Abstract
Introduction
A hyperinflammatory environment has been a hallmark of COVID‐19 infection and is thought to be a key mediator of morbidity. Elevated ferritin has been observed in many patients with COVID‐19. Several retrospective studies have shown ferritin levels can be correlated and predictive of poor outcomes in COVID‐19, though a rigorous analysis has been lacking.
Methods
A retrospective analysis of 942 adult COVID‐19 patients admitted in March 2020 at a large New York City health system with available ferritin levels.
Results
The primary outcome, all‐cause mortality, was observed in 265 (28.1%) patients. Patients who died had a significantly higher median admission and maximum ferritin levels than those who did not. However, death was poorly predicted by admission and maximum ferritin levels on receiver operator curve (ROC) analysis, with AUCs of 0.677 and 0.638, respectively. AUCs increased when the cohort was limited to progressively younger patients. Ferritin levels were minimally better at predicting our secondary outcomes. These included mechanical ventilation, observed in 280 (29.7%) patients with an ROC yielding an area under the curve (AUC) of 0.769, and new renal replacement therapy, observed in 80 (8.5%) of patients with an ROC yielding an AUC of 0.787. We also performed a subset analysis on 22 patients with ferritins >20 000 ng/mL. None of the patients met HLH‐2004 diagnostic criteria. Fifteen (68.2%) of these patients had suspected or confirmed bacterial infections.
Conclusions
Though many patients with COVID‐19 present with hyperferritinemia, elevated ferritin levels are not accurate predictors of outcomes and do not appear to be indicative of hemophagocytic lymphohistiocytosis.
Keywords: COVID‐19, cytokines, ferritin, hemophagocytic lymphohistiocytosis, interleukin‐6
1. INTRODUCTION
The disease outbreak of coronavirus‐19 (COVID‐19) continues to affect a large swath of the global population. However, while the first details of the disease were initially reported in December 2019, there is still much unknown about its pathophysiology. 1 The virus responsible, severe acute respiratory coronavirus 2 (SARS‐CoV‐2), has been shown to cause a constellation of symptoms affecting multiple organ systems. 2 One of the earlier and more dramatic findings has been the association of an acute inflammatory syndrome (“cytokine storm”) associated with COVID‐19, with certain extreme patients having a clinical picture consistent with secondary hemophagocytic lymphohistiocytosis. 3 , 4 Ferritin, the major intracellular iron storage protein, is an acute phase reactant elevated in many inflammatory conditions, including acute infections. 5 Extremely high ferritin levels are the hallmark of hyperferritinemic syndromes, an umbrella term for macrophage activation syndrome, catastrophic antiphospholipid syndrome, adult onset Still's disease, and septic shock. 6 , 7 In general, highly elevated ferritin levels portend poor prognoses in hospitalized patients. 8 There are multiple studies correlating elevated ferritin levels and other pro‐inflammatory markers in COVID‐19 with poor outcomes. 9 , 10 , 11 , 12 , 13 , 14 , 15 Current efforts in treating COVID‐19 include trialing various anti‐inflammatory biologic agents to inhibit this robust immune response. 16 , 17 , 18 , 19 However, the utility of ferritin to predict outcomes has not yet been established. 20 We report here on a large retrospective analysis of patients admitted for COVID‐19, evaluating the predictive value of presentation and maximum ferritin values. In patients with the highest ferritin values, we looked to see if patients met HLH criteria and how they responded to different anti‐inflammatory treatments.
2. METHODS
This study was approved by the Program for Protection of Human Subjects of the Icahn School of Medicine at Mount Sinai. We reviewed the records of all laboratory‐confirmed COVID‐19 patients across a large multi‐hospital New York City health system from March 1 to April 1, 2020. COVID‐19‐positive patients were identified based on a positive reverse‐transcriptase polymerase chain reaction SARS‐CoV‐2 assay of a specimen obtained from a nasopharyngeal swab. Serum ferritin levels were assayed using an electrochemiluminescence immunoassay with a Roche COBAS analyzer system. All included sites used the same means of ferritin measurement. Of all the COVID‐19‐positive patients, only hospitalized patients with a ferritin level available over admission were included in the analysis. Patients who had a ferritin level obtained within 3 days of admission were considered to also have a presentation ferritin. If multiple ferritin levels were obtained, then the one closest to admission was used for the admission ferritin, and the highest ferritin over the admission was chosen as the maximum ferritin.
