Abstract
Background
Tocilizumab (TCZ) has been used in the management of COVID‐19‐related cytokine release syndrome (CRS). Concerns exist regarding the risk of infections and drug‐related toxicities. We sought to evaluate the incidence of these TCZ complications among COVID‐19 patients.
Methods
All adult inpatients with COVID‐19 between 1 March and 25 April 2020 that received TCZ were included. We compared the rate of late‐onset infections (>48 hours following admission) to a control group matched according to intensive care unit admission and mechanical ventilation requirement. Post‐TCZ toxicities evaluated included: elevated liver function tests (LFTs), GI perforation, diverticulitis, neutropenia, hypertension, allergic reactions, and infusion‐related reactions.
Results
Seventy‐four patients were included in each group. Seventeen infections in the TCZ group (23%) and 6 (8%) infections in the control group occurred >48 hours after admission (P = .013). Most infections were bacterial with pneumonia being the most common manifestation. Among patients receiving TCZ, LFT elevations were observed in 51%, neutropenia in 1.4%, and hypertension in 8%. The mortality rate among those that received TCZ was greater than the control (39% versus 23%, P = .03).
Conclusion
Late onset infections were significantly more common among those receiving TCZ. Combining infections and TCZ‐related toxicities, 61% of patients had a possible post‐TCZ complication. While awaiting clinical trial results to establish the efficacy of TCZ for COVID‐19 related CRS, the potential for infections and TCZ related toxicities should be carefully weighed when considering use.
Keywords: coronavirus, cytokine/chemokine, immune responses, Il‐6 inhibition, SARS coronavirus, tocilizumab
Highlights
Infectious complications and drug‐related toxicities are concerns associated with TCZ use.
Among COVID‐19 patients presenting with a hyper‐inflammatory response receiving TCZ, 61% had a possible post‐TCZ complication.
Additional studies are needed to further evaluate the safety and efficacy of TCZ in the management of patients with COVID‐19.
1. INTRODUCTION
Immune‐modulators targeting interleukin‐6 (IL‐6) have been implemented in the management of patients with severe coronavirus disease 2019 (COVID‐19) presenting with a hyperinflammatory response resembling cytokine release syndrome (CRS). COVID‐19 related CRS is associated with elevated levels of several inflammatory markers, including IL‐6. 1 , 2 Tocilizumab (TCZ), an IL‐6 receptor monoclonal antibody, has gained attention as a potential option to treat the hyperinflammatory state that develops in patients with severe COVID‐19 based predominantly on case reports and non‐randomized studies. 3 , 4 , 5 , 6 , 7 , 8 However, concerns exist regarding the potential for an increased risk of infection, as TCZ can blunt the immune response, in addition to concerns for other toxicities known to be associated with its use (eg, liver dysfunction, gastrointestinal perforation, diverticulitis, hypertension, neutropenia, and infusion‐related reactions). 9 , 10 The occurrence of these complications following the administration of off‐label TCZ among patients with COVID‐19 related hyperinflammatory response is not well established. We sought to evaluate the incidence of these potential complications following the use of TCZ among COVID‐19 patients.
2. METHODS
This was a single‐center, retrospective, observational study comparing COVID‐19 patients who received TCZ to those who did not receive TCZ. All adult inpatients with COVID‐19 admitted between 1 March 1 2020 to 25 May 2020 who received at least one 400 mg dose of TCZ for CRS were included. During the study period, the institutional guideline‐recommended administration of TCZ 400 mg intravenous once (with the option of redosing based on a clinical response within 12‐24 hours) if patients presented with severe and rapidly progressing hypoxia in addition to elevated inflammatory markers (eg, D‐Dimer >2 mg/L, C‐reactive protein [CRP] >100 mg/L, and/or ferritin >600 mcg/L [or >300 mcg/L if ferritin doubled in the previous 24 hours]). TCZ use was avoided in patients with confirmed or suspected bacterial infections, neutropenia, thrombocytopenia, or if liver function tests (LFTs) were >10x upper limit of normal. TCZ for this indication required approval by the inpatient infectious diseases consultation service. Patients receiving TCZ as part of a clinical trial were excluded. Our protocol for the management of COVID‐19 patients did not recommend corticosteroids or other immune‐modulating therapies during the included analysis period. This project received a formal determination of quality improvement status according to the University of Chicago Medicine institutional policy. As such, this initiative was deemed not human subjects research and was therefore not reviewed by the Institutional Review Board.
