Abstract
The coronavirus disease 2019 (COVID‐19) pandemic spread by the single‐stranded RNA severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) belongs to the seventh generation of the coronavirus family. Following an unusual replication mechanism, its extreme ease of transmissivity has put many countries under lockdown. With the uncertainty of developing a cure/vaccine for the infection in the near future, the onus currently lies on healthcare infrastructure, policies, government activities, and behaviour of the people to contain the virus. This research uses exponential growth modelling studies to understand the spreading patterns of SARS‐CoV‐2 and identifies countries that showed early signs of containment until March 26, 2020. Predictive supervised machine learning models are built using infrastructure, environment, policies, and infection‐related independent variables to predict early containment. COVID‐19 infection data across 42 countries are used. Logistic regression results show a positive significant relationship between healthcare infrastructure and lockdown policies, and signs of early containment. Machine learning models based on logistic regression, decision tree, random forest, and support vector machines are developed and show accuracies between 76.2% and 92.9% to predict early signs of infection containment. Other policies and the decisions taken by countries to contain the infection are also discussed.
Keywords: coronavirus, COVID‐19, exponential growth model, machine learning, pandemic, SARS‐CoV‐2
1. INTRODUCTION
Coronaviruses, though uncommon, are serious pathogens responsible for infections that cause flu‐like symptoms in infected individuals. These symptoms sometimes resemble the cold and cough symptoms caused by the rhinovirus. Recently, the family has added its seventh‐generation coronavirus – severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) (Chengxin, Zheng, Huang, Bell, & Zhang, 2020). The virus shares 79% identity to severe acute respiratory syndrome (SARS) and 50% identity to Middle East respiratory syndrome (MERS) epidemic outbreaks in 2003 and 2012 (Salute, 2020).
SARS‐CoV‐2 that causes coronavirus disease 2019 (COVID‐19) mutated to transmit from animal to human. This virus is believed to have transferred to humans through bats from a meat market in Wuhan, China (Rajendran et al., 2020; Shereen, Khan, Kazmi, Bashir, & Siddique, 2020). In March 2020, the WHO declared COVID‐19 a pandemic; a pandemic being described as an infection that has spread across countries and international borders rather than within a local region or neighbouring countries.
The SARS‐CoV‐2 is a deadly coronavirus that is transmitted readily between humans and has already infected more than 530,000 people all over the world in 198 countries as on March 26, 2020 which led to global shutdowns (WHO, 2020a,2020b,2020c). The fatality rate has varied among countries and age groups. Until June 2020, the fatality rate averaged 5.5% with Italy recording the highest with 14.49%. The fatality rates of the USA, Germany, and India were 5.56%, 4.72%, and 2.86%, respectively, until June 2020 (Our World in Data, 2020). Of the total deaths, less than 5% belonged to the age group of less than 45 years thereby indicating that the younger population is much more resilient to the COVID‐19 (Worldmeters, 2020). While these fatality rates are significantly less than those of MERS (34.4%) and SARS (9.5%) (Petrosillo, Viceconte, Ergonul, Ippolito, & Petersen, 2020), COVID‐19 has severe transmissivity because of the possibility of asymptomatic people being carriers and spreaders of the virus (Daw, Medicine, & Disease, 2020). The reproduction number R 0 for SARS‐CoV‐2 has been found to be between 2.06 and 2.52 (Sheng, Diao, Yu, Pei, & Chen, 2020). A value of R 0 greater than 1 indicates that the disease can invade the human population, and the higher the value, the easier is its spread.
SARS‐CoV‐2 is the largest single‐stranded RNA virus known to humankind; while other viruses have a single protein spike for attachment to the human cell, this coronavirus family has 10 to 12 spike proteins, which makes it easier for the virus to attach itself to the angiotensin‐converting enzyme 2 protein in humans (Paraskevis, Kostaki, Magiorkinis, Panayiotakopoulos, & Sourvinos, 2020). The virus follows an unusual double‐step replication mechanism, which leads to high rates of proliferation (Luan, Lu, Jin, & Zhang, 2020). The incubation period is typically 2 to 14 days, and the infected person often does not have serious symptoms, but rather common symptoms associated with flu and pneumonia (Rodeny, 2020). General symptoms of pneumonia include fever, cough, chest pain, shortness of pain, fatigue, headache, myalgia, and arthralgia (Sattar SBA, 2020). In addition to symptoms of pneumonia, COVID‐19‐infected individuals may experience a loss of taste or smell, nausea, congestion, and diarrhoea (CDC, 2020).
There are a few drugs that are being recommended and used to manage the symptoms of COVID‐19, but there have, as yet, been no vaccines that are proven to be effective against the coronavirus family, including COVID‐19 (Gautret et al., 2020; Sexton et al., 2016). In the absence of vaccines, it is imperative to check transmission of the virus by alternative ways (Dey, Rahman, Siddiqi, & Howlader, 2020). Policy changes in pandemic and epidemic situations involve social distancing, lockdowns, travel restrictions, awareness campaigns, etc. It has been speculated in past research that environmental conditions of countries like temperature and humidity also sometimes play a significant role in controlling pandemics (Lin et al., 2020).
Quantitative COVID‐19 impact analyses are scarce in literature, given the recency of the pandemic, and more studies in this area are necessary, given the seriousness of the infection. Epidemics are assumed to have an exponential growth at an early stage, and the number of infections reduces over time due to interventions like lockdowns, travel restrictions, awareness programs, etc. Mathematical modelling studies using exponential growth analysis coupled with machine learning could provide a better prediction model for COVID‐19 transmission (Keeling & Danon, 2009; Siettos & Russo, 2013; Victor, 2020). Such models must incorporate the various precautionary measures taken during the viral outbreak.
The objective of this research is to develop a mathematical model using exponential growth analysis coupled with machine learning to predict worldwide early signs of containment of COVID‐19. The models have been developed based on data collected from 42 countries. The objectives of this work are twofold. First, it seeks to identify countries that were successful in early containment of SARS‐CoV‐2. Second, the research aims at building supervised machine learning models with high accuracies for predicting signs of early containment with infrastructure availability, environmental factors, infection severity factors, and government policies of countries as independent variables (Table 1). In the process of modelling, the significance of the above variables in containing the infection at early stages is also studied. This report also includes a discussion on other activities undertaken by the governments of various nations to flatten the infection curves and their corresponding effectiveness.
TABLE 1.
Country‐wise data on infrastructure, weather, policy, and infection as on March 26, 2020
| Variable centricity | Infrastructure | Weather | Policy | Infection | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Country | Doctors per 1,000 population | Beds per 1,000 population | Average temperature in Celsius | Average humidity in percent | Days since official lockdown | Lockdown days in percent of days since contact | Total cases per million population | Deaths per million population | Days since the first contact | Percentage of serious cases of infected |
| Austria | 5.1 | 7.6 | 15 | 65 | 9 | 26.47 | 909 | 8 | 34 | 1.56 |
| Brazil | 2.1 | 2.2 | 25 | 74 | 9 | 26.47 | 16 | 0.4 | 34 | 8.51 |
| Canada | 2.6 | 2.7 | 8 | 34 | 19 | 29.23 | 146 | 1 | 65 | 2.17 |
| Chile | 1.1 | 2.2 | 21 | 60 | 15 | 55.56 | 100 | 0.3 | 27 | 0.37 |
| China | 1.8 | 4.2 | 14 | 54 | 62 | 78.48 | 57 | 2 | 79 | 1.09 |
| Czechia | 4.8 | 6.5 | 10 | 51 | 12 | 41.38 | 237 | 1 | 29 | 9.84 |
| Denmark | 4.5 | 2.5 | 12 | 61 | 17 | 53.13 | 380 | 11 | 32 | 4.95 |
| Ecuador | 2 | 1.5 | 15 | 64 | 7 | 23.33 | 103 | 3 | 30 | 3.18 |
| France | 3.2 | 6.5 | 12 | 57 | 12 | 18.18 | 576 | 35 | 66 | 11.37 |
| Germany | 4.2 | 8.3 | 14 | 53 | 8 | 12.70 | 671 | 5 | 63 | 2.81 |
| Greece | 4.6 | 4.3 | 9 | 56 | 8 | 24.24 | 102 | 3 | 33 | 6.22 |
| Iceland | 4 | 3.2 | 3 | 59 | 3 | 9.68 | 2,822 | 6 | 31 | 1.87 |
| India | 0.8 | 0.7 | 30 | 94 | 7 | 11.67 | 0.7 | 0.01 | 60 | 0.00 |
| Indonesia | 0.4 | 1.2 | 30 | 91 | 2 | 7.14 | 4 | 0.4 | 28 | 0.00 |
| Iran | 1.1 | 1.5 | 15 | 58 | 7 | 17.50 | 422 | 30 | 40 | 9.05 |
| Ireland | 3.1 | 2.8 | 7 | 93 | 15 | 50.00 | 489 | 7 | 30 | 2.44 |
| Israel | 3.2 | 3.1 | 29 | 25 | 9 | 23.68 | 418 | 1 | 38 | 1.38 |
| Italy | 4.1 | 3.4 | 15 | 88 | 18 | 30.00 | 1529 | 166 | 60 | 4.17 |
| Japan | 2.4 | 13.4 | 17 | 83 | 0 | 0.00 | 12 | 0.4 | 75 | 3.74 |
| Luxembourg | 3 | 4.8 | 12 | 50 | 11 | 36.67 | 2,925 | 29 | 30 | 1.37 |
| Malaysia | 1.5 | 1.9 | 34 | 94 | 12 | 18.46 | 72 | 0.8 | 65 | 2.33 |
| Netherlands | 3.5 | 4.7 | 9 | 48 | 12 | 37.50 | 570 | 37 | 32 | 7.80 |
| Norway | 4.6 | 3.9 | 6 | 80 | 16 | 48.48 | 737 | 4 | 33 | 2.10 |
| Pakistan | 1 | 0.6 | 20 | 95 | 7 | 21.21 | 7 | 0.05 | 33 | 0.47 |
| Panama | 1.6 | 2.3 | 32 | 44 | 5 | 25.00 | 182 | 3 | 20 | 2.54 |
| Peru | 1.3 | 1.6 | 23 | 70 | 6 | 25.00 | 20 | 0.5 | 24 | 4.92 |
| Philippines | 1.3 | 1 | 34 | 82 | 3 | 5.00 | 10 | 0.6 | 60 | 0.09 |
| Poland | 2.4 | 6.5 | 12 | 36 | 15 | 57.69 | 43 | 0.5 | 26 | 0.18 |
| Portugal | 3.3 | 3.4 | 15 | 67 | 10 | 35.71 | 507 | 10 | 28 | 1.72 |
| Qatar | 0.1 | 1.2 | 27 | 65 | 8 | 26.67 | 205 | 0.3 | 30 | 1.02 |
| Republic of Korea | 2.4 | 11.5 | 10 | 17 | 0 | 0.00 | 185 | 3 | 70 | 0.62 |
| Romania | 2.3 | 6.3 | 8 | 70 | 4 | 12.12 | 75 | 2 | 33 | 2.34 |
| Saudi Arabia | 2.4 | 2.7 | 35 | 17 | 3 | 10.71 | 35 | 0.1 | 28 | 0.50 |