The primary outcome was all‐cause mortality, with secondary outcomes including the need for renal replacement therapy (RRT) and for invasive mechanical ventilation (intubation). These outcomes were determined based on study investigator's review of the electronic health record (EHR).
We calculated the descriptive statistics used in this study, including percentages and frequencies, along with their 95% confidence intervals for categorical variables. For continuous variables, we used median and interquartile ranges (IQR). Comparison of independent medians and distributions were carried out via the Mann‐Whitney U test.
The specificities and sensitivities of admission and maximum ferritin levels for each outcome of interest across all ferritin cutoffs were calculated. These specificity and sensitivity values were used to generate a receiver operator curve (ROC). We calculated the area under the curve (AUC) for the resultant ROC to evaluate the performance and accuracy of admission and maximum ferritins in predicting each outcome of interest. Youden's J statistic was used to determine the optimum cutoff values of admission ferritins in the prediction of each outcome of interest.
3. RESULTS
3.1. Patient characteristics
From our initial cohort of 2032 COVID‐19 hospitalized patients, we identified 838 patients with an available presentation ferritin level and 942 total patients who had an available ferritin during their admission. The baseline characteristics of our patient population are listed in Table 1. The median duration of follow‐up was 15 days, with a range of 0‐62 days (IQR of 9‐27 days). The median ferritin on presentation and of the maximum value over admission were 711 ng/mL (IQR 318‐1567 ng/mL) and 1148 ng/mL (IQR 446‐2664 ng/mL), respectively. A ferritin >500 ng/mL on presentation was seen in 512 patients (54.4%), and 683 patients (72.5%) had a maximum ferritin >500 ng/mL over their hospital stay. Only 13 patients (1.4%) had a ferritin >10 000 ng/mL on presentation, while 44 patients (4.7%) had a ferritin >10 000 ng/mL at some time over their admission. The primary outcome of interest, all‐cause mortality, occurred in 265 patients (28.1%), while secondary outcomes including the need for mechanical ventilation occurred in 280 patients (29.7%) and for new RRT occurred in 80 patients (8.5%).
TABLE 1.
Baseline characteristics and outcomes
| Patients with any ferritin value (n=) | 942 |
| Patients with presentation ferritin (n=) | 838 |
| Median age, years (range) [IQR] | 66 (18‐99) [54‐77] |
| Percent Male (N) | 60.1% (566) |
| Race | Non‐Hispanic Black: 28.7% (270) |
| Non‐Hispanic White: 24.9% (235) | |
| Other: 42.0% (396) | |
| Unknown: 4.4% (41) | |
| Patients <50 y old (n=) | 170 |
| Patients <55 y old (n=) | 242 |
| Patients <60 y old (n=) | 333 |
| Median Max ferritin, ng/mL (range) [IQR] | 1148 (7‐33 511) [446‐2664] |
| Max ferritin >500 ng/mL, N (%) | 683 (72.5%) |
| Max ferritin >1000 ng/mL, N (%) | 503 (53.4%) |
| Max ferritin >10 000 ng/mL, N (%) | 44 (4.7%) |
| Median admission ferritin (range) [IQR] | 711 (7‐33 511) [318‐1567] |
| Presentation ferritin >500 ng/mL, N (%) | 512 (54.4%) |
| Presentation ferritin >1000 ng/mL, N (%) | 311 (33.0%) |
| Presentation ferritin >10 000 ng/mL, N (%) | 13 (1.4%) |
| Death, N (%) | 265 (28.1%) |
| New renal replacement therapy, N (%) | 80 (8.5%) |
| Mechanical ventilation, N (%) | 280 (29.7%) |
Abbreviations: IQR, interquartile range; Max, maximum.
