Dear Editor, Coronavirus disease 2019 (COVID‐19) is already well known globally.1 Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection causes a spectrum of severe clinical manifestations, which lead to a high rate of intensive care unit admissions and mortality. We report a first case of COVID‐19 with reactivation of the human herpesvirus (HHV) alpha subfamily – herpes simplex virus (HSV)‐1 and varicella zoster virus (VZV). Due to the immunosuppressive state associated with COVID‐19, infection with the HHV alpha subfamily could be potentially life‐threatening.
A 73‐year‐old man, with respiratory symptoms and positive nucleic acid test of throat swab specimens, was diagnosed with SARS‐CoV‐2 infection on 2 February 2020. As the respiratory symptoms deteriorated, he was admitted to intensive care. Due to the rapid aggravation of respiratory distress, onset of septic shock, and progression of chest imaging, the application of venovenous extracorporeal membrane oxygenation for respiratory support was performed on 15 February. After comprehensive treatment and assisted ventilation, the oxygenation index and relevant indicators improved. The patient’s clinical condition stabilized with downtrending SARS‐CoV‐2 viral load and improvement in his sepsis biomarkers.
For continuous monitoring of the infection status, serial follow‐up pathogen next‐generation sequencing (NGS) tests were performed (Figure 1a), including tests on blood, sputum and bronchoalveolar lavage fluid (BALF). On 8 March, the patient had a second septic shock. The blood pathogen NGS report (8 March) revealed the onset of VZV and HSV‐1 reactivation. Two days later, multiple clusters of haemorrhagic blisters, bullae and diffuse erosions covered the right lateral arm, shoulder and neck of the patient (Figure 1b). Meanwhile, liver function showed the second highest peak during the treatment course.
Figure 1.
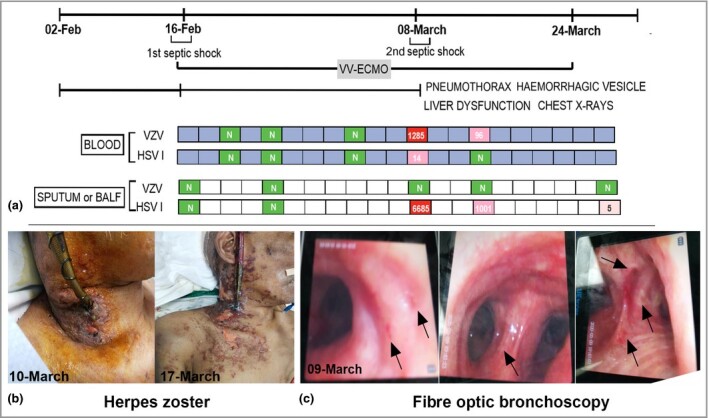
(a) Next‐generation sequencing tests of pathogen from blood and deep sputum or bronchoalveolar lavage fluid (BAFL) samples. Viraemia [varicella zoster virus (VZV) and herpes simplex virus (HSV)‐1] and BAFL (HSV‐1) were detected on 8 March 2020 (N, not detected; red, high; pink, low), which might have resulted in aggravation of the disease course. The symptoms and laboratory indicators both improved after acyclovir treatment. (b) Clinical manifestation of herpes zoster. (c) Several small ulcers on the bronchial mucosa during the fibre optic bronchoscopy. VV‐ECMO, venovenous extracorporeal membrane oxygenation.
The report from BALF also showed a higher sequence number of HSV‐1 on 8 March. Accordingly, his chest radiograph suddenly worsened with a left pneumothorax. In addition, we found several small ulcers on the bronchial mucosa during fibre optic bronchoscopy, as shown in Figure 1c. After antiviral treatment (aciclovir 0·5 g intravenous drip, every 8 h), all parameters gradually improved.
HSV‐1 and VZV are DNA viruses belonging to the neurotropic alpha herpesvirus subfamily.2 After primary infection, they can both formulate a latent infection. However, once the interactional balance between the host immunity and the virus is broken, the following battle in the immune system might be complicated and fierce, involving multiple organs (cutaneous, liver, kidney and brain).3 In our case, the onset of viraemia (VZV and HSV‐1) might have triggered the second septic shock. During fibre optic bronchoscopy, we found several small ulcers on the mucosal membrane of bronchi (Figure 1c), and the reports of BAFL and blood tests illustrated the onset of VZV and HSV‐1 reactivation. Meanwhile, the serial chest radiographs suddenly aggravated. Besides the manifestation in lung and skin, the second highest peak of liver dysfunction might have been related to the reactivation of the HHV alpha subfamily.
The immunological characteristics of patients with COVID‐19 are variable. They show a cytokine storm syndrome and immunosuppression, especially in a subgroup of critically ill patients,4 which suggests an unbalanced immune response and exhaustion of multiple cytokines; this could be one of the reasons for the HSV‐1 and VZV reactivation. The SARS‐CoV‐2 infection affects T lymphocytes, particularly CD4+ T cells, CD8+ T cells4 and natural killer cells,5 resulting in functional exhaustion and decreases in numbers. The resulting immunosuppressive state may encourage reactivation of latent viral infection, resulting in sudden worsening of symptoms in the course of recovery.