Infection, other than SARS‐CoV‐2, was defined as a positive culture or nucleic acid amplification test (NAAT) for which directed therapy was initiated. Late‐onset infections were those that occurred >48 hours after admission. We evaluated respiratory cultures, blood cultures, tissue cultures, fluid cultures, cytomegalovirus infection, and Clostridioides difficile. Positive urine cultures were excluded, as many positive urine cultures represent colonization, and assessment of symptoms to determine the presence of true infection was often not possible. A random group of patients who did not receive TCZ was selected using a random number generator to provide a comparator group for the analysis. The comparator group was matched according to requirements of intensive care unit (ICU) admission and mechanical ventilation during the clinical course.
We also assessed if patients developed GI perforation, diverticulitis, hypertension, neutropenia (absolute neutrophil count (ANC) <500 cells/m3), or increased liver enzymes, LFTs (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)) following TCZ. We evaluated specifically for an elevation resulting a doubling of baseline liver enzymes or an increase to >5 times upper limit normal following the TCZ dose. Hypertension following TCZ was identified by evaluating progress notes noting new‐onset hypertension at any point after the TCZ dose. Many patients had hypertension at baseline, in these patients, we evaluated whether additional interventions were needed to maintain control of patient blood pressures following TCZ. Progress notes and nursing notes were also reviewed to assess if any allergic reaction or infusion‐related reaction occurred.
Baseline characteristics and comorbidities that are known to be associated with more severe COVID‐19 (hypertension, cardiovascular disease, diabetes, obesity, chronic kidney disease or end‐stage renal disease, asthma or chronic obstructive pulmonary disease, HIV regardless of CD4 count and any other immune‐deficiency) 11 , 12 , 13 , 14 , 15 , 16 were evaluated on all included patients in the TCZ evaluation as well as the non‐TCZ comparator group for the post‐TCZ infection analysis. Immune‐deficiency included any of the following: leukemia, lymphoma, solid tumor if recent chemotherapy or radiation therapy (past 3 months), HIV with CD4 < 200/mm3, neutropenia <1000/mm3, primary immune deficiency, autoimmune or idiopathic condition requiring a biological agent or steroids, the equivalent of ≥prednisone 20 mg daily for >30 days. To characterize the patients’ clinical presentation, course, and outcomes in the group that received TCZ, we also evaluated time to TCZ from symptom onset, intensive care unit (ICU) admission at the time of the TCZ dose, the TCZ weight‐based dose, mechanical ventilation at the time of the TCZ dose, whether they also received remdesivir or a hydroxychloroquine based regimen, time to defervesce following TCZ (if the patient was febrile before the dose), time to positive culture (if they had positive cultures post TCZ), length of hospital stay (LOS), and mortality (all‐cause). We also reviewed the mean baseline and daily (for 5 days following the initial TCZ dose) C‐reactive protein (CRP), ferritin, and D‐Dimer trends.
2.1. Statistical analysis
Descriptive statistics were used to summarize the observed infectious and toxicity analysis for TCZ. For the post‐TCZ infection analysis, χ² or Fisher's exact test were used to compare the categorical baseline characteristics, comorbidities, and identified infections among those that received TCZ versus those in the control group that did not receive TCZ. For age, as a continuous variable that was determined to be normally distributed a Student t‐test was performed. LOS was not normally distributed and evaluated using Mann Whitney U. All statistical analyses were performed using STATA, version 16, College Station, TX.
3. RESULTS
A total of 74 patients received TCZ for COVID‐19‐associated CRS consistent with our protocol. The majority of patients (89%) in the TCZ group received only one dose. The mean follow‐up period for all patients reviewed was 58 days. The baseline characteristics and comorbidities among those that received TCZ in addition to the control group are noted in Table 1. There were no statistically significant differences between groups for the comorbidities assessed. A higher proportion of patients were male (42% vs 33% control) and were immune‐compromised (12% vs 4% control) in the TCZ group. Of the 25 TCZ patients that required mechanical ventilation, 24 (96%) received their dose after intubation. The Late‐onset infection rate compared to that in the control group is shown in Table 2. All of the infections following TCZ (n = 17 infections identified in 12 patients) and six infections (identified in three patients) in the control occurred more than 48 hours following admission (P = .013). Overall infection rate, regardless of onset of infection, was similar between groups, 12 (16.2%) versus 13 (17.5%) in the TCZ and control groups, respectively. Most of the infections identified were bacterial pneumonia. Three patients in the TCZ group were found to have an invasive fungal infection (Mucor pneumonia, C. albicans fungemia, fungal sternal wound infection), while one patient in the control group had an invasive fungal infection (Aspergillus pneumonia).