| Singapore | 2.3 | 2.4 | 30 | 89 | 0 | 0.00 | 137 | 0.3 | 67 | 2.37 |
| Slovenia | 3 | 4.6 | 15 | 76 | 7 | 26.92 | 329 | 4 | 26 | 3.65 |
| South Africa | 0.9 | 2.8 | 25 | 78 | 2 | 8.00 | 20 | 0.03 | 25 | 0.59 |
| Spain | 4.1 | 3 | 8 | 75 | 14 | 23.73 | 1545 | 124 | 59 | 5.76 |
| Switzerland | 4.2 | 4.7 | 12 | 67 | 10 | 29.41 | 1626 | 31 | 34 | 2.14 |
| Thailand | 0.8 | 2.1 | 35 | 83 | 5 | 6.49 | 18 | 0.09 | 77 | 0.88 |
| UK | 2.8 | 2.8 | 13 | 61 | 5 | 8.47 | 252 | 15 | 59 | 0.95 |
| Turkey | 1.8 | 2.7 | 11 | 81 | 3 | 15.00 | 88 | 1 | 20 | 4.17 |
| USA | 2.6 | 2.9 | 20 | 50 | 13 | 18.84 | 358 | 6 | 69 | 2.25 |
2. THEORY
COVID‐19 is believed to have originated in an animal meat market in Wuhan, China, and it is thought to have been transmitted from bats (Shereen et al., 2020). Within few months, the virus has rapidly spread across the world through transmissions of fluids and aerosol particles between humans. Initially, all diagnosed cases outside China had a travel history to the Wuhan market. Soon, community transfer caused exponential increases in infections in countries like Italy, USA, UK, Korea, Japan, etc. The ability of SARS‐COV‐2 to double replicate with the spike protein has posed significant challenges to the development of vaccines (Shereen et al., 2020). While hydroxychloroquine and azithromycin have been recommended by some researchers to treat COVID‐19‐infected people, there have not been many clinical trials to validate the claim (Gautret et al., 2020). Thus, until a scientifically validated cure or vaccine is developed, countries have to rely on preventive measures to contain the spread. This, in turn, depends on epidemiological studies that can predict spreading patterns so that policymakers can take appropriate protective measures. Several viruses including SARS have been reported to be vulnerable to hot temperatures, which results in differences in spreading patterns across geographic locations (Zhang et al., 2020). However, such geographic variations have not yet been analysed for COVID‐19. Other factors like government policies and interventions, infrastructure availability, and the severity of the infection itself can affect the ability of a country to contain epidemics and pandemics. This research seeks to explore all the above factors.
2.1. Factors affecting early containment of the COVID‐19 pandemic
2.1.1. Environment
Climatic conditions such as temperature and humidity play an important role in both airborne and aerosol virus transmissions. The 30‐year human relationship with the influenza virus has proven that the mortality rate is directly related to temperature and humidity (Lowen & Steel, 2014). Hence, in order to minimize transmission of diseases, isolation wards in hospitals generally tend to have optimized pressure, temperature, and humidity (WHO, 2014). Research on the virus on the Diamond Princess cruise ship off the coast of Japan showed that a 1°C rise in temperature and a 1% increase in pressure could reduce the reproduction number R 0 down by 0.0224–0.0383. It must be mentioned that the generalizability of the study is questionable because the ship was a contained environment and the results may not apply to the real world (Sheng et al., 2020). Certain studies in China and Indonesia have investigated the relationship between temperature and the spread of infection and resultant deaths and have reported a low to medium level of correlation (Yueling Ma et al., 2020; Tosepu et al., 2020). Relative humidity was found to have low to no correlation with infection spread or deaths. Global warming has also been a reason for recent temperature increases, and certain studies indicate that this can reduce flu‐based viral infections (Actuaries, 2010; Dincer, Midilli, Hepbasli, & Karakoc, 2010; National Research Council, 2001). However, these statements need to be further validated.
While the spread of the virus may be affected by climatic conditions, once the virus enters the human body, it is independent of the outside environment. However, since the virus lives outside the human body for a period of at least 12 hr under normal conditions (Richard, 2020), it is necessary to study the effects of the environment on the spreading patterns itself.
2.1.2. Policies and interventions
Social distancing
Social distancing, although a new terminology for the twenty‐first century, is not a new approach to epidemic control. It was used by the UK in 1918 to control the influenza virus outbreak that caused about 100 million deaths. Social distancing involves the avoidance of mass gatherings and distancing of at least 6 feet between people. Such measures are combined with enhanced personal hygiene through regular hand washing and wearing protective masks for flu‐like outbreaks (Leung, Ball, Sirl, & Britton, 2018; Yu, Lin, Chiu, & He, 2017). This is done primarily because flu‐causing viruses are spread through aerosols generated from saliva and nasal fluid, which can be transmitted across distances as much as 3 feet. The average lifetime of SARS‐CoV‐2 in the outer environment is believed to be about 12 hr, which increases transmissivity because aerosols from infected people can settle on doorknobs, lifts, etc. in places such as means of transport, hotels, and malls and stay active for a long time, thus increasing the window of transmission. Direct physical contact, such as handshaking, is also an avenue of transmission of the virus.
The reduction of social contact has been proven to significantly reduce flu‐like diseases (Maharaj & Kleczkowski, 2012). The closure of schools and malls flattened the spread curve during the influenza pandemic in 2009 (MOH, 2014; Rashid et al.,n.d.). Thus, governments worldwide have stressed on social distancing and quarantining measures for at least 14 days – the typical incubation period of SARS‐CoV‐2 – to contain its spread (Prem et al., 2020).
Lockdowns
Lockdown is a preventive strategy taken by local, central, or global administrations during the spread of epidemic or pandemic diseases and involves stopping transportation between cities, provinces, or countries. The world has so far seen four major pandemics, viz. the plague in the fourteenth century, influenza in 1918, SARS in 2009, and the current COVID‐19 in 2019 as reported by the WHO (East et al., 2020; Pi, 2020; Porta, 2008). In all these cases, lockdowns were implemented by various countries to control the outbreaks. China announced a lockdown as early as January 2020 to flatten the curve of the COVID‐19 infections over time. In March, most countries around the globe announced lockdowns of local transport, offices, industries, and city and national borders to contain the virus (Callaway, Cyranoski, Mallapaty, Stoye, & Tollefson, 2020). Although quarantine centres for the infected are available in hospitals, large‐scale infections necessitate self‐quarantines and lockdown measures in addition to the hospital‐based quarantines (Wuhan, 2020).
2.1.3. Healthcare infrastructure
During epidemic and pandemic viral outbreaks, the availability of and access to healthcare infrastructure such as hospitals, beds, healthcare workers, clinical equipment, first aid kits, ventilators, and protective equipment are vital to pandemic management (Bambas & Drayton, 2000; Persoff, Ornoff, & Little, 2018). During the massive influenza outbreak of 1918, even developed countries had inadequate healthcare infrastructure, which further expanded the outbreak (Miller, Randolph, & Patterson, 2008). The Ebola outbreak in West Africa became uncontrollable due to lack of infrastructure facilities (Paweska et al., 2017). After the outbreak, the WHO in South Africa had asked the hospitals to report their available facilities to plan for future infections optimally (Murrin, 2018). Innovative measures have been recommended, to create necessary healthcare infrastructure during pandemic and epidemic situations by converting schools, colleges, theatres, and stadiums into hospitals and quarantine centres (Nuzzo et al., 2019; Wimberly, 2018). Healthcare workers supported by NGOs, youth, and volunteers also play a significant role in containing outbreaks (Itzwerth, 2013). Hence, studying healthcare infrastructure availability across countries can predict COVID‐19 containment at an early stage.
2.2. Predictive modelling
Predictive modelling using machine learning and growth models can provide actionable insights for policy makers and governments to contain epidemic and pandemic infections (Thompson, Brooks‐pollock, & Thompson, 2019). During the onset of an epidemic, it is crucial to use exponential growth models to understand the infection rates, and with proper policy implementation and behavioural changes among the susceptible groups of the population, the slope reduces and the curve flattens over time (Keeling & Danon, 2009). For other outbreaks like smallpox, Ebola, SARS, and influenza, various studies have used mathematical and statistical modelling to understand the growth of infections (Dietz, 2002; Kerkhove & Ferguson, 2020; Nishiura, 2011). In fact, the Centres for Disease Control and Prevention published an exclusive book with established procedures for analysing disease outbreaks, stressing on the importance of such modelling studies (Dicker, 2006). In outbreaks, epidemiologists generally use the exponential growth model at the onset of an outbreak and proceed with prediction and classification techniques like regression, decision trees, neural networks and deep learning, etc. to forecast outbreaks (Sameni, 2020; Victor, 2020).
There are few studies on modelling and predicting containment of COVID‐19 so far (Lin et al., 2020; Prem et al., 2020). The research work reported in this paper sought to integrate crucial variables concerning infrastructure, environment, policies, and severity of the disease to predict initial signs of containment. The study used a machine learning and exponential growth model. The variables used as part of the predictive mode were: doctors per 1,000 population, beds per 1,000 population, average temperature, average humidity, days since official lockdown, percentage of lockdown days, total cases per million population, deaths per million population, days since the first contact, and percentage of serious cases of infection.
