Abstract
Myocardial injury is frequently detected in coronavirus disease 2019 (COVID‐19) patients. However, up to one‐third of COVID‐19 patients showing ST‐segment elevation on the electrocardiogram have angiographically normal coronary arteries. We present a case of an acute coronary syndrome due to a coronary spasm in a severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) patient. This pathophysiological mechanism was clearly demonstrated by intracoronary imaging techniques (optical coherence tomography) and invasive vasospasm test.
Keywords: AMI ‐ Acute myocardial infarction, CAD ‐ Coronary Artery Disease
1. INTRODUCTION
ST‐segment elevation leads to myocardial injury in coronavirus disease 2019 (COVID‐19) patients. 1 One‐third of COVID‐19 patients showing ST‐segment elevation on the electrocardiogram (ECG) have angiographically normal coronary arteries. 2 We present a patient with severe acute respiratory syndrome (SARS)‐COVID‐19 who presented with a transient ST‐segment elevation acute coronary syndrome secondary to coronary vasospasm (CV).
2. CASE REPORT
A 66‐year‐old man recently diagnosed of bilateral pneumonia due to COVID‐19 presented with rest chest pain and inferolateral ST‐segment elevation on the ECG (Figure 1a). He had no previous history of coronary artery disease or coronary risk factors. He had been treated with hydroxychloroquine (5 days), ritonavir/liponavir (5 days), azithromycin (3 days), and oxygen. After intravenous nitroglycerin administration, the chest pain disappeared and the ECG changes completely normalized. Urgent coronary angiography revealed significant lesions in the proximal and distal left circumflex coronary artery (LCX) and the left anterior descending coronary artery (LAD), with a normal coronary flow. Lesion severity remained unchanged after intracoronary nitroglycerin administration (Figure 1c,d, arrows). Owing to the clinical suspicion of a complicated plaque in the proximal LCX (“transient” ST‐segment elevation), optical coherence tomography (OCT) was performed. OCT disclosed a stable, mainly fibrotic atheromatous plaque, with some macrophage clusters and a minimal lumen area of 1.9 mm2. No signs of plaque erosion or rupture, or residual intracoronary thrombus, could be detected (Figure 1b, asterisk denotes wire artifact). The lesions in the LCX were treated with everolimus‐eluting stents, initially resulting in some vessel spams at the stent edges that completely disappeared after repeated intracoronary nitroglycerin administration. A diagnosis of CV over a stable coronary plaque was made. The patient evolved with a minimal rise in cardiac markers (Troponin T–US: 74 ng/L) but shoved a marked increase in inflammatory markers, including elevated levels of D‐dimer (43 μg/ml), fibrinogen (815 mg/dl), C‐reactive protein (55 mg/L), and interleukin‐6 (65 pg/ml). Four days later, elective treatment of the LAD lesion was scheduled. However, given the high clinical suspicion of associated CV, a pharmacological provocation test was first performed by administering intracoronary ergonovine at increasing doses. After the 10‐μg dose, the patient complained of severe chest pain and universal ST‐segment elevation was observed on the ECG. Coronary angiography revealed a nearly occlusive CV involving both the LAD and LCX. Strikingly, only the two previously stented segments of the LCX‐maintained lumen unchanged (Figure 2a, Video S1). After intracoronary nitroglycerin administration, the electrical changes completely disappeared and the LCX and LAD rapidly regained their normal caliber (Figure 2b, Video S1). A drug‐eluting stent was successfully implanted in the LAD with excellent result. Clinical outcome was uneventful with vasodilator treatment (including both calcium‐channel blockers and nitrates), the pneumonia progressively resolved, and eventually the patient could be discharged 6 days after admission.
FIGURE 1.
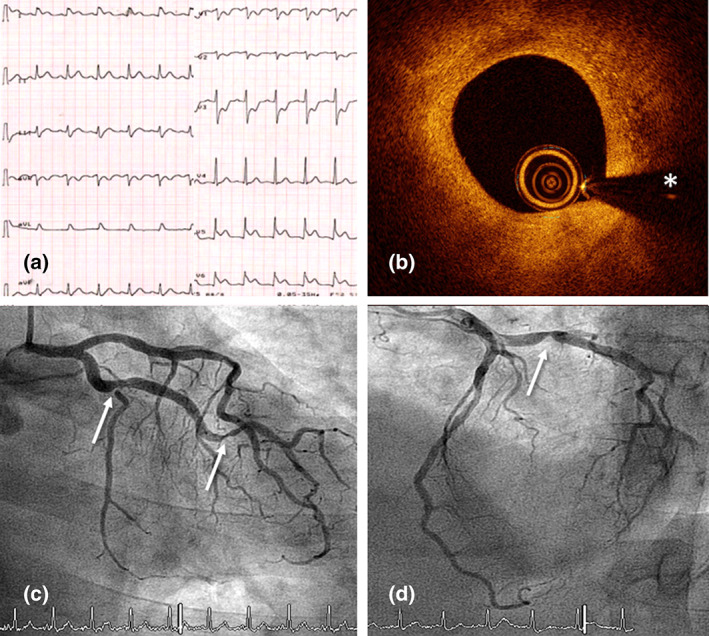
(a) Electrocardiogram during the chest pain episode showing elevation of the inferolateral ST‐segment with specular descent in right precordial leads. (b) Optical coherence tomography intracoronary image of the proximal left circumflex coronary artery (LCX) showing the presence of a stable fibrous plaque with cellular infiltration adjacent to the site with minimal lumen area. Notably, presence of erosion or rupture as a possible trigger of acute coronary syndrome was excluded (asterisk denotes wire artifact). (c and d) Urgent coronary angiogram showing lesions in the proximal (arrow) and distal (arrow) LCX
FIGURE 2.