Table 1.
Comparison of TCZ patients to control group
| Baseline characteristics/comorbidities | TCZ (n = 74) | Control (n = 74) | P‐value |
|---|---|---|---|
| Age (mean ± standard deviation) | 66 ± 13.7 | 65 ± 16.3 | .8 |
| Male (%) | 43 (58) | 33 (45) | .14 |
| DM (%) | 24 (32) | 28 (38) | .61 |
| HTN (%) | 41 (55) | 47 (64) | .40 |
| CVD (%) | 23 (31) | 32 (43) | .17 |
| Asthma or COPD (%) | 10 (13.5) | 18 (24) | .14 |
| CKD or ESRD (%) | 7 (9.4) | 11 (15) | .45 |
| HIV (%) | 0 (0) | 1 (1.4) | 1.0 |
| Immunodeficiency (%) | 9 (12) | 3 (4) | .13 |
| Obesity (%) | 38 (51) | 34 (46) | .62 |
| ICU Admission (%) | 52 (70) | 52 (70) | 1.0 |
| Mechanical Ventilation (%) | 25 (34) | 23 (31) | .86 |
| Concomitant COVID‐19 therapy | |||
| Remdesivir (Trial, EUA, or compassionate use) | 21 (28) | 27 (36.5) | .38 |
| HCQ based regimen | 42 (57)a | 15 (20)b | .001 |
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; ESRD, end‐stage renal disease; EUA, emergency use authorization, ICU, intensive care unit; HCQ, hydroxychloroquine; HIV, human immunodeficiency virus; HTN, hypertension; TCZ, tocilizumab.
HCQ alone (17), HCQ + ribavirin (5), HCQ + lopinavir/ritonavir (20).
HCQ alone (3), HCQ + ribavirin (2), HCQ + lopinavir/ritonavir (10).
Table 2.
Late onset infections post‐TCZ compared to control groupa
| TCZ (n = 74) | Control (n = 74) | P‐value | |
|---|---|---|---|
| Late‐onset Infections (>=48 h from admission)b | 17 (23) | 6 (8) | .013 |
| Time to positive culture, d (mean)c | 11.3 | 6.5 | .04 |
| Pneumonia | 7 (9.5) | 5 (6.8) | .76 |
| MSSA | 4 | 1 | … |
| MRSA | 0 | 1 | … |
| E. coli | 1 | 0 | … |
| Enterobacter | 1 | 0 | … |
| Pseudomonas | 2 | 1 | … |
| Acinetobacter | 0 | 1 | … |
| Burkholderia | 1 | 0 | … |
| Serratia | 0 | 1 | … |
| Aspergillus | 0 | 1 | … |
| Mucor | 1 | 0 | … |
| … | |||
| Bacteremia/Fungemia | 4 (5.4) | 0 (0) | .12 |
| Coagulase negative Staphylococcus | 1 | 0 | … |
| (Unknown source, Line‐related) | |||
| MSSA | 1 | 0 | … |
| (Pulmonary source) | |||
| C. perfringens | 1 | 0 | … |
| (Unclear source, possibly Sacral ulcer) | |||
| C. albicans | 1 | 0 | … |
| (Unclear source, possibly line‐related) | |||
| SSTI/BJI | 2 (2.7) | 0 (0) | .5 |
| MSSA (osteomyelitis) | 1 | 0 | … |
| Yeast, not speciated (sternal wound) | 1 | 0 | … |
| Other | 4 (5.4) | 1 (1.4) | .37 |
| C. difficile | 3 | 1 | … |
| CMV (viremia) | 1 | 0 | … |
Abbreviations: BJI, bone and joint infection; CMV, cytomegalovirus; MRSA, methicillin resistant staphylococcus aureus; MSSA, methicillin susceptible staphylcoccus aureus; SSTI, skin and soft tissue infection; TCZ, tocilizumab.
All pathogens isolated in relevant cultures are listed, some patients may have grown more than one organism, only organisms specifically requiring treatment are included.
Total number of patients with an infection, some had more than 1 following TCZ (or during admission at any point for controls). 17 total infections were identified in 12 patients the TCZ group and 6 infections were identified in three patients in the control group.