3. DATA COLLECTION, ANALYSIS, and RESULTS
3.1. Data collection
Data associated with the variables were collected from different official sources for a total of 42 countries with respect to COVID‐19 infections as on March 26, 2020. This accounts for 448,989 COVID‐19 cases representing 84.78% of the total infections worldwide. The daily number of infections, recovery, and deaths were collected from the WHO website. The data for infrastructure‐centred variables like the number of hospitals and the number of doctors were taken from the World Bank website (World Bank, 2020). Environment‐based variables like average temperature and humidity since the onset of COVID‐19 were taken from the Weather Underground website (Weather Underground, 2020). Day‐wise COVID‐19 case distributions extracted from the WHO were used to identify countries that showed signs of containment of the virus based on a novel exponential growth modelling approach. Raw data from the sources were also consolidated, and the variables physicians per thousand individuals, hospitals per thousand individuals, percentage of lockdown days since the first contact, cases per million population, deaths per million population, days since the first case, serious cases per thousand infections, average temperature since the first infection, and average humidity since the first infection were calculated to train the machine learning models.
3.2. Analysis plan
Most epidemic and pandemic diseases grow exponentially in the initial stages of the outbreak in a country (Ma, 2020). A popular modelling technique that demonstrates this is the susceptible‐infectious‐recovered (SIR) model (Kermack & McKendrick, 1927). If S denotes the fraction of susceptible individuals to a pandemic, I indicates the fraction of infectious people, R is the fraction of recovered patients, β indicates the transmission rate per infectious individual, and the recovery rate is denoted by γ, the infectious period is exponentially distributed with a mean of 1/γ as shown below:
Linearizing this about the disease‐free equilibrium, we get the following.
Hence from the above expression, if , then the infection function I(t) grows exponentially about the disease‐free equilibrium point. In addition to this, at the onset of the infection, , and hence the incidence rate also grows exponentially. Hence, modelling the initial stages on a pandemic like COVID‐19 is both relevant and crucial in understanding the growth of the infection. Although sub‐exponential and polynomial modelling have worked in cases of outbreaks like Ebola, HIV, and foot and mouth diseases (Chowell, Viboud, Simonsen, & Moghadas, 2016), they generally work well with proceeding generations too. For pandemics like COVID‐19, the exponential growth model is relevant, and the use of least squares at the initial stages can afford precise insights.
The exponential growth model bears the expression , where “a” is a function of the initial cases reported and “b” depends on the rate at which the infection spreads. This model is extremely sensitive to the initial few periods, and analysis of the last few data points concerning the model itself can assist in understanding if the interventions and policy implementations by the government of a country have been effective in terms of containing the infection. Other factors like infrastructure, availability of doctors, and temperature and humidity of the country during the spread can also significantly affect the growth rates of the infection. However, the reason for using the exponential growth model for this research is to understand if the actual infections in a country are lower than the predicted infections for the past few days and to classify countries accordingly, thereby forming base data to design a classifier with this as the dependent variable. The past seven time periods were considered for comparison with the predicted values of the corresponding model for a particular country. If these actual data points of a particular country were significantly lower than the predicted values with the exponential growth model, it indicated the initial signs of containment owing to several factors like policies, infrastructures, behavioural changes, actions, etc.
This sign of containment was then used as a dependent variable for machine learning models like logistic regression, support vector machines, decision trees, and random forest algorithms. The independent variables for the study included physicians per thousand individuals, hospitals per thousand individuals, percentage of lockdown days since the first contact, cases per million population, deaths per million population, days since the first case, serious cases per thousand infections, average temperature since the first infection, and average humidity since the first infection. A combination of infrastructure, infection, policies, and environmental‐related variables was used to train the model. A comparative analysis of the accuracy and error metrics in terms of predicting the country's ability to contain the infection for the corresponding algorithms are reported. Python was used to perform all the analysis and learning model developments for this research. Figure 1 shows the analysis plan to achieve the objectives of the research.
FIGURE 1.
Analysis plan [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Exponential growth model
The exponential growth model assumes that the onset of any outbreak follows an exponential distribution. However, due to government interventions, medical innovations, behavioural changes, etc., at a later stage, the growth curve flattens and the rate of infections gradually reduces (Kermack et al., 1927). To identify such signs, we looked at the infections in the last seven‐day period, and the deviation of the data points from the modelled exponential curve was captured using the mean absolute percentage error metric. Based on the errors and the direction in which the actual data points were to the modelled growth curve, the countries were classified according to whether they showed initial signs of containment or not.
3.4. Machine learning models
In line with the objectives of the study, classifiers were built based on a set of independent variables to predict if a country that has COVID‐19 infections showed early signs of infection containment as a reflection of policy implementations and behaviour changes. Logistic regression was used to understand the list of independent variables significantly affecting the infection containment and their corresponding importance in the model. Then, to predict signs of early containment, machine learning algorithms like logistic regression, decision trees, and random forest and support vector machines were used and their corresponding accuracies were compared. For all models, fivefold cross‐validation was done to address overfitting.
3.4.1. Logistic regression
Logistic regression by le Cessie and van Houwelingen (1992) is a generalized linear model (GLM) and is one of the most widely used classifiers. According to Kondofersky & Theis (2018), when there is binary response, as in this research, by using logistic regression one typically aims at estimating the conditional probability , where . As with simple linear regression, bearing equation , estimating the dependent variable y directly, the logistic regression estimates p using the following equation:
As with multiple linear regression, logistic regression can also handle multiple independent variables, and its probability estimate can be represented as follows:
The conditional probability can be calculated using the odds ratio . The significance of the beta coefficient values in the above equation can be tested to see if their corresponding independent variables are influencers of the dependent variable. A Wald test is generally conducted to evaluate the statistical significance of the coefficients in the model. Since logistic regression falls under the category of GLM, the significance of each independent variable in predicting the outcome of the dependent variable, signs of early containment, can be studied.
3.4.2. Decision tree
A decision tree is a decision support model that illustrates the consequences, chances, and event outcomes of certain decisions. Decision trees are used as a predictive model to make statistical conclusions about an item's target value, based on observations. In this tree structure, leaves represent class labels and branches represent conjunctions of features that lead to those class labels. There are both classification trees where the response variable takes on a set of categorical values and regression trees where the response variable takes on a set of continuous values. The collective name for such trees is classification and regression trees (CART), first introduced and developed by Breiman, Friedman, Olshen, & Stone (1984). Decision trees use two metrics, namely entropy and information gain, to arrive at the final tree. Entropy is the measure of the total amount of uncertainty in the data set and is given as follows:
where
S is the data set for which entropy is to be calculated,
C the set of classes in the data set S, and
p(c) the ratio of the number of elements in class c to the number of elements in set S.
When the entropy value is equal to 0, the data set S is perfectly classified. The information gain metric is defined as the measure of the difference in the entropy from before to after the data set S is split based on an attribute A and is given as follows:
where
H(S) is the entropy of the data set S,
T the subsets created by splitting the data set S by attribute A so that ,
p(t) the ratio of the number of elements in class t to the number of elements in set S, and
H(t) the entropy of set t.
Once the information gain and entropy are calculated, decision trees follow a series of steps as follows to arrive at the final tree:
Step 1: Compute entropy for the data set.
-
Step 2: For every feature in the data set, compute the following:
Calculate the entropy for all the categorical values.
Find the average information entropy for current attributes.
Calculate the gain for current attributes.
Step 3: Select the attribute with the highest gain.
Step 4: Repeat from step 1 till the desired tree is achieved.
3.4.3. Random forest
Introduced by Breiman (2001), random forest is a statistical supervised machine learning technique that we used for both regression and classification. This is an ensemble learning technique that uses an averaged combination of many decision trees for the final prediction. The technique of averaging a statistical machine learning model is called bagging, and it improves stability and avoids overfitting (Hastie, Tibshirani, & Friedman, 2009). Normally, decision trees are not competitive to the best‐supervised learning approaches in terms of prediction accuracy since they tend to have high variance and low bias. This is because building two different decision trees can yield two different trees. Bagging is therefore well suited for decision trees since it reduces the variance. The idea behind random forests is to draw bootstrap samples from the training data set and then build several different decision trees on the different training samples. This method is called random forest because it chooses random input variables before every split when building each tree. By doing this, each tree would have reduced covariance, which, in turn, would lower the overall variance even further (Hastie et al., 2009). The random forest algorithm has two stages – random forest creation followed by random forest prediction. The steps involved in the stages are as follows:
Stage I: random forest creation
Step 1: Randomly select “k” features from the total “m” features available in the data set where k << m.
Step 2: Using the best split point, calculate the node “d” among the selected “k” features.
Step 3: Split the node into daughter nodes using the best split.
Step 4: Repeat steps 1 to 3 until “l” node is reached.
Step 5: Repeat steps 1 to 4 for “n” number of times to create a forest of “n” number of trees.
Stage II: random forest prediction
Step 1: Using the features and applying the rules of randomly selected decision trees, predict the outcome and store it as a predicted target.
Step 2: Calculate the votes for each predicted target.
Step 3: The highest voted predicted target will be the prediction of the random forest algorithm.
3.4.4. Support vector machine
The objective of the support vector machine (SVM) is to find a line that best separates the data into multiple groups. This is achieved by an optimization process supported by the data in the training set. These instances are called support vectors, and they play a crucial role in the classification process (Flake & Lawrence, 2002). Finally, few data sets can be separated with just a straight line. Sometimes a line with curves or even polygonal regions must be marked. This is achieved with the SVM by projecting the data into a higher‐dimensional space to draw the lines and make predictions. SVMs calculate a maximum margin around the boundary that ultimately results in a homogenous partition. The ultimate goal is to establish a margin as wide as possible. In order to do so, a Lagrange multiplier has to be constructed as follows and maximized:
where
w is a vector of arbitrary length with a constraint of being perpendicular to the median of the margin, and
x is the sample feature data.
Not in all cases are the data linearly separable, and hence a transformation onto a new space is necessary. For this purpose, a variety of kernel functions is used. A sample linear kernel function is as follows:
3.5. Results
3.5.1. Exponential growth model
Table 2 shows the results of exponential growth models for different countries and whether the countries showed a sign of containment or not based on the results. Figures 2 and 3 show the infections for the countries and the infections predicted by the corresponding exponential growth model. Brazil, Canada, Ecuador, France, Germany, India, Italy, Luxembourg, Malaysia, Pakistan, Panama, Philippines, Romania, South Africa, Spain, the UK, Turkey, and the USA did not show any signs of early containment as the infections in the last seven‐day periods exceeded or followed the exponential growth model. The countries that showed initial signs of containment were Austria, Chile, China, Czechia, Denmark, Greece, Iceland, Indonesia, Iran, Ireland, Israel, Japan, Netherlands, Norway, Peru, Poland, Portugal, Qatar, Republic of Korea, Saudi Arabia, Singapore, Slovenia, Switzerland, and Thailand.