The median presentation and maximum ferritins were significantly different in patients who did and did not survive COVID‐19 (P < .0001). As detailed in Table S1 in the supplementary data, of the 265 patients who did not survive, the median presentation ferritin was 915 ng/mL and median maximum ferritin was 1648 ng/mL. For the 677 patients who survived, the median presentation ferritin was 634 ng/mL and median maximum ferritin was 928 ng/mL.
3.2. Receiver operator curves
The ROCs for presentation and maximum ferritin in predicting all‐cause mortality are shown in Figure 1A,B, respectively. The AUC for presentation ferritin was 0.638, with an optimal cutoff of 799 ng/mL in predicting all‐cause mortality. This AUC represents a poor ability to discriminate predictions for death. Performance characteristics include a positive predictive value (PPV) of 0.356 and negative predictive value (NPV) of 0.776, which indicate a rather poor predictor of all‐cause mortality. The AUC for maximum ferritin was 0.677, with an optimal cutoff of 862 ng/mL in predicting all‐cause mortality. This AUC represents a poor ability to discriminate predictions for death. Performance characteristics include a PPV of 0.364 and NPV of 0.829, indicating a poor test in predicting all‐cause mortality.
FIGURE 1.
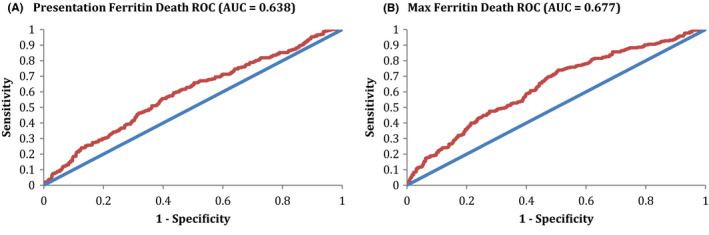
Ferritin ROC for mortality. A, The ROC for presentation ferritin in predicting all‐cause mortality is shown. The AUC for this ROC was 0.638, consistent with a poor discriminating ability for prediction of all‐cause mortality. Optimal Cutoff Ferritin was 799, with performance characteristics at the optimal cutoff of Sensitivity 0.557, Specificity 0.603, PPV 0.356, & NPV 0.776. B, The ROC for maximum ferritin in predicting all‐cause mortality is shown. The AUC for this ROC was 0.677, consistent with a poor discriminating ability for prediction of all‐cause mortality. Optimal Cutoff Ferritin was 862, with performance characteristics at the optimal cutoff of Sensitivity 0.740, Specificity 0.493, PPV 0.364, & NPV 0.829. AUC, area under the curve; Max, maximum; ROC, receiver operator curve
The ROCs for presentation and maximum ferritin in predicting the need for mechanical ventilation are shown in Figure 2A,B, respectively. The AUC for presentation ferritin was 0.681, with an optimal cutoff of 619 ng/mL in predicting the need for mechanical ventilation. This AUC represents a poor ability to discriminate predictions for mechanical ventilation. Performance characteristics include a PPV of 0.371 and NPV of 0.813, which indicate a poor predictor of mechanical ventilation. The AUC for maximum ferritin was 0.769, with an optimal cutoff of 1014 ng/mL in predicting mechanical ventilation. This AUC represents a moderate ability to discriminate predictions for mechanical ventilation. Performance characteristics include a PPV of 0.446 and NPV of 0.869, which indicate a poor predictor of mechanical ventilation.
FIGURE 2.