For patients that received TCZ; time from TCZ to the first culture positive, for patients in the control group; time from date of admission to date of first culture positive.
Safety analysis was performed among the 74 patients receiving TCZ. Transient elevations in LFTs were documented in 38 patients (51%). Ten patients (26%) with LFT elevations experienced an increase to >5 times upper limit normal. Among those with elevated LFTs, the mean AST was 176 U/L and mean ALT was 101 U/L. The mean number of days for an increase in LFTs to either double from baseline or increase to >5 times upper limit normal was 7.2 days. Neutropenia and hypertension occurred in one (1.4%) and six (8%) patients, respectively. No patients developed a GI perforation or diverticulitis post‐TCZ and none experienced an allergic or infusion‐related reaction. The total number of patients that received TCZ with either post‐dose infection or at least one toxicity was 45 (61%).
The mean time for the TCZ administration from symptom onset was 9 days, with a mean weight‐based dose of 4.5 milligrams per kilogram. The mean time for patients that were febrile before the TCZ dose (n = 15) to defervesce was 6.3 hours, and mean time to culture positivity (from the date of the first TCZ dose) among those with a culture‐positive infection was 11 days. The overall mean LOS was 15.5 days vs 10.3 days in the TCZ and control groups, respectively (P = .04). The mortality rate among those that received TCZ was 39% (n = 29) vs 23% (n = 17) in the control group, P = .03. The inflammatory marker trends following TCZ are shown in Figures 1 to 3. At 5 days following the TCZ dose, the mean CRP decreased by 80% and mean ferritin by 30%. D‐Dimer levels did not decrease following TCZ.
Figure 1.
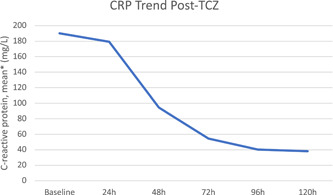
C‐reactive protein (CRP) trends post TCZ. * Excludes CRP values below the limit of detection (<3mg/L). TCZ, Tocilizumab
Figure 2.
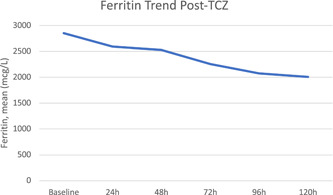
Ferritin trend post‐TCZ. TCZ, Tocilizumab
4. DISCUSSION
The rate of infections following TCZ administration among patients with COVID‐19 related CRS observed in this study, both overall and late onset, is similar to the findings of recently published studies evaluating the use of TCZ for this indication reporting an infection rate ranging between 0% to 27%. 3 , 6 , 7 , 8 , 10 One retrospective study comparing rates of infections among patients with COVID‐19 between those that received TCZ versus a standard of care (SOC) control group, found no difference in the rates of infections between these groups (13% TCZ and 12% SOC). 8 In our analysis however, we identified that while overall infection rate was similar between groups, late on‐set infections occurred in significantly more patients that received TCZ compared to the control (23% vs 8%, P = .013). This is not surprising given the long half‐life of the drug (11 days with 4 mg/kg doses). 17 And while not significantly different between groups, of interest, culture‐confirmed invasive fungal infections were present in three patients that received TCZ in our study and one additional patient had invasive fungal sinusitis diagnosed by CT imaging, no culture data could be obtained; however, this additional patient was not included in our analysis. A recent report found that among 43 patients that received TCZ, three (6.9%) were later diagnosed with candidemia. 10 This is of concern in that previous studies have shown that mice deficient in IL‐6 were more susceptible to candida infections and had increased fungal burdens, compared to nondeficient controls. 18
As far as the other toxicities evaluated, the observed rate of LFT elevations (51%) is higher compared to other studies findings reporting a rate of 15% to 29%, however, the rate of neutropenia (1.4%) is much lower than reported elsewhere (16%). 8 GI perforation, diverticulitis, infusion reactions, and severe allergic reactions are rarely reported in the literature evaluating the use of TCZ for COVID‐19, congruent with the fact that we did not observe any of these toxicities in our study. 3 , 6 , 7 , 8