TABLE 2.
Exponential growth model and signs of early containment
| Country | Number of time periods, n | Coefficient a | Coefficient b | Standardized coefficient β | F value | R 2 | Lower than predicted/sign of early containment |
|---|---|---|---|---|---|---|---|
| Austria | 31 | 1.302 | 0.337 | 0.991*** | 781.241 | 0.982 | YES |
| Brazil | 30 | 0.481 | 0.291 | 0.978*** | 303.991 | 0.956 | NO |
| Canada | 61 | 1.176 | 0.128 | 0.937*** | 101.457 | 0.879 | NO |
| Chile | 24 | 0.750 | 0.366 | 0.991*** | 758.273 | 0.982 | YES |
| China | 65 | 490.301 | 0.283 | 0.986*** | 498.685 | 0.973 | YES |
| Czechia | 26 | 2.093 | 0.319 | 0.995*** | 1,332.385 | 0.990 | YES |
| Denmark | 29 | 0.452 | 0.456 | 0.984*** | 413.802 | 0.967 | YES |
| Ecuador | 26 | 5.807 | 0.104 | 0.955*** | 144.175 | 0.911 | NO |
| France | 63 | 2.484 | 0.078 | 0.907*** | 64.943 | 0.823 | NO |
| Germany | 60 | 2.548 | 0.135 | 0.863*** | 40.822 | 0.745 | NO |
| Greece | 30 | 1.266 | 0.305 | 0.971*** | 234.059 | 0.944 | YES |
| Iceland | 28 | 1.540 | 0.324 | 0.937*** | 100.483 | 0.878 | YES |
| India | 57 | 1.331 | 0.068 | 0.737** | 16.614 | 0.543 | NO |
| Indonesia | 25 | 0.818 | 0.349 | 0.981*** | 360.233 | 0.963 | YES |
| Iran | 37 | 2.963 | 0.474 | 0.989*** | 621.521 | 0.978 | YES |
| Ireland | 27 | 0.691 | 0.347 | 0. 976*** | 277.177 | 0.952 | YES |
| Israel | 35 | 0.450 | 0.280 | 0.981*** | 349.631 | 0.961 | YES |
| Italy | 56 | 1.826 | 0.038 | 0.861*** | 40.091 | 0.741 | NO |
| Japan | 65 | 1.106 | 0.229 | 0.968*** | 206.096 | 0.936 | YES |
| Luxembourg | 27 | 0.314 | 0.295 | 0.938*** | 102.131 | 0.879 | NO |
| Malaysia | 62 | 3.259 | 0.103 | 0.959*** | 160.352 | 0.920 | NO |
| Netherlands | 29 | 1.341 | 0.441 | 0.968*** | 207.786 | 0.937 | YES |
| Norway | 30 | 1.436 | 0.411 | 0.969*** | 216.713 | 0.939 | YES |
| Pakistan | 30 | 1.628 | 0.141 | 0.931*** | 91.720 | 0.868 | NO |
| Panama | 17 | 4.036 | 0.320 | 0.942*** | 110.033 | 0.887 | NO |
| Peru | 21 | 1.119 | 0.375 | 0.980*** | 347.076 | 0.961 | YES |
| Philippines | 57 | 1.109 | 0.078 | 0.879*** | 47.546 | 0.773 | NO |
| Poland | 23 | 1.107 | 0.389 | 0.980*** | 347.425 | 0.961 | YES |
| Portugal | 25 | 1.693 | 0.354 | 0.990*** | 720.564 | 0.981 | YES |
| Qatar | 27 | 0.794 | 0.394 | 0.952*** | 134.907 | 0.906 | YES |
| Republic of Korea | 65 | 0.880 | 0.215 | 0.977*** | 289.019 | 0.954 | YES |
| Romania | 30 | 0.787 | 0.244 | 0.972*** | 241.849 | 0.945 | NO |
| Saudi Arabia | 25 | 0.659 | 0.368 | 0.983*** | 394.381 | 0.966 | YES |
| Singapore | 64 | 1.681 | 0.202 | 0.968*** | 206.524 | 0.937 | YES |
| Slovenia | 22 | 3.721 | 0.330 | 0.954*** | 141.131 | 0.910 | YES |
| South Africa | 22 | 0.600 | 0.381 | 0.987*** | 520.753 | 0.974 | YES |
| Spain | 55 | 0.812 | 0.065 | 0.868*** | 42.667 | 0.753 | NO |
| Switzerland | 31 | 1.404 | 0.421 | 0.969*** | 215.328 | 0.939 | YES |
| Thailand | 65 | 3.113 | 0.152 | 0.935*** | 97.258 | 0.874 | YES |
| UK | 56 | 1.213 | 0.132 | 0.914*** | 71.525 | 0.836 | NO |
| Turkey | 61 | 0.644 | 0.573 | 0.982*** | 370.097 | 0.964 | NO |
| USA a | 31 | 526.875 | 0.293 | 0.996*** | 1749.121 | 0.992 | NO |
Dataset time period – 22 January to 26 March 2020.
Data from onset not available.
p < .05.
p < .01.
p < .001.
FIGURE 2.
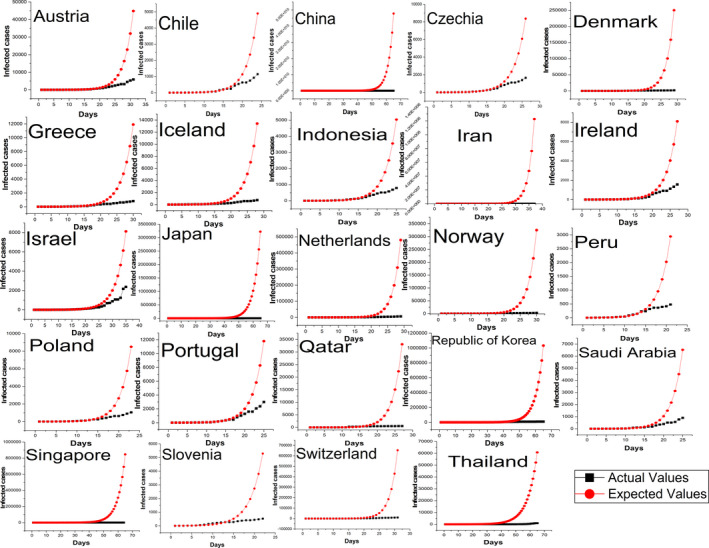
Countries showing initial level of containment of COVID‐19 [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
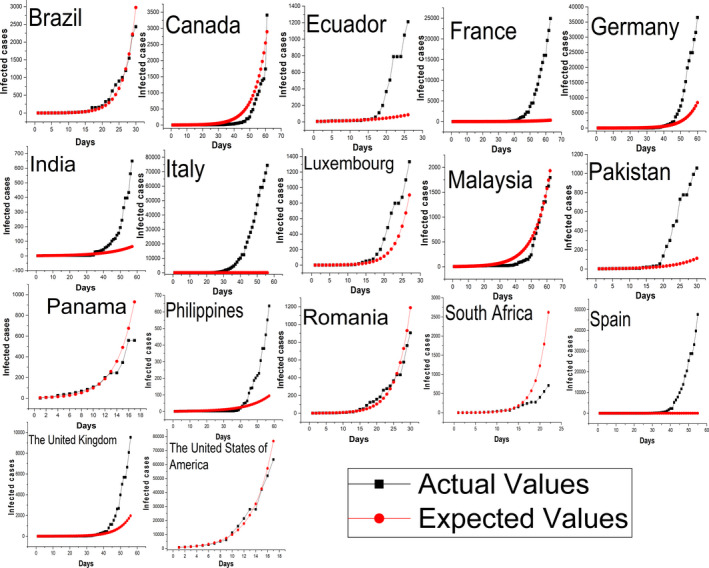
Countries not showing initial level of containment of coronavirus disease 2019 (COVID‐19) [Colour figure can be viewed at wileyonlinelibrary.com]
3.5.2. Logistic regression
Table 3 shows the results for logistic regression with early containment as the dependent variable. Of all the independent variables, the availability of beds in hospitals and the percentage of lockdown days significantly and positively affected the signs of early containment. Other variables did not significantly influence the dependent variables. The model had an accuracy of 78.57% in the classification. The true positive and false negative rates were found to be 78.6% and 21.6%, respectively. Precision and recall values were 0.788 and 0.786. The F1 score and receiver operating characteristic (ROC) values were found to be 0.786 and 0.755, respectively.
TABLE 3.
Logistic regression results
| Independent variable | Regression coefficient | Wald |
|---|---|---|
| Doctors per 1,000 population | 0.215 | 0.209 |
| Beds per 1,000 population | 0.241** | 2.773 |
| Average temperature | 0.052 | 0.766 |
| Average humidity | −0.004 | 0.040 |
| Days since official lockdown | −0.098 | 0.757 |
| Percentage of lockdown days | 9.998*** | 3.410 |
| Total cases per million population | 0.000 | 0.025 |
| Deaths per million population | −0.026 | 0.799 |
| Days since first contact | 0.001 | 0.001 |
| Percentage of serious cases of infected | −1.151 | 0.005 |
Accuracy – 78.6%. Dependent variable – early containment.
p < .05.
p < .01.
p < .001.
3.5.3. Decision tree
A J48 decision tree was constructed for predicting early infection containment with the independent variables listed in Figure 1. The batch size was set to 10, and the confidence factor was selected as 0.25. The minimum number of objects on the tree was set to 2. The accuracy of the tree was found to be 80.95%. The variables in the decision tree were percentage of lockdown days, days since official lockdown, and death rate per million population. The decision tree is shown in Figure 4. The true positive and false negative rates were found to be 81% and 25.4%, respectively. Precision and recall values were 0.857 and 0.81. The F1 score and ROC values were found to be 0.796 and 0.852, respectively.
FIGURE 4.