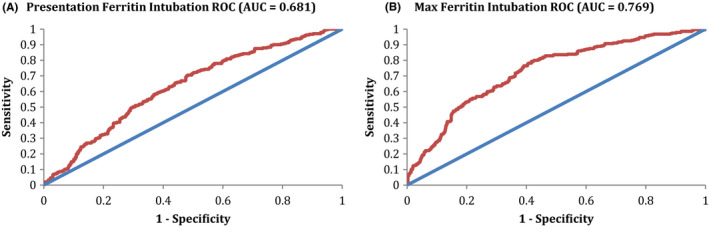
Ferritin ROC for intubation. A, The ROC for presentation ferritin in predicting intubation is shown. The AUC for this ROC was 0.681, consistent with a poor discriminating ability for prediction of intubation. Optimal Cutoff Ferritin was 619, with performance characteristics at the optimal cutoff of Sensitivity 0.705, Specificity 0.518, PPV 0.371, & NPV 0.813. B, The ROC for maximum ferritin in predicting intubation is shown. The AUC for this ROC was 0.769, consistent with a minimally effective discriminating ability for prediction of intubation. Optimal Cutoff Ferritin was 1014, with performance characteristics at the optimal cutoff of Sensitivity 0.793, Specificity 0.583, PPV 0.446, & NPV 0.869. AUC, area under the curve; Max, maximum; ROC, receiver operator curve
The ROCs for presentation and maximum ferritin in predicting the need for new RRT are shown in Figure 3A,B, respectively. The AUC for presentation ferritin was 0.704, with an optimal cutoff of 596 ng/mL in predicting the need for new RRT. This AUC represents a moderate ability to discriminate predictions for developing a new RRT requirement. Performance characteristics include a PPV of 0.117 and NPV of 0.973, which suggests some utility in being able to predict those patients who will not require new RRT over their admission via the cutoff value. The AUC for maximum ferritin was 0.787, with an optimal cutoff of 2365 ng/mL in predicting the need for new RRT. This AUC represents a moderate ability to discriminate predictions for developing a new RRT requirement. Performance characteristics include a PPV of 0.189 and NPV of 0.956, which suggests some utility in being able to predict those patients who will not require new RRT over their admission via the cutoff value.
FIGURE 3.
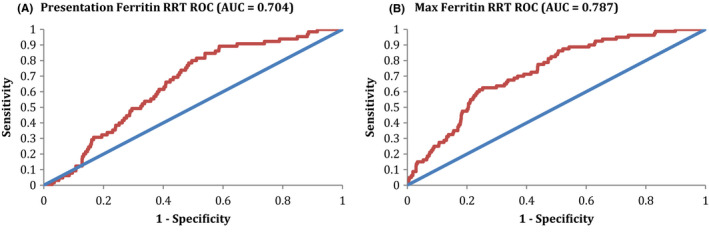
Ferritin ROC for RRT. A, The ROC for presentation ferritin in predicting new RRT is shown. The AUC for this ROC was 0.704, consistent with a minimally effective discriminating ability for prediction of intubation. Optimal Cutoff Ferritin was 596, with performance characteristics at the optimal cutoff of Sensitivity 0.846, Specificity 0.461, PPV 0.117, & NPV 0.973. B, The ROC for maximum ferritin in predicting new RRT is shown. The AUC for this ROC was 0.704, consistent with a minimally effective discriminating ability for prediction of intubation. Optimal Cutoff Ferritin was 2365, with performance characteristics at the optimal cutoff of Sensitivity 0.625, Specificity 0.747, PPV 0.189, & NPV 0.956. AUC, area under the curve; ROC, receiver operator curve; RRT, renal replacement therapy
As ferritin values can increase with age (“inflammaging”), we evaluated the ROCs for maximum ferritin in predicting death at several age cutoffs, as seen in Figure 4A‐C. As previously mentioned, the AUC for all‐cause mortality for the entire cohort was 0.677 (Figure 1B). However, when broken down into progressively younger cohorts, there was a noticeable increase in the AUC, representing a moderate discriminatory ability in predicting death. For patients younger than 60 years old, the AUC was 0.730. For those younger than 55 years old, the AUC was 0.740. Finally, for those younger than 50 years old, the AUC was 0.756.
FIGURE 4.