The observed mortality rate in our study was 39%, higher than the control group (23%), and what has been reported in other studies evaluating the use of TCZ for COVID‐19 CRS (15%‐27%). 5 , 6 , 7 , 8 While the reason for this is unclear, it should be considered that our protocol required both rapidly progressing hypoxemia and presence of elevated inflammatory markers, and among the patients that required mechanical ventilation in the TCZ group, the majority received their TCZ after intubation. The required parameters for patients to be considered for TCZ and the fact that 32% of patients in the TCZ group received their dose after being placed on mechanical ventilation resulted in us reserving use only in patients where poor outcomes may already be imminent, hence contributing to a higher rate of mortality and possibly to the longer length of stay as well. It also pertinent to consider that we implemented a lower flat dose (average dose 4.5 mg/kg), some recommend dosing 8 mg/kg per dose for COVID‐19 related CRS, which could have had an impact on the outcomes observed in our study. 7 The mean time to dose the TCZ in our study was 9 days following symptom onset. While the optimal timing of TCZ for COVID‐19 is not established, this could have potentially contributed to our observed mortality rate as well. Additionally, while the differences were not statistically significant, a larger proportion of patients in the TCZ group were male (42% TCZ vs 33% control), and were immune‐compromised at baseline (12% TCZ vs 4% control). Both male gender, and immune‐compromised have been identified as factors that may be associated with more severe disease or mortality among COVID‐19 patients. 19 , 20 Finally, a larger proportion of patients in the TCZ group also received hydroxychloroquine (57% vs 20%, P = .001) which could also contribute to increased mortality, however, studies have not consistently demonstrated whether the use of this agent increases mortality among patients with COVID‐19. 21 , 22 , 23
We also observed an evident decline in CRP and ferritin following the administration of TCZ, consistent with other studies evaluating the use of TCZ in the setting of COVID‐19 CRS. 5 , 7 However, the D‐Dimer levels continued to increase following TCZ, also consistent with observations of a previous study. 7 The discrepancy in the effect on the levels of these inflammatory markers highlights the fact that TCZ is only acting partially on the inflammatory response cascade. Figure 3
Figure 3.
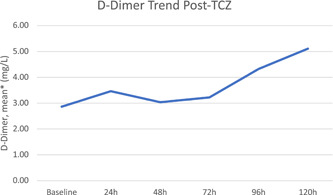
D‐dimer trend post‐TCZ. *Excludes D‐Dimer values above (>20 mg/L) or below (<0.27 mg/L) the limit of detection/reporting. TCZ, Tocilizumab
There are several limitations to this study to consider. As a retrospective analysis, bias and confounders may influence the observed outcomes, including differences in baseline characteristics and length of stay. All TCZ use for the indication of COVID‐19 CRS was overseen by our Infectious Diseases consult service, following the criteria that had been outlined in a clinical protocol, which promoted consistent usage of TCZ among those meeting outlined criteria. Though this may also have contributed to selection bias, including the difference in early‐onset infection, as patients who presented with infection were less likely to receive TCZ based on our protocol. To avoid potential bias in the control group, we matched the patients to the TCZ group according to the requirement of intensive care unit admission and mechanical ventilation. While matching according to clinical scores that may be applicable to the critically‐ill patient population (eg, SOFA or APACHE II) could also help to establish a more evenly matched control group, the applicability of these scales among COVID‐19 patients with suspected CRS are lacking. We feel that matching according to requiring ICU admission and mechanical ventilation are more clinically relevant in day‐to‐day practice. Evaluating toxicities related to a drug retrospectively is also a limitation in that we cannot establish a true causality, many of these patients already had hypertension and elevated LFTs at baseline, and COVID‐19 itself may cause LFTs to be elevated as well. 24 More patients in the TCZ group also received hydroxychloroquine which also could have contributed to the observed rates of hepatotoxicity in this group as this agent can be associated with hepatotoxicity, although rare. 25 We also did not have a control arm for the safety analysis. Additionally, while we had a clinical protocol outlining when to consider TCZ based on progressive hypoxia and elevated inflammatory markers, the criteria to use for the diagnosis of COVID‐19 related CRS and whether it requires the administration of TCZ is not well established. Our criteria are based on observations from direct patient care and studies identifying inflammatory marker thresholds for more severe disease. 19 IL‐6 levels are not included in this analysis as it was not consistently available on all patients, and the sample had to be sent out to an external laboratory for it to be performed at our medical center; therefore, the clinical utility of an IL‐6 level was limited in our cohort of patients.