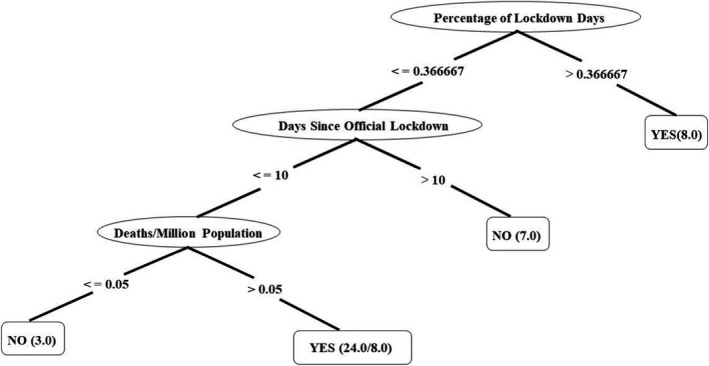
Decision tree for infection containment
3.5.4. Random forest
A random forest ensemble algorithm was created with 100 combined trees. The batch size was selected as 10, and the depth of the trees was set to unlimited. Other metrics for the random forest algorithm are given in Table 4. This model reported a high accuracy figure of 92.9% in correctly classifying countries that showed signs of early containment. The true positive and false negative rates were found to be 92.9% and 8.1%, respectively. Precision and recall values were 0.929 and 0.929. The F1 score and ROC values were found to be 0.928 and 0.993, respectively.
TABLE 4.
Accuracy metrics for machine learning models
| True positive rate | False positive rate | Precision | Recall | F measure | ROC | Accuracy | |
|---|---|---|---|---|---|---|---|
| Logistic regression | 0.786 | 0.216 | 0.788 | 0.786 | 0.786 | 0.775 | 0.786 |
| Decision tree | 0.81 | 0.254 | 0.857 | 0.81 | 0.796 | 0.852 | 0.810 |
| Random forest | 0.929 | 0.081 | 0.929 | 0.929 | 0.928 | 0.993 | 0.929 |
| Support vector machine | 0.762 | 0.262 | 0.761 | 0.762 | 0.76 | 0.75 | 0.762 |
Abbreviation: ROC, receiver operating characteristic.
3.5.5. Support vector machine
In order to make predictions for signs of early containment, an SVM was modelled for 500 epochs and a learning rate of 0.01. The hinge loss function with stochastic gradient descent was used to model the loss, and batch size was set to 10. The epsilon threshold was set to 0.001, and the regularization constant was set to 10–4. The resultant model produced an accuracy of 76.19% in classifying the dependent variable. The true positive and false negative rates were found to be 76.2% and 26.2%, respectively. Precision and recall values were 0.761 and 0.762. The F1 score and ROC values were found to be 0.76 and 0.75, respectively.
On fivefold cross‐validation with the data for all the algorithms and models, it can be inferred that the random forest design produces the minimum error and maximum accuracy as reported in Table 4. It outshines all the other machine learning algorithms constructed in the study. The J48 decision tree, logistic regression, and SVM produce almost similar levels of accuracy in predicting the signs of containment of COVID‐19.
4. DISCUSSION
This research is one of the first of its kind to integrate exponential growth modelling with machine learning techniques for predicting the spread of COVID‐19. The research presents machine learning models based on variables such as infrastructure, environment, policies, and the infection itself to predict early signs of containment in a country. For the purpose, disease data from 42 leading countries in COVID‐19 infections were taken, and exponential growth modelling was used to see if the countries showed signs of containment. Then, with the signs of the early containment of the infection as a dependent variable, supervised machine learning predictive models including logistic regression, decision tree, random forest, and support vector machine were developed. This research can directly be of use to countries and policymakers to understand if their proposed interventions are effective in containing infections even during early stages. Table 5 shows the steps taken by countries to contain COVID‐19 infections.
TABLE 5.
Actions and policies of governments to contain COVID‐19
| Country | Other actions taken by the government |
|---|---|
| Austria | Tightened rules to contain spread of coronavirus, people were quarantined, and a fine of up to 30,000 Euros was levied for violating the rules (Gesley, 2020) |
| Brazil | Employees at the airport were asked to wear a mask. Borders were closed for flights from affected countries (CDCP, 2020) |
| Canada | All travellers were forced to self‐isolate for 14 days upon entry to control the outbreak (GC, 2020) |
| Chile | Screening at the airport was enhanced, and people with symptoms were isolated (U.S Embassy in Chile, 2020) |
| China | Transmission dynamics of SARS‐CoV‐2 in different settings was analysed, and eventually the country went for a lockdown to control the exponential outbreak (WHO, 2020c) |
| Czechia | Instructions were provided by Medicaid Service in all hospitals to protect kids, adults, and aged people from infection (Grafton, 2020) |
| Denmark | Testing and quarantining at an early stage itself (Carstensen, 2020b) |
| Ecuador | Maximized control by implementing innovative licensing strategies on products to fight COVID‐19 (Silverman, 2020) |
| France | Closed down borders and special employment forces were used to contain infections. Necessary precautions were taken for the localities as well (Barbière, 2020) |
| Germany | Employed a rigorous door‐to‐door testing procedure. The infected and suspected people were quarantined with special care leaving the mobility undisturbed (Sepkowitz, 2020) |
| Greece | Greece tightened measures, all new arrivals to be quarantined (Euractiv, 2020) |
| Iceland | Infected persons were immediately transferred to a special infection control section and were quarantined from their relatives and general public (Kyzer, 2020) |
| India | Tracked travellers from affected countries and quarantined them including family members in their own home (Diwanji, 2020) |
| Indonesia | Made public calls for people to self‐isolate if they have symptoms (Nyoman Sutarsa, 2020) |
| Iran | Followed strict social distancing and lockdown (Duddu, 2020) |
| Ireland | Invested in massive testing facilities. Treated all patients equally irrespective of their income strata. All hospitals operated on a not‐for‐profit basis (BBC, 2020 |
| Israel | Used technology to track the movement of infected individuals with their mobiles and quarantined the people who came in contact with the individual (Lomas, 2020) |
| Italy | Though Italy closed borders during the onset, lack of proper testing facilities caused a massive outbreak. This was followed by a strict lockdown (Gary, 2020) |
| Japan | Managed the outbreak with rules and excellent medical infrastructure (Japan, 2020) |
| Luxembourg | Quarantined people over 60 years to reduce casualties (Piscitelli, 2020) |
| Malaysia | Banned entry of people from infected countries followed by additional screening measures at the airport. Promoted personal hygiene eventually followed by a lockdown (Garda World, 2020a) |
| Netherlands | Travellers returning from affected countries were advised to visit doctors and medical facilities if symptoms were felt. Post outbreak, the country went under lockdown (Garda World, 2020b) |
| Norway | Travel bans and closure of schools, public services like gyms, malls, theatres, etc. (Norway Panorama, 2020) |
| Pakistan | Formed a team to monitor the situation and take necessary actions on a daily basis (Pakistan, 2020) |
| Portugal | Employed strict lockdown (Oliveira, 2020) |
| Qatar | Proper tracking and strict screening and testing of travellers (Master of Public Health, 2020) |
| Republic of Korea | Proactively built a centralized testing and quarantine facility before an outbreak in the country. China's reports triggered this action (Beaubien, 2020) |
| Romania | Lockdown and border closing (Gherasim, 2020) |
| Singapore | With previous experience from SARS pandemic, the country had a proper infrastructure facility with negative pressure room for pandemic control. Testing was done rigorously, and the infected were not let back into society. Migrants from other countries were not allowed to work until the pandemic was controlled (Fisher, 2020b) |
| Slovenia | Used innovative ways to spread COVID‐19 control messages before going into lockdown (Slovenija, 2020) |
| South Africa | Immediately restricted entry and exit to affected countries. Declared as a national disaster and went into lockdown to prevent a major outbreak (Fihlani, 2020) |
| Spain | Local movement controlled by social distancing. Travel to an affected country completely banned. Enhanced medical attention at arrival to control the spread (Kate Mayberry, 2020) |
| Switzerland | After closing school, colleges, and non‐essential businesses, the country used their military and civilian support to enhance infrastructure and healthcare needs to contain the infection (Keystone, 2020) |
| UK | People with symptoms were asked to self‐quarantine. Cancelled overseas travel and only tested people who were admitted. Followed social distancing, lockdown, isolation, and house quarantine. The country did not force people for testing (Yong, 2020) |
| USA | Enforced travel restrictions and implemented mandatory quarantine in New York. A level of screening and lockdown was implemented (Brittany Shammas et al., 2020) |
Abbreviations: COVID‐19, coronavirus disease 2019; SARS, severe acute respiratory syndrome; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
This research identifies countries that have been successful or have shown signs of containing COVID‐19 since infection using the exponential growth modelling in stage I of the research. Various studies have used statistical models like exponential growth, decay and exponential adjustments, computational modelling, and stochastic simulations to model the reproductive number of the COVID‐19 infections (Dhungana, 2020; Liu, Gayle, Wilder‐Smith, & Rocklöv, 2020; Wu, Leung, & Leung, 2020). However, the estimations have varied from 1.50 to 6.47 for the same data set. Hence, at the onset of a pandemic, it is challenging to accurately calculate the reproduction number. The only possible conclusion that we can derive at this point from the exponential growth model or any statistical model is an understanding of whether a country shows signs of early containment or not. This research classified the 42 countries considered for the study according to their signs of early containment.
Logistic regression results prove the need for infrastructure and the percentage of lockdown days as significant factors to contain infections. This research also proves that environmental factors like temperature and humidity do not significantly affect spreading patterns or contain COVID‐19 infections in the early stages. The results come in line with studies conducted in China and Indonesia that have reported low to no correlation of temperature and humidity to the infection growths and deaths (Yueling Ma et al., 2020; Tosepu et al., 2020). However, the long‐term effect of environmental factors on the infection rates may prove to be significant. Decision tree analysis also shows that early signs of containment are possible if the number of lockdown days is at least 33.7% of the days since the first contact till the containment of the infection. If that is not the case, countries show recovery signs if the lockdown is at least 10 days or more. For countries with a lockdown period less than 10 days, the variable depicting the number of deaths per million population plays a significant role in containing the infection. This variable is indirectly related to the healthcare infrastructure of countries like beds, physician, ventilators, intensive care units, etc. Hence, in any pandemic situation, governments must be proactive and frame policies even at the onset, thereby reducing the risk of spread, which would ultimately lead to early containment. This also emphasizes on the need for resilient healthcare infrastructure to contain infections at an early stage. The machine learning models random forest and support vector machines were able to classify the countries with respect to their signs of early containment with an accuracy of 92.9 and 76.2 percentages, respectively, proving random forest to be the best machine learning algorithm for the problem studied. Although this research applies data from only 42 countries, the proposed models with their corresponding hyperparameters can be extended to predict early containment for the other countries as well. Similarly, although these models were built only for the COVID‐19 pandemic, they can serve as a base for other future pandemics that have similar characteristics and reproduction numbers thereby giving governments the necessary information to take timely actions to protect both people and the economy.