Until now, most reports of inpatients with COVID‐19 have characterized the data without including screening tests for other viral infections. In this case, we identified a viraemia (HSV‐1 and VZV) that resulted in an aggravated disease course. Simultaneous infection with VZV and HSV‐1 has rarely been reported. The clinical presentation of co‐reactivation could be skin manifestations, localized in the same or different dermatomal areas,6 or, due to their different anatomical latent sites, they could be separately or simultaneously detected in isolated organs. Severe and lethal cases are prone to occur in older immunocompromised patients.7
In conclusion, co‐reactivation of latent viruses could aggravate the COVID‐19 disease course. Viral‐related screening and detection tests should take cognizance of the following points. Firstly, despite improvements in chest imaging or decreases in SARS‐CoV‐2 RNA load or IgG and IgM, respiratory symptoms and chest imaging can suddenly worsen along with symptoms related to other organ system injuries (unexplainable liver or kidney injury). Thus, in addition to suspicion of secondary bacterial infection, viral infection should also be considered. Secondly, if scattered vesicles or ulcers are found during fibre optic bronchoscopy, HSV‐1‐ and VZV‐related pulmonary infection should be suspected.
Author Contribution
Rui Xu: Conceptualization (equal); Data curation (equal); Formal analysis (lead); Funding acquisition (equal); Investigation (equal); Resources (supporting); Validation (lead); Visualization (lead); Writing‐original draft (lead). Yanbin Zhou: Conceptualization (lead); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Validation (equal); Visualization (equal); Writing‐original draft (lead). Lihua Cai: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Methodology (equal); Resources (equal); Visualization (equal); Writing‐original draft (lead). Wang Lingyan: Conceptualization (equal); Data curation (lead); Formal analysis (equal); Investigation (equal); Methodology (equal); Resources (equal); Visualization (equal); Writing‐original draft (lead). Jiande Han: Conceptualization (equal); Data curation (equal); Supervision (equal); Writing‐review & editing (equal). Xue Yang: Data curation (equal); Investigation (equal); Validation (equal); Visualization (equal). Jie Chen: Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal). Junfeng Chen: Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal). Ma Chunguang: Data curation (equal); Investigation (equal); Methodology (equal). Lihan Shen: Conceptualization (lead); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Methodology (equal); Supervision (lead); Visualization (lead); Writing‐original draft (lead); Writing‐review & editing (lead).
Contributor Information
R. Xu, Department of DermatologyThe First Affiliated Hospital of Sun GuangzhouChina
Y. Zhou, Department of Pulmonary and Critical Care MedicineThe First Affiliated Hospital of Sun GuangzhouChina
L. Cai, Department of Critical Care Medicine Affiliated Dongguan People’s HospitalSouthern Medical University Dongguan China
L. Wang, Department of Critical Care Medicine The First Affiliated Hospital of Sun Yat‐sen University Guangzhou China
J. Han, Department of DermatologyThe First Affiliated Hospital of Sun GuangzhouChina
X. Yang, Department of Critical Care Medicine Affiliated Dongguan People’s HospitalSouthern Medical University Dongguan China
J. Chen, Department of Critical Care Medicine Affiliated Dongguan People’s HospitalSouthern Medical University Dongguan China
J. Chen, Department of Critical Care Medicine Affiliated Dongguan People’s HospitalSouthern Medical University Dongguan China
C. Ma, Department of DermatologyThe First Affiliated Hospital of Sun GuangzhouChina
L. Shen, Department of Critical Care Medicine Affiliated Dongguan People’s HospitalSouthern Medical University Dongguan China.
References
- Zhang JJ, Dong X, Cao YY et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy 2020; 75:1730–41. [DOI] [PubMed] [Google Scholar]
- Ouwendijk WJ, Laing KJ, Verjans GM, Koelle DM. T‐cell immunity to human alphaherpesviruses. Curr Opin Virol 2013; 3:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BM, Bloom DC, Cohrs RJ et al. Herpes simplex virus‐1 and varicella‐zoster virus latency in ganglia. J Neurovirol 2003; 9:194–204. [DOI] [PubMed] [Google Scholar]
- Chen G, Wu D, Guo W et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130:2620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Gao Y, Wang G et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol 2020; 17:533–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giehl KA, Muller‐Sander E, Rottenkolber M et al. Identification and characterization of 20 immunocompetent patients with simultaneous varicella zoster and herpes simplex virus infection. J Eur Acad Dermatol Venereol 2008; 22:722–8. [DOI] [PubMed] [Google Scholar]
- Curley MJ, Hussein SA, Hassoun PM. Disseminated herpes simplex virus and varicella zoster virus coinfection in a patient taking thalidomide for relapsed multiple myeloma. J Clin Microbiol 2002; 40:2302–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


