug Administration . In Vitro Diagnostics EUAs ‐ Serology Template for Commercial Manufacturers (updated June 26, 2020). https://www.fda.gov/media/137698/download. Accessed August 8, 2020.
- 7. Kelly H, Peck HA, Laurie KL, et al. The age‐specific cumulative incidence of infection with pandemic influenza H1N1 2009 was similar in various countries prior to vaccination. PLoS One. 2011;6(8):e21828. 10.1371/journal.pone.0021828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS‐CoV‐2 in 10 sites in the United States [published online ahead of print July 21, 2020]. JAMA Intern Med. 2020. 10.1001/jamainternmed.2020.4130 [DOI] [PubMed] [Google Scholar]