While almost all countries practised lockdowns to contain the virus, certain countries have also taken some unique additional measures to contain the infection. China is one of the very first countries in the world to contain and control COVID‐19. China used policy changes in terms of lockdowns, travel restrictions, infrastructure development, and machine learning to properly predict and flatten the infection curve during its first wave. Studying the transmission dynamics of SARS‐CoV‐2 in different settings and continually measuring the ongoing progress and impact lead to containment (WHO, 2020b). Austria enforced strict rules such as social distancing, closure of schools and colleges, and the closing of entertainment and grouping places, and this has led to initial signs of containment. The country passed a special act called COVID‐19 Act which has proven to be effective to contain the infection (Library of Congress, 2020). The number of hospital beds per 1,000 population in Austria was also high, which facilitated early recovery. Chile has implemented sanitary barriers and intense screening mechanisms to track and quarantine the infected (US Embassy, 2020). In addition to tough quarantine measures, Denmark closed down schools and also announced a lockdown in March. Employers were also instructed not to cut the salaries of the employees in quarantine, thereby encouraging social distancing and hence containing the infection (Carstensen, 2020a). Japan, South Korea, and Singapore did not announce any lockdowns. South Korea used processes that led to early detection of the COVID‐19 and quarantining the infected, thus stopping spread. They also predicted the movement of viruses, and tactical interventions were taken to minimize spread (NPR, 2020). Singapore had a ready infrastructure with isolation wards in place during the SARS outbreak and was readily equipped, which led to early containment of COVID‐19. Strong community engagement messages and communications from the government also led to better pandemic management in Singapore (Fisher, 2020a). Most other countries that showed early signs of recovery rigorously followed lockdowns, social distancing, travel restrictions, and testing to contain infections. Another reason for countries like Japan, Korea, and Austria to contain the infection was the availability of strong healthcare infrastructures in these countries to address the infections. The various actions taken by the government to control the transmission of COVID‐19 are shown in Table 5.
Countries like Italy, Brazil, India, Malaysia, Pakistan, UK, etc. do not have the necessary healthcare infrastructure to support mass admission of COVID‐19 patients and hence need to rely on intense lockdowns to contain the infections. The increase in the number of COVID‐19 cases in the USA and the inability to contain it is also due to the late lockdown decision of the government post outbreak. The percentage of lockdown days since the first infection continues to be too low for these countries to be on a recovery path against the infection. With time, there is a high probability that the infection will be contained. However, in the long run, these countries must invest in improving healthcare facilities to reduce causalities during pandemics. Countries must be prepared for epidemics and pandemics, and proactive policies and infrastructure as in the case of Singapore can save more lives than reactive measures. It is evident that COVID‐19, unlike SARS, will not be controlled by environmental factors, and any future outbreaks will still rely on healthcare infrastructures, timely lockdowns, and social distancing for containment.
CONFLICT OF INTEREST
There is no conflict of interest for any of the authors of this paper.
ETHICAL APPROVAL
This research adhered to the highest level of ethics.
Kasilingam D, Sathiya Prabhakaran SP, Rajendran DK, Rajagopal V, Santhosh Kumar T, Soundararaj A. Exploring the growth of COVID‐19 cases using exponential modelling across 42 countries and predicting signs of early containment using machine learning. Transbound Emerg Dis.2021;68:1001–1018. 10.1111/tbed.13764
DATA AVAILABILITY STATEMENT
The data are openly available in the World Health Organization reports.
REFERENCES
- Actuaries, M. B. A. (2010). Potential Impact of Pandemic Influenza On the U. S. Health Insurance Industry Sponsored By Committee on Life Insurance Research Health Section Joint Risk Management Section Society of Actuaries Prepared By.
- Bambas, A. , & Drayton, H. A. (2000). Health & Human Development in the New Global Economy: The Contributions and Perspectives of Civil Society in the Americas.American Civil Society; [Google Scholar]
- Barbière, C. (2020). Euractiv, COVID‐19: France calls unemployed to work in fields as borders stay closed [Online]. Retrieved from https://www.euractiv.com/section/coronavirus/news/covid‐19‐france‐calls‐unemployed‐to‐work‐in‐fields‐as‐borders‐stay‐closed/ [Google Scholar]
- BBC (2020). BBC, Coronavirus: Republic of Ireland introduces stronger measures. Retrieved from https://www.bbc.com/news/uk‐northern‐ireland‐52026545 [Google Scholar]
- Beaubien, J. (2020). Goats and soda, how south korea reined in the outbreak without shutting everything down [Online]. https://www.npr.org/sections/goatsandsoda/2020/03/26/821688981/how‐south‐korea‐reigned‐in‐the‐outbreak‐without‐shutting‐everything‐down [Google Scholar]
- Breiman, L. (2001). Random Forests, Vol. 45. Page 5‐32 [Google Scholar]
- Breiman, L. , Friedman, J. H. , Olshen, R. A. , & Stone, C. (1984). Classification and regression trees, Vol. 432 (pp.151–166). Belmont, CA: Wadsworth. [Google Scholar]
- Brittany Shammas, E. , Rauhala, K. , Bellware, L. , Beachum, S. , Goff, J. D. , & Knowles, J. D. (2020). The Washington Post, Trump says quarantine for New York area ‘will not be necessary’; U.S. coronavirus‐related deaths double in two days [Online]. Retrieved from https://www.washingtonpost.com/world/2020/03/28/coronavirus‐latest‐news/ [Google Scholar]
- Callaway, B. E. , Cyranoski, D. , Mallapaty, S. , Stoye, E. , & Tollefson, J. (2020). Coronavirus by the numbers. News in Focus.576, 482–483. [DOI] [PubMed] [Google Scholar]
- Carstensen, T. (2020a). The World, Denmark takes swift action against coronavirus: “You can’t do enough to contain this epidemic” [Online]. Retrieved from https://www.pri.org/stories/2020‐03‐17/denmark‐takes‐swift‐action‐against‐coronavirus‐you‐cant‐do‐enough‐contain [Google Scholar]
- Carstensen, T. (2020b). The World, Denmark takes swift action against coronavirus: ’You can’t do enough to contain this epidemic [Online]. Retrieved from https://www.pri.org/stories/2020‐03‐17/denmark‐takes‐swift‐action‐against‐coronavirus‐you‐cant‐do‐enough‐contain [Google Scholar]
- CDC (2020). https://www.cdc.gov/coronavirus/2019‐ncov/symptoms‐testing/symptoms.html, 2019‐ncov symptoms [Online].
- CDCP (2020). CDCP, Travel Health Notices [Online]. Retrieved from https://wwwnc.cdc.gov/travel/destinations/traveler/none/brazil [Google Scholar]
- Chengxin, W. , Zheng, X. , Huang, E. W. , Bell, X. Z. , & Zhang, Y. (2020) Protein structure and sequence re‐analysis of 2019‐nCoV genome does not indicate snakes as its intermediate host or the unique similarity between its spike protein insertions and HIV‐1, 1–13. 10.1021/acs.jproteome.0c00129 [DOI] [PMC free article] [PubMed]
- Chowell, G. , Viboud, C. , Simonsen, L. , & Moghadas, S. M. (2016). Characterizing the reproduction number of epidemics with early subexponential growth dynamics. Journal of the Royal Society, Interface, 13, 20160659. 10.1098/rsif.2016.0659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw, M. A. , Medicine, T. , & Disease, I. (2020). Preliminary epidemiological analysis of suspected cases of corona virus infection in Libya. Travel Medicine and Infectious Disease, 35, 101634. 10.1016/j.tmaid.2020.101634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey, S. K. , Rahman, M. M. , Siddiqi, U. R. , & Howlader, A. (2020). Analyzing the epidemiological outbreak of COVID‐19: A visual exploratory data analysis (EDA) approach. Journal of Medical Virology, 92, 1–7. 10.1002/jmv.25743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhungana, H. N. (2020). Comments on “Preliminary estimation of the basic reproduction number of novel Coronavirus (2019‐nCoV) in China, from 2019 to 2020: A data‐driven Analysis in the early phase of the outbreak”. International Journal of Infectious Diseases, 94, 72–73. 10.1016/j.ijid.2020.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker, R. C. (2006). Principles of Epidemiology in Public Health Practice. CDC Glossary of Epidemiology Terms. [Google Scholar]
- Dietz, K. , & Heesterbeek, J. (2002). Daniel Bernoulli’s epidemiological model revisited. Mathematical Biosciences, 180(1‐2), 1–21. 10.1016/S0025-5564(02)00122-0 [DOI] [PubMed] [Google Scholar]
- Dincer, I. , Midilli, A. , Hepbasli, A. , & Karakoc, T. H. (2010). Global warming: Engineering solutions. Green Energy Technology, 31, 643–657. 10.1007/978-1-4419-1017-2 [DOI] [Google Scholar]
- Diwanji, S. (2020). Statistica, Precautionary measures to prevent spread of COVID‐19 India 2020 [Online]. Retrieved from https://www.statista.com/statistics/1098404/india‐precautionary‐measures‐against‐covid‐19/ [Google Scholar]
- Duddu, P. (2020). Focus, COVID‐19 in Iran: Coronavirus outbreak, measures and impact [Online]. Retrieved from https://www.pharmaceutical‐technology.com/features/iran‐coronavirus‐covid‐19‐death‐toll‐cases‐ncov‐measures‐impact/ [Google Scholar]
- East, M. , Res, V. , Guardian, T. , Health, T. O. , Health, O. , Food, U. N. , … Health, O. (2020). Emerging zoonoses: A one health challenge. EClinicalMedicine, 19, 100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euractiv (2020). Euractiv, Greece on total lockdown all new arrivals will be quarantined [Online]. Retrieved from https://www.euractiv.com/section/coronavirus/news/greece‐on‐total‐lockdown‐all‐new‐arrivals‐will‐be‐quarantined/ [Google Scholar]
- Fihlani, P. (2020). BBC, Coronavirus: African states impose strict restrictions [Online]. Retrieved from https://www.bbc.com/news/world‐africa‐51906053 [Google Scholar]
- Fisher, D. (2020a). Why Singapore isn’t in a coronavirus lockdown — as told by a doctor of the country [Online]. The Print. Retrieved from https://theprint.in/world/why‐singapore‐isnt‐in‐a‐coronavirus‐lockdown‐as‐told‐by‐a‐doctor‐of‐the‐country/384784/ [Google Scholar]
- Fisher, D. (2020b). The Print, Why Singapore isn’t in a coronavirus lockdown ‐ as told by a doctor of the Country [Online]. Retrieved from https://theprint.in/world/why‐singapore‐isnt‐in‐a‐coronavirus‐lockdown‐as‐told‐by‐a‐doctor‐of‐the‐country/384784/
- Flake, G. W. , & Lawrence, S. (2002). Efficient SVM regression training with SMO. Machine Learning, 46, 271–290. [Google Scholar]