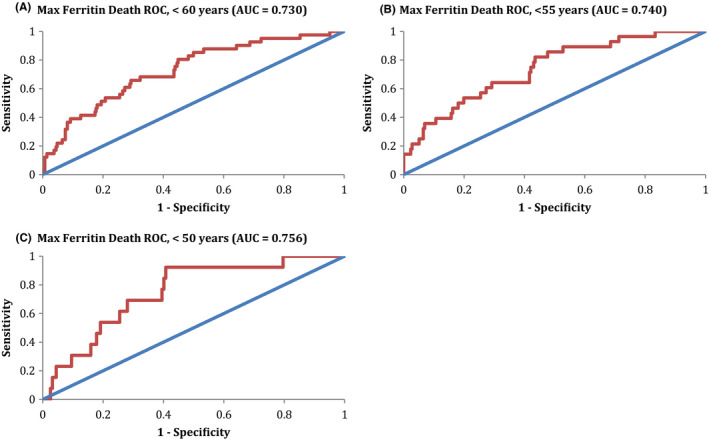
Maximum ferritin ROC for death, stratified by age. A, The ROC for maximum ferritin in predicting all‐causemortality in patients <60 y‐old is shown. The AUC for this ROC was 0.730, consistent with a minimally effective discriminating ability for prediction of death. B, The ROC in patients <55 y‐old is shown. The AUC for this ROC was 0.740, consistent with a minimally effective discriminating ability for prediction of death. C, The ROC in patients <50 y‐old is shown. The AUC for this ROC was 0.756, consistent with a minimally effective discriminating ability for prediction of death. AUC, area under the curve; ROC, receiver operator curve
3.3. Secondary hemophagocytic lymphohistiocytosis
The clinical characteristics and outcomes of the patients with the highest maximum ferritin values (>20 000 ng/mL) are detailed in Table S2. Notably, six (27.3%) of the patients were on hemodialysis (HD) prior to admission. As expected, the outcomes of these patients were poor: 16 (72.7%) of the patients died, 18 (81.8%) required mechanical intubation, and five (22.7%) required new RRT. Fifteen (68.2%) of the patients had suspected or confirmed bacterial infections, which likely contributed to their highly elevated ferritin levels. Interleukin 6 (IL‐6) and c‐reactive protein (CRP) levels were drawn on 20 and 21 patients, respectively. The medians of the maximum IL‐6 and CRP obtained over the hospitalization were highly elevated to 408.8 pg/mL and 306.7 mg/L, respectively, correlating with a pro‐inflammatory environment. The majority of patients received hydroxychloroquine, azithromycin, and/or corticosteroids. Outcomes were not improved in the 10 patients (45.5%) who received tocilizumab, as eight (36.4%) of them died. Two of the patients who received tocilizumab also received anakinra; unfortunately, both of these patients died as well. The patients were evaluated to see if they would meet HLH‐2004 diagnostic criteria. No patients were able to fulfill at least five of the criteria, though only one patient was tested for soluble IL‐2 receptor, no patients had natural killer cell activity assessed, no bone marrows or other organ biopsies were performed, and full abdominal imaging was rarely performed.
4. DISCUSSION
This retrospective analysis of 942 COVID‐19 patients admitted to a large New York City health system shows that ferritin levels, either obtained at presentation or the maximum value over admission, are a relatively poor test in predicting the evaluated outcomes, namely all‐cause mortality, new mechanical ventilation, and a new RRT requirement. This was shown via several analytic techniques, including ROC analysis and through performance characteristics (specificity, sensitivity, NPV, PPV) obtained at optimal cutoff ferritins. There seemed to be some moderate predictive power in ferritin being able to discriminate the need for a new RRT requirement, as this test had AUCs > 0.7 and NPVs > 0.95 for both presentation and maximum ferritins, but this was not seen for our other outcomes of interest. Though higher median ferritin levels were seen in those patients who died than those who survived, the test remains a poor clinical predictor overall.
Ferritin has been proposed to be a useful marker in predicting patient outcomes in those with COVID‐19. There are multiple publications showing that higher ferritin levels, along with other pro‐inflammatory markers, including CRP and IL‐6, are correlated with worse outcomes and may even help predict these outcomes. 9 , 10 , 11 , 12 , 13 , 14 , 15 Several of these studies have limitations, including small sample populations, lack of clarity as to when during the admission the laboratories of impact were drawn, weak statistical analysis, and poor comparator arms. Notably, the one study that conducted an ROC for ferritin was only used to predict mild vs critically ill patients. It demonstrated an AUC of only 0.812, from among sample size of only 389 patients. Furthermore, our analysis used objective criteria including death and mechanical ventilation to evaluate predictive power, whereas this study used more nebulous criteria, including septic shock and multi‐organ dysfunction, to classify patients into the critically ill category. 20
Of note, there have been late‐breaking reports of a Kawasaki‐like inflammatory condition affecting children associated with COVID‐19, including a warning from the United States Centers for Disease Control and Prevention. 21 Though our cohort only included patients ≥18 years, we showed that ferritin was better at predicting all‐cause mortality in progressively younger cohorts of patients, though the test still remained lacking overall. This may suggest that inflammation plays a larger role leading to mortality in younger vs older adults. Of note, as detailed in Table 1, these patients only represent a small fraction of our total cohort. However, even among patients <50 years old, there are still 170 patients included in the analysis.