Forty‐five (61%) patients that received TCZ had at least one of the evaluated complications and we observed a 39% mortality rate, higher than our control group and other studies. 3 , 5 , 6 , 7 , 8 Based on these findings, infection risk (considering the higher rate of late‐onset infections and invasive fungal infections observed) and other drug‐related toxicities should be factored in when considering the use of TCZ for COVID‐19‐associated CRS. Until results from randomized and controlled prospective clinical trials evaluating TCZ for this indication are available, there remain limited data to definitively establish efficacy or safety in this setting. With the potential for harm and unclear evidence to support efficacy, clinicians should consider limiting off‐label use of TCZ for COVID‐19 CRS and only using in the setting of clinical trials.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
NNP: Study design, data collection, analysis of data, write‐up of manuscript. CTN: Study design, data collection, review of manuscript. DW, LK, GMM, DP, and KP: Study design, review of manuscript.
Pettit NN, Nguyen CT, Mutlu GM, et al. Late onset infectious complications and safety of tocilizumab in the management of COVID‐19. J Med Virol. 2021;93:1459–1464. 10.1002/jmv.26429
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Chen X, Zhao B, Qu Y, et al. Detectable serum SARS‐CoV‐2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL‐6) level in critically ill COVID‐19 patients [published online ahead of print April 17, 2020]. Clin Infect Dis. 2020:ciaa449. https://pubmed.ncbi.nlm.nih.gov/32301997/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. 10.1016/j.jaut.2020.102433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117(20):10970‐10975. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dastan F, Nadji SA, Saffaei A, Tabarsi P. Tocilizumab administration in a refractory case of COVID‐19. Int J Antimicrob Agents. 2020;56:106043. 10.1016/j.ijantimicag.2020.106043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol. 2020;92(7):814‐818. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morena V, Milazzo L, Oreni L, et al. Off‐label use of tocilizumab for the treatment of SARS‐CoV‐2 pneumonia in Milan, Italy. Eur J Intern Med. 2020;76:36‐72. 10.1016/j.ejim.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID‐19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568. 10.1016/j.autrev.2020.102568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campochiaro C, Della‐Torre E, Cavalli G, et al. Efficacy and safety of tocilizumab in severe COVID‐19 patients: a single‐centre retrospective cohort study. Eur J Intern Med. 2020;76:43‐49. 10.1016/j.ejim.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheppard M, Laskou F, Stapleton PP, Hadavi S, Dasgupta B. Tocilizumab (Actemra). Hum Vaccin Immunother. 2017;13(9):1972‐1988. 10.1080/21645515.2017.1316909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Antinori S, Bonazzetti C, Gubertini G, et al. Tocilizumab for cytokine storm syndrome in COVID‐19 pneumonia: an increased risk for candidemia? Autoimmun Rev. 2020;19(7):102564. 10.1016/j.autrev.2020.102564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richardson S MD, MPH, Hirsch JS J MD,MA, MSB, M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the NewYork City area. JAMA. 2020;232(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang YC, Luo H, Liu S, et al. Clinical course and outcomes of critically Ill patients with SARS‐CoV2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV2 in Singapore. JAMA. 2020;323:1488‐1494. 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. COVID‐19 in critically Ill patients in the Seattle region – case series. N Engl J Med. 2020;382:2012‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tocilizumab (Actemra®) Prescribing Information. South San Francisco, CA: Genentech, Inc. Revised October 2013.
- 18. van Enckevort FH, Netea MG, Hermus AR, et al. Increased susceptibility to systemic candidiasis in interleukin‐6 deficient mice. Med Mycol. 1999;37(6):419‐426. 10.1046/j.1365-280x.1999.00247.x [DOI] [PubMed] [Google Scholar]
- 19. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Center for Immunization and Respiratory Disease (NCIRD). Division of Viral Diseases . Evidence Used to Update the List of Underlying Medical Conditions that Increase a Persons Risk of Severe Illness from COVID‐19. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html. Accessed June 25, 2020. [PubMed]
- 21. Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in‐hospital mortality in patients with COVID‐19 in New York state. JAMA. 2020;323(24):2493‐2502. 10.1001/jama.2020.8630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with COVID‐19. N Engl J Med. 2020;382(25):2411‐2418. 10.1056/NEJMoa2012410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in united states veterans hospitalized with COVID‐ 19. Med. 2020. 10.1016/j.medj.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feng G, Zheng KI, Yan QQ, et al. COVID‐19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020;8(1):18‐24. 10.14218/JCTH.2020.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LiverTox: Clinical and research information on drug‐induced liver injury [Internet]. Hydroxychloroquine. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases. 2012. https://www.ncbi.nlm.nih.gov/books/NBK548738/. Accessed March 25, 2018. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