- Garda World (2020a). Garda World, Malaysia: New travel restrictions introduced [Online]. Retrieved from https://www.garda.com/crisis24/news‐alerts/312061/malaysia‐new‐travel‐restrictions‐introduced‐february‐6‐update‐2 [Google Scholar]
- Garda World (2020b). Garda World, Netherlands: Government confirms first case of COVID‐19 [Online]. Retrieved from https://www.garda.com/crisis24/news‐alerts/318341/netherlands‐government‐confirms‐first‐case‐of‐covid‐19‐february‐27‐update‐2 [Google Scholar]
- Gary, R. S. (2020). HBR, Lessons from Italy’s Response to Coronavirus [Online]. Retrieved from https://hbr.org/2020/03/lessons‐from‐italys‐response‐to‐coronavirus [Google Scholar]
- Gautret, P. , Lagier, J.‐C. , Parola, P. , Hoang, V. T. , Meddeb, L. , Mailhe, M. , … Raoult, D. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID‐19: Results of an open‐label non‐randomized clinical trial. Journal of Antimicrobial Agents, 56(1), 105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- GC (2020). gc, Prime Minister announces new actions under Canada’s COVID‐19 response [Online]. Retrieved from https://pm.gc.ca/en/news/news‐releases/2020/03/16/prime‐minister‐announces‐new‐actions‐under‐canadas‐covid‐19‐response [Google Scholar]
- Gesley, J. (2020). Library of Congress, Emergency management, Epidemics, Government, Infectious and parasitic diseases, Public health [Online]. Retrieved from http://www.loc.gov/law/foreign‐news/article/austria‐government‐tightens‐rules‐to‐contain‐spread‐of‐coronavirus/ [Google Scholar]
- Gherasim, C. (2020). Euobserver, Romania’s Orban sworn in again amid corona emergency [Online]. Retrieved from https://euobserver.com/coronavirus/147762 [Google Scholar]
- Grafton . (2020). Grafton, GRAFTON’S RESPONSE TO COVID‐19 (CORONAVIRUS) [Online]. Retrieved from https://www.grafton.org/frequently‐asked‐questions‐about‐covid‐19/ [Google Scholar]
- Hastie, T. , Tibshirani, R. , & Friedman, J. (2009). The elements of statistical learning: Data mining, inference, and prediction, Verlag: Springer Science & Business Media. [Google Scholar]
- Itzwerth, R. (2013). Critical infrastructure and preparedness perspectives on pandemic influenza. PhD thesis. Sydney, NSW: THe University of New South Wales. [Google Scholar]
- Japan (2020). Retrieved from https://thediplomat.com/2020/03/japans‐limited‐response‐to‐the‐covid‐19‐pandemic/
- Kate Mayberry, T. V. (2020). Aljazeera, WHO: “Test every suspected case” of COVID‐19 ‐ Live updates [Online]. Retrieved from https://www.aljazeera.com/news/2020/03/toll‐rises‐coronavirus‐tightens‐global‐grip‐live‐updates‐200315231500487.html [Google Scholar]
- Keeling, M. J. , & Danon, L. (2009). Mathematical modelling of infectious diseases. British Medical Bulletin, 92(1), 33–42. 10.1093/bmb/ldp038 [DOI] [PubMed] [Google Scholar]
- Kerkhove, M. D. V. , & Ferguson, N. M. (2020). Epidemic and intervention modelling – a. 1–9. 10.2471/BLT.11.097949 [DOI]
- Kermack, W. O. , & McKendrick, A. G. (1927). Containing papers of a mathematical, and physical character, 1927: A contribution to the mathematical theory of epidemics. Proceedings of the Royal Society of London, 115, 700–721. [Google Scholar]
- Keystone, F. (2020): SwissInfo, Coronavirus: the situation in Switzerland [Online]. Retrieved from https://www.swissinfo.ch/eng/covid‐19_coronavirus–the‐situation‐in‐switzerland/45592192 [Google Scholar]
- Kondofersky, I. , & Theis, F. (2018). TREVOR HASTIE, ROBERT TIBSHIRANI, AND MARTIN WAINWRIGHT. Statistical Learning with Sparsity: The Lasso and Generalizations. Boca Raton: CRC Press. Biometrics 74, 769. [Google Scholar]
- Kyzer, L. (2020). Iceland Review, Steps taken to prevent spread of covid‐19 in iceland [Online]. Retrieved from https://www.icelandreview.com/news/steps‐taken‐to‐prevent‐spread‐of‐covid‐19‐in‐iceland/ [Google Scholar]
- Le Cessie, S. , & Van Houwelingen, J. C. (1992). Ridge estimators in logistic regression. Applied Statistics, 41(1), 191–201. 10.2307/2347628 [DOI] [Google Scholar]
- Leung, K. Y. , Ball, F. , Sirl, D. , & Britton, T. (2018). Individual preventive social distancing during an epidemic may have negative population‐level outcomes. Journal of the Royal Society Interface, 15(145), 20180296. 10.1098/rsif.2018.0296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Library Of Congress (2020). Austria: Government Tightens Rules to Contain Spread of Coronavirus [Online]. Global Legal Monitor. Retrieved from http://www.loc.gov/law/foreign‐news/article/austria‐government‐tightens‐rules‐to‐contain‐spread‐of‐coronavirus/ [Google Scholar]
- Lin, Q. , Zhao, S. , Gao, D. , Lou, Y. , Yang, S. , Musa, S. S. , … He, D. (2020). International Journal of Infectious Diseases A conceptual model for the coronavirus disease 2019 ( COVID‐19) outbreak in Wuhan, China with Individual Reaction and Governmental Action. International Journal of Infectious Diseases, 93, 211–216. 10.1016/j.ijid.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Gayle, A. A. , Wilder‐Smith, A. , & Rocklöv, J. (2020). The reproductive number of COVID‐19 is higher compared to SARS coronavirus. Journal of Travel Medicine, 27(2), 1–4. 10.1093/jtm/taaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas, N. (2020). Tech Crunch, Israel passes emergency law to use mobile data for COVID‐19 contact tracing [Online]. Retrieved from https://techcrunch.com/2020/03/18/israel‐passes‐emergency‐law‐to‐use‐mobile‐data‐for‐covid‐19‐contact‐tracing/ [Google Scholar]
- Lowen, A. C. , & Steel, J. (2014). Roles of Humidity and Temperature in Shaping Influenza Seasonality. Journal of Virology, 88, 7692–7695. 10.1128/JVI.03544-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan, J. , Lu, Y. , Jin, X. , & Zhang, L. (2020). Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS‐CoV‐2 infection. Biochemical and Biophysical Research Communications, 526(1), 165–169. 10.1016/j.bbrc.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J. (2020). Estimating epidemic exponential growth rate and basic reproduction number. Infectious Disease Modelling, 5, 129–141. 10.1016/j.idm.2019.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. , Zhao, Y. , Liu, J. , He, X. , Wang, B. O. , Fu, S. , … Luo, B. (2020). Effects of temperature variation and humidity on the death of COVID‐19 in Wuhan, China. Science of the Total Environment, 724, 138226. 10.1016/j.scitotenv.2020.138226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharaj, S. , & Kleczkowski, A. (2012). Controlling epidemic spread by social distancing: Do it well or not at all, United Kingdom: BioMed Centre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master of Public Health (2020). State of Qatar, Coronavirus Disease 2019 [Online]. Retrieved from https://www.moph.gov.qa/english/Pages/Coronavirus2019.aspx [Google Scholar]
- Miller, G. , Randolph, S. , & Patterson, J. E. (2008). Responding to Simulated Pandemic Influenza in San Antonio, Texas. Infection Control & Hospital Epidemiology, 29(4), 320–326. 10.1086/529212. [DOI] [PubMed] [Google Scholar]
- MOH (2014). MOH pandemic readiness and response plan for influenza and other acute respiratory diseases, USA: MOH. [Google Scholar]
- Murrin, S. (2018). Office of inspector general hospitals reported improved preparedness for emerging infectious diseases after the Ebola outbreak, USA: Department of Health. [Google Scholar]
- National Research Council (2001). Linkages between climate, ecosystems, and infectious disease. Under Weather Climate Ecosystem Infectious Disease, Washington DC: National Academy Press. [Google Scholar]
- Nishiura, H. (2011). Real‐time forecasting of an epidemic using a discrete time stochastic model: a case study of pandemic influenza (H1N1‐2009). Biomedical Engineering Online, 10, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norway Panorama (2020). The Nordic Page, Norway Government Takes Radical Decisions against Spread of Coronavirus: First Time Since WW2 [Online]. Retrieved from https://www.tnp.no/norway/panorama/norway‐government‐takes‐radical‐decisions‐against‐spread‐of‐coronavirus [Google Scholar]
- NPR (2020). How South Korea Reined In The Outbreak Without Shutting Everything Down [Online]. National Public Radio. Retrieved from https://www.npr.org/sections/goatsandsoda/2020/03/26/821688981/how‐south‐korea‐reigned‐in‐the‐outbreak‐without‐shutting‐everything‐down [Google Scholar]
- Nuzzo, J. B. , Meyer, D. , Snyder, M. , Ravi, S. J. , Lapascu, A. , Souleles, J. , … Bishai, D. (2019). What makes health systems resilient against infectious disease outbreaks and natural hazards? Results from a Scoping Review. BMC PublicHealth, 19, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyoman Sutarsa, A. P. (2020). Channel News Asia, COVID‐19 self‐isolation is punishing the poor in Indonesia [Online]. Retrieved from https://www.channelnewsasia.com/news/commentary/covid‐19‐coronavirus‐indonesia‐poor‐gig‐work‐gojek‐self‐isolate‐12567552 [Google Scholar]
- Oliveira, I. (2020). Pololitico, Portugal shuts down to tackle coronavirus [Online]. https://www.politico.eu/article/portugal‐quarantine‐measures‐coronavirus‐covid19‐antonio‐costa‐shutdown‐state‐of‐emergency/ [Google Scholar]
- Our World in Data (2020). https://ourworldindata.org/mortality‐risk‐covid
- Pakistan (2020). Gulf News, 10 steps Pakistan is taking to contain coronavirus [Online]. Retrieved from https://gulfnews.com/world/asia/pakistan/10‐steps‐pakistan‐is‐taking‐to‐contain‐coronavirus‐1.70403640 [Google Scholar]
- Paraskevis, D. , Kostaki, E. G. , Magiorkinis, G. , Panayiotakopoulos, G. , & Sourvinos, G. (2020). Full‐genome evolutionary analysis of the novel corona virus (2019‐nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infection, Genetics and Evolution, 79, 104212– 10.1016/j.meegid.2020.104212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paweska, J. T. , Jansen van Vuren, P. , Meier, G. H. , le Roux, C. , Conteh, O. S. , Kemp, A. , … Madhi, S. A. (2017). South African Ebola diagnostic response in Sierra Leone: A modular high biosafety field laboratory. PLoS Neglected Tropical Diseases, 11(6), e0005665. 10.1371/journal.pntd.0005665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persoff, J. , Ornoff, D. , & Little, C. (2018). The role of hospital medicine in emergency preparedness: A framework for hospitalist leadership in disaster preparedness, response, and recovery. Journal of Hospital Medicine, 713–718. 10.12788/jhm.3073 [DOI] [PubMed] [Google Scholar]
- Petrosillo, N. , Viceconte, G. , Ergonul, O. , Ippolito, G. , & Petersen, E. (2020). Since January 2020 Elsevier has created a COVID‐19 resource centre with free information in English and Mandarin on the novel coronavirus COVID‐ 19. The COVID‐19 resource centre is hosted on Elsevier Connect, the company ’ s public news and information.