There have been reports of secondary HLH associated with COVID‐19, though exact diagnostic details and large patient samples have been lacking. 3 , 16 , 22 We therefore decided to perform a more detailed analysis in the 22 patients in our cohort with the highest ferritin values (>20 000 ng/mL), evaluating whether they could fit with the common HLH‐2004 diagnostic criteria. 23 None of the 22 patients were able to meet the minimum five criteria, with only one patient achieving four. Only seven patients met the criteria for ≥2 cytopenias, and no patients had any neutropenia. A limitation of this evaluation is that few of these patients had a complete workup for HLH, including NK cell activity assays, abdominal imaging, and tissue or bone marrow biopsies. As expected in patients with such high ferritin values, the mortality rate was high. Of interest, we noticed that 6 of the 22 patients were on HD prior to diagnosis. Renal disease is a risk factor for poorer COVID‐19 outcomes 24 ; it is unclear how HD might impact the inflammatory environment. Additionally, the higher ferritin values were likely not solely driven by SARS‐CoV‐2 infection, as 15 of the patients had suspected or confirmed bacterial infections, indicative that a secondary infectious process should be ruled out in patients with such high ferritin levels.
As of the writing of this report, there are currently a significant number of trials of different anti‐inflammatory drugs, including the IL‐6 receptor inhibitors tocilizumab (44 clinical trials per clinicaltrials.gov) and sarilumab (n = 14), the IL‐6 inhibitor siltuximab (n = 2), the interleukin‐1 (IL‐1) receptor antagonist anakinra (n = 13), the interferon gamma inhibitor emapalumab (n = 1), and etoposide (n = 1). There are a number of trials involving different corticosteroids and nonsteroidal anti‐inflammatory agents as well. Several retrospective reports from Italy and China on the positive clinical impact of tocilizumab and consequent reduction in inflammatory markers have been published. 17 , 18 , 19 Sample sizes in the studies ranged from 21 to 100 patients, and oxygen requirements varied from mild oxygen supplementation to ventilation, depending on the study. The ferritin levels reported in the two Italian studies ranged from 1000 to 4000 ng/mL. 17 , 18 In our cohort of patients with ferritin levels >20 000 ng/mL, the use of tocilizumab did not appear to affect mortality, albeit in a small sample size of 10 patients.
Our study has a number of potential limitations, mostly deriving from its retrospective nature. Less than half of the patients in our original data set had a ferritin value recorded, which was required to be included in this study. These missing patients could have been less sick and/or incidentally found to have COVID‐19, and therefore not had a ferritin drawn, perhaps enriching our cohort in patients with poorer outcomes. Furthermore, there are different practice patterns among the different hospitals across our health system. We only evaluated a small cohort of patients with the highest ferritin values to determine what COVID‐19 anti‐inflammatory medications they received. This study only evaluated ferritins drawn within 3 days of presentation and the maximum ferritin values obtained over admission. We did not explore the impact of serial ferritins over time and how changes in ferritin values could predict outcomes. This may be studied in the future. Finally, we did not assess the impact of baseline ferritins pre‐COVID‐19 diagnosis. Some patients may have had an already high ferritin level because of co‐morbid conditions, including pre‐existing neoplasms, autoimmune conditions, and infections.
The strengths of this study comprise the large patient population with a high number of patients achieving our outcomes of interest within a relatively short period of time. This would lessen the effect of the changes in practice that have occurred throughout the health system over time as our practitioners have become unfortunately more common with the treatment of this novel disease.