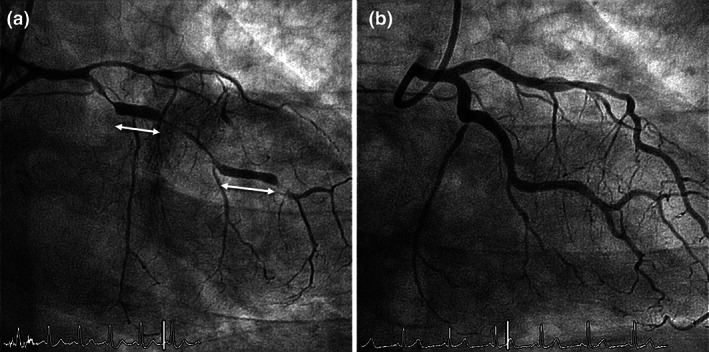
Coronary angiogram during invasive pharmacological coronary vasospasm (CV) provocation test. After intracoronary administration of 10 μg of ergonovine, nearly occlusive multisegment CV involving both the left anterior descending coronary artery and left circumflex coronary artery (LCX) was demonstrated. Only the two previously stented segments of the LCX (double head arrows) maintained lumen unchanged (a). After the administration of intracoronary nitroglycerin, the spasm was reversed with a recovery of the previous arterial caliber (b)
3. DISCUSSION
SARS‐CoV‐2 is currently causing a pandemic with an exponential increase in cases worldwide. Notably, myocardial injury is frequently detected in COVID‐19 patients. 1 Episodes of severe ST‐segment elevation leading to myocardial injury or myocardial infarction have been recently reported in these patients. However, up to one‐third of COVID‐19 patients showing ST‐segment elevation on the ECG have angiographically normal coronary arteries. 2 The underlying pathophysiological mechanism of myocardial damage in these patients remains unknown but angiographically silent plaque rupture or erosion, CV, microthrombi, or hypoxic injury have been proposed. 2 However, to the best of our knowledge, this is the first description of severe CV associated to an uncomplicated coronary lesion, resulting in transient ST‐segment elevation, in a COVID‐19 patient. The causes of this phenomenon remain unclear but might be multifaceted. CV results from hypercontraction of coronary wall smooth muscle triggered by an increase of intracellular calcium particularly in the presence of increased calcium sensitivity. It is well known that CV may occur in patients with preexisting atherosclerotic coronary lesions. Importantly, inflammation plays an important role in the pathogenesis of CV and interleukin‐6 polymorphism, as well as oxidative stress, has been associated with this phenomenon. 3 Severe COVID‐19 disease is characterized by a major systemic inflammatory response. Moreover, COVID‐19 activates the angiotensin‐converting enzyme 2 receptor, largely expressed in vascular tissues, and may induce endothelial dysfunction. 4 Altogether, the intense inflammatory milieu of severe COVID‐19 infection may provide a trigger able to elicit CV in some of these patients. A high degree of clinical suspicion is essential to be able to confirm the diagnosis of CV in patients with angiographically normal coronary arteries but also in those with associate stable coronary lesions. Appropriate intense vasodilator treatment appears crucial to prevent CV in patients with severe COVID‐19 disease presenting with episodes of dynamic ST‐segment elevation.
4. CONCLUSION
CV may be a cause of myocardial damage in patients with SARS‐COVID‐19 infection in the context of the pro‐inflammatory response to the disease.
Supporting information
Video S1: Coronary angiogram during CV provocation test. After intracoronary 10 μgrams of ergonovine were administered, a nearly occlusive multisegment CV involving both the LAD and LCX. Only the two previously stented segments maintained lumen unchanged. After the administration of intracoronary nitroglycerin, the spasm was reversed with a recovery of the previous arterial caliber.
Rivero F, Antuña P, Cuesta J, Alfonso F. Severe coronary spasm in a COVID‐19 patient. Catheter Cardiovasc Interv. 2021;97:E670–E672. 10.1002/ccd.29056
REFERENCES
- 1. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bangalore S, Sharma A, Slotwiner A, et al. ST‐segment elevation in patients with Covid‐19 – a case series. N Engl J Med. 2020;382(25):2478‐2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murase Y, Yamada Y, Hirashiki A, et al. Genetic risk and gene‐environment interaction in coronary artery spasm in Japanese men and women. Eur Heart J. 2004;25:970‐977. [DOI] [PubMed] [Google Scholar]
- 4. Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1: Coronary angiogram during CV provocation test. After intracoronary 10 μgrams of ergonovine were administered, a nearly occlusive multisegment CV involving both the LAD and LCX. Only the two previously stented segments maintained lumen unchanged. After the administration of intracoronary nitroglycerin, the spasm was reversed with a recovery of the previous arterial caliber.