- Pi, B. R. (2020). Life lessons from the history of lockdowns (pp. 1–77). India: Livemint. [Google Scholar]
- Piscitelli, C. (2020). MSAN, Measures taken by the Government Council on 12 March 2020 in response to the Coronavirus [Online]. Retrieved from https://msan.gouvernement.lu/en/actualites.gouvernement%2Ben%2Bactualites%2Btoutes_actualites%2Bcommuniques%2B2020%2B03‐mars%2B12‐cdg‐extraordinaire‐coronavirus.html [Google Scholar]
- Porta, M. (2008). Dictionary of epidemiology (p. 179). Oxford: Oxford University Press. [Google Scholar]
- Prem, K. , Liu, Y. , Russell, T. W. , Kucharski, A. J. , Eggo, R. M. , & Davies, N. (2020). The effect of control strategies to reduce social mixing on outcomes of the COVID‐19 epidemic in Wuhan, China: A Modelling Study. The Lancet, 2667, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran, D. K. , Rajagopal, V. , Alagumanian, S. , Santhosh Kumar, T. , Sathiya Prabhakaran, S. P. , & Kasilingam, D. (2020). Systematic literature review on novel corona virus SARS‐CoV‐2: a threat to human era. VirusDisease, 31(2), 161–173. 10.1007/s13337-020-00604-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid, H. , Ridda, I. , King, C. , & Booy, R. (n.d.). Social distancing Evidence summary. [DOI] [PubMed]
- Richard (2020). UPS Battery Center, Coronavirus Epidemic, Humidity and Temperature [Online]. Retrieved from https://www.upsbatterycenter.com/blog/coronavirus‐epidemic‐and‐temperature/%0AWhy [Google Scholar]
- Rodeny, R. (2020). 2019 Novel Coronavirus (2019‐nCoV) Update: Uncoating the Virus. American Society for Microbiology, 24, 1–9. [Google Scholar]
- Salute, M. D. (2020). Nuovo Coronavirus Covid‐19. COVID‐19, 1–73). Italy: Ministry of Health. [Google Scholar]
- Sameni, R. (2020). Mathematical modeling of epidemic diseases; A case study of the COVID‐19 coronavirus. 1–11.
- Sattar SBA, S. S. (2020). Bacterial Pneumonia [Online]. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK513321/ [Google Scholar]
- Sepkowitz, K. . (2020. ) CNN, Why is Covid‐19 death rate so low in Germany? [Online]. Retrieved from https://edition.cnn.com/2020/03/24/opinions/germany‐low‐death‐rate‐for‐coronavirus‐sepkowitz/index.html [Google Scholar]
- Sexton, N. R. , Smith, E. C. , Blanc, H. , Vignuzzi, M. , Peersen, O. B. , & Denison, M. R. (2016). Homology‐Based Identification of a Mutation in the Coronavirus RNA‐Dependent RNA Polymerase That Confers Resistance to Multiple Mutagens. Journal of Virology, 90, 7415–7428. 10.1128/jvi.00080-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng, M. , Diao, W. , Yu, L. , Pei, Z. L. , & Chen, D. (2020). Estimation of the reproductive number of novel coronavirus ( COVID‐19) and the probable outbreak size on the Diamond Princess cruise ship: A data‐driven analysis. International Journal of Infectious Diseases, 93, 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen, M. A. , Khan, S. , Kazmi, A. , Bashir, N. , & Siddique, R. (2020). COVID‐19 infection: Origin, transmission, and characteristics of human coronaviruses. Journal of Advanced Research, 24, 91–98. 10.1016/j.jare.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siettos, C. I. , & Russo, L. (2013). Mathematical modeling of infectious disease dynamics. Virulence, 4(4), 295–306. 10.4161/viru.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman, E. D. (2020). Statnews, Ecuador becomes the latest country to eye compulsory licensing for Covid‐19 products [Online]. https://www.statnews.com/pharmalot/2020/03/23/ecuador‐compulsory‐licensing‐covid19‐coronavirus/ [Google Scholar]
- Slovenija, R. (2020) Gov of Slovenija, [Online]. Retrieved from https://www.gov.si/en/topics/coronavirus‐disease‐covid‐19/koronavirus‐preparedness‐and‐measures‐in‐slovenia/ [Google Scholar]
- Thompson, R. N. , Brooks‐pollock, E. , & Thompson, R. N. (2019). Detection, forecasting and control of infectious disease epidemics: modelling outbreaks in humans, animals and plants. Philosophical Transactions of the Royal Society B: Biological Sciences.374, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosepu, R. , Gunawan, J. , Effendy, D. S. , Ahmad, L. O. A. I. , Lestari, H. , Bahar, H. , & Asfian, P. (2020). Correlation between weather and Covid‐19 pandemic in Jakarta, Indonesia. Science of the Total Environment, 725. 138436. 10.1016/j.scitotenv.2020.138436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S Embassy in Chile (2020). U.S Embassy in Chile, Global Level 4 Health Advisory [Online]. Retrieved from https://cl.usembassy.gov/announcement‐of‐curfew‐and‐increased‐sanitary‐protection‐measures‐by‐the‐government‐of‐chile/ [Google Scholar]
- US Embassy (2020). COVID‐19 Update – March 25. Retrieved from https://cl.usembassy.gov/announcement‐of‐curfew‐and‐increased‐sanitary‐protection‐measures‐by‐the‐government‐of‐chile/ U.S. Embassy in Chile [Google Scholar]
- Victor, A. O. (2020). Mathematical predictions for Covid‐19 as a global pandemic. MedRxiv. 10.13140/RG.2.2.12081.53600 [DOI] [Google Scholar]
- Weather Underground (2020). wunderground [Online] Retrieved from https://www.wunderground.com/ [Google Scholar]
- WHO (2014). Infection prevention and control of epidemic‐ and pandemic‐prone acute respiratory infections in health care, Swiss: WHO. [PubMed] [Google Scholar]
- WHO (2020a). Coronavirus disease (COVID‐19) outbreak situation [Online]. World Health Organization. Retrieved from https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019 [Google Scholar]
- WHO (2020b). Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19), Swiss: World Health Organization. [Google Scholar]
- WHO (2020c). Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19), Swiss: WHO. [Google Scholar]
- Wimberly, A. (2018). Pandemic Planning: Estimating Disease Burden of Pandemic Influenza to Guide Preparedness Planning Decisions for Nebraska Medicine.DigitalCommons@UNMC
- World Bank (2020). Physicians (per 1,000 people) [Online]. The World Bank. Retrieved from https://data.worldbank.org/indicator/SH.MED.PHYS.ZS [Google Scholar]
- Worldmeters (2020). Retrieved from https://www.worldometers.info/coronavirus/coronavirus‐age‐sex‐demographics/
- Wu, J. T. , Leung, K. , & Leung, G. M. (2020). Nowcasting and forecasting the potential domestic and international spread of the 2019‐nCoV outbreak originating in Wuhan, China: A modelling study. Lancet, 395, 689–697. 10.1016/S0140-6736(20)30260-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhan, L. (2020). Locked down Wuhan and why we always overplay the threat of the new Kenan Malik China ’ s reaction to the coronavirus outbreak may have the opposite effect to what ’ s needed. 1–7.
- Yong, E. (2020). The Atlantic, The U.K’.s Coronavirus ‘Herd Immunity’ Debacle [Online]. Retrieved from https://www.theatlantic.com/health/archive/2020/03/coronavirus‐pandemic‐herd‐immunity‐uk‐boris‐johnson/608065/, https://www.weforum.org/agenda/2020/03/3‐charts‐that‐changed‐coronavirus‐policy‐in‐the‐uk‐and‐us/ [Google Scholar]
- Yu, D. , Lin, Q. , Chiu, A. P. Y. , & He, D. (2017). Effects of reactive social distancing on the 1918 influenza pandemic. PLoS One, 12(7), 1–16. 10.1371/journal.pone.0180545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Yan, K. , Ye, H. , Lin, J. , Zheng, J. , Cai, T. , … J‐jun, Z. (2020). SARS‐CoV‐2 turned positive in a discharged patient with COVID‐19 arouses concern regarding the present standard for discharge. International Journal of Infectious Disease.75, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are openly available in the World Health Organization reports.