In conclusion, our retrospective study of over 900 patients admitted with COVID‐19 shows that though higher ferritin levels are associated with all‐cause mortality. However, ferritin cannot reliably predict several important outcomes, including death. Furthermore, though HLH has been commonly reported in association with COVID‐19, we were unable to find any patient who met diagnostic criteria among our patients with the highest ferritin values.
CONFLICT OF INTEREST
The authors have no competing interests.
AUTHOR CONTRIBUTION
JF, DT, and LN designed the study, performed the research, analyzed the data, and wrote the manuscript. ST and AK performed the research and approved the final manuscript.
Supporting information
Table S1
Table S2
ACKNOWLEDGEMENTS
The authors would like to thank everyone who assisted in reviewing the EHR and inputted data for this project.
Feld J, Tremblay D, Thibaud S, Kessler A, Naymagon L. Ferritin levels in patients with COVID‐19: A poor predictor of mortality and hemophagocytic lymphohistiocytosis. Int J Lab Hematol. 2020;42:773–779. 10.1111/ijlh.13309
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Puelles VG, Lutgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS‐CoV‐2. New Engl J Med. 2020;383(6):590–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including Interleukin‐6 in COVID‐19 induced pneumonia and macrophage activation syndrome‐like disease. Autoimmun Rev. 2020;19(6):102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29(9):401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colafrancesco S, Alessandri C, Conti F, Priori R. COVID‐19 gone bad: a new character in the spectrum of the hyperferritinemic syndrome? Autoimmun Rev. 2020; 19:102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosario C, Zandman‐Goddard G, Meyron‐Holtz EG, D'Cruz DP, Shoenfeld Y. The hyperferritinemic syndrome: macrophage activation syndrome, Still's disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia PC, Longhi F, Branco RG, Piva JP, Lacks D, Tasker RC. Ferritin levels in children with severe sepsis and septic shock. Acta Paediatr. 2007;96(12):1829‐1831. [DOI] [PubMed] [Google Scholar]
- 9. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Investig. 2020;130(5):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giamarellos‐Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID‐19 patients with severe respiratory failure. BMJ Open Diabetes Res Care. 2020;27(6):992–1000.e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y, Hu Y, Yu J, Ma T. Retrospective analysis of laboratory testing in 54 patients with severe‐ or critical‐type 2019 novel coronavirus pneumonia. Lab Investig. 2020;100:794‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun L, Shen L, Fan J, et al. Clinical features of patients with coronavirus disease 2019 (COVID‐19) from a designated hospital in Beijing, China. Pediatr Allergy Immunol. 2020. 10.1002/jmv.25966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang F, Hou H, Luo Y, et al. The laboratory tests and host immunity of COVID‐19 patients with different severity of illness. JCI insight. 2020;5:e137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dimopoulos G, de Mast Q, Markou N, et al. Favorable Anakinra responses in severe COVID‐19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020;28:117‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sciascia S, Aprà F, Baffa A, et al. Pilot prospective open, single‐arm multicentre study on off‐label use of tocilizumab in patients with severe COVID‐19. Clin Exp Immunol. 2020;38:529‐532. [PubMed] [Google Scholar]
- 18. Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID‐19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19:102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970‐10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hou H, Zhang B, Huang H, et al. Using IL‐2R/lymphocytes for predicting the clinical progression of patients with COVID‐19. Clin Exp Immunol. 2020;201:76‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Falk J, Friedman O, Zaman T, et al. Storm, typhoon, cyclone or hurricane in patients with COVID‐19? Beware of the same storm that has a different origin. Basic research in cardiology. RMD Open. 2020;6(1):e001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Radmanesh F, Rodriguez‐Pla A, Pincus MD, Burns JD. Severe cerebral involvement in adult‐onset hemophagocytic lymphohistiocytosis. J Clin Neurosci. 2020;76:236‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henter JI, Horne A, Arico M, et al. HLH‐2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124‐131. [DOI] [PubMed] [Google Scholar]
- 24. Rombola G, Brunini F. COVID‐19 and dialysis: why we should be worried. J Nephrol. 2020;33(3):401‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


