Abstract
What is known and Objective
Controversy has arisen in the scientific community on whether the use of renin‐angiotensin system (RAS) inhibitors in the context of COVID‐19 would be beneficial or harmful. A meta‐analysis of eligible studies comparing the occurrence of severe and fatal COVID‐19 in infected hypertensive patients who were under treatment with angiotensin‐converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) vs no treatment or other antihypertensives was conducted.
Methods
PubMed, Google Scholar, the Cochrane Library, medRxiv and bioRxiv were searched for relevant studies. Fixed‐effects models or random‐effects models were used depending on the heterogeneity between estimates.
Results and discussion
A total of eighteen studies with 17 311 patients were included. The use of RAS inhibitors was associated with a significant 16% decreased risk of the composite outcome (death, admission to intensive care unit, mechanical ventilation requirement or progression to severe or critical pneumonia): RR: 0.84 (95% CI: 0.73‐0.95), P = .007, I2 = 65%.
What is new and conclusion
The results of this pooled analysis suggest that the use of ACEI/ARB does not worsen the prognosis of COVID‐19, and could even be protective in hypertensive subjects. Hypertensive patients should continue these drugs even if they become infected with SARS‐CoV‐2.
Keywords: angiotensin receptor blockers, angiotensin‐converting enzyme inhibitors, hypertension, SARS‐CoV‐2, severity
Controversy exists on whether RAS inhibitors are beneficial or harmful in COVID‐19. In this meta‐analysis, the use of RAS inhibitors was not associated with a worse COVID‐19 prognosis and was even protective in hypertensive patients. Patients should continue these drugs during their COVID‐19 illness.

1. WHAT IS KNOWN AND OBJECTIVE
The coronavirus disease 2019 (COVID‐19) outbreak originated in Wuhan in December 2019 and caused by the betacoronavirus SARS‐CoV‐2, was declared a pandemic by the World Health Organization in March 2020. Since then, it has affected more than 6 600 000 people and has caused more than 390 000 deaths. 1
Interestingly, COVID‐19 seems to manifest as a more severe disease in people with cardiovascular comorbidities, such as hypertension, 2 , 3 although is not yet very clear whether this association is independent from advanced age. 4 Myocardial injury has been proposed as the link between the inflammatory pathogenesis during the progress of the disease and the poorer prognosis. 5 , 6 It has been postulated that the virus could damage myocardial cells through several mechanisms including direct damage and systemic inflammatory responses. 6 Subjects with preexisting cardiovascular diseases might be more susceptible to COVID‐19–induced heart injury.
SARS‐CoV‐2 gains entrance to cells through the angiotensin‐converting enzyme 2 (ACE2), 7 a carboxypeptidase that converts angiotensin II into angiotensin‐(1‐7) and counterbalances the renin‐angiotensin‐aldosterone system, exerting protective effects in the cardiovascular system. Given that there are limited reports that ACE inhibitors affect the expression of ACE2 in the heart and the kidney, 8 there has been a growing concern about angiotensin‐converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) increasing patient susceptibility to viral host cell entry and propagation. 8 , 9 , 10 Of note, many patients with cardiovascular comorbidities, particularly hypertension, are treated with these drug classes. On the other hand, it is hypothesized that SARS‐CoV‐2, like SARS‐CoV, not only gains initial entry through ACE2 but also subsequently downregulates ACE2 expression, 11 and deregulated ACE2 may theoretically mediate acute lung injury. 12 In fact, some experts have advocated for the use of ACEI and ARB to prevent organ injury and there are currently several registered clinical trials that will evaluate the potential benefit of ARB or ACEI in either hospitalized or not hospitalized COVID‐19–infected patients.
To date, there is insufficient clinical or scientific evidence to recommend the discontinuation or maintenance of ACEI/ARB treatment in hypertensive patients in face of COVID‐19. Therefore, in this article, we conducted a systematic literature search to determine a possible association between the use of ACEI/ARB in hypertensives who become infected with COVID‐19 and the progression of the disease to severe forms or death.
2. METHODS
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement 13 was followed for the conduct and reporting of this systematic review (PRISMA checklist provided as Supporting Information).
2.1. Data source, search strategy and eligibility criteria
To identify publications regarding the clinical outcomes of COVID‐19 in infected hypertensive patients under treatment or not under treatment with ACEI/ARB, an extensive search of the literature was conducted in MEDLINE (through PubMed interface), Cochrane Library, Google Scholar and the preprint servers for the health sciences medRxiv and bioRxiv, from December 2019 to 5 June 2020. In addition, we manually searched from the reference lists of all relevant retrieved studies (snowball technique) to identify any other studies that may have been missed by our search strategy.
The following search strategy was implemented:
#1: SARS‐CoV‐2 OR COVID 19 OR coronavirus disease 2019 OR coronavirus 2 OR novel coronavirus OR 2019‐nCoV OR receptor to SARS‐CoV‐2 OR coronavirus entry OR virulence of SARS‐CoV‐2.
#2: hypertension OR blood pressure OR antihypertensive OR angiotensin OR angiotensin converting enzyme inhibitor OR angiotensin receptor blocker OR ACEi OR ARB OR antihypertensive drugs OR withdrawal of RAS inhibitors OR medication use in COVID‐19 OR initiation or discontinuation of RAS blockade OR angiotensin‐converting enzyme 2 OR ace2 OR renin‐angiotensin system OR RAS OR RAS blockers OR RAS treatment OR RAS activity OR cardiovascular disease OR cardiovascular conditions OR diabetes OR chronic kidney disease OR renal disease OR heart failure OR myocardial infarction OR comorbidity.
#3: cardiac injury OR respiratory failure OR mortality OR fatality rates OR death OR complications OR survival OR prognosis OR respiratory support OR mechanical ventilation OR noninvasive ventilation OR intensive care unit OR ICU OR cardiomyopathy OR pneumonia OR acute respiratory distress OR ARDS OR myocarditis OR pulmonary disease.
#1 AND #2 AND #3
Articles were limited to human studies, original articles including comparative studies, both randomized controlled trials (RCTs) or non‐RCTs where treatment with ACEI and/or ARB in hypertensive patients was compared with no treatment or other antihypertensive drugs in terms of COVID‐19 infection serious adverse outcomes. Case reports, non‐human studies and studies without adequate information were excluded. Abstracts of articles were then scanned for relevance.
2.2. Outcome measures
Our outcome was a combination of death, admission to intensive care unit (ICU), mechanical ventilation requirement or progression to severe or critical pneumonia. According to the Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19), 14 severe pneumonia was defined as dyspnoea, respiratory frequency ≥ 30/min, blood oxygen saturation ≤ 93%, PaO2/FiO2 ratio < 300 and/or lung infiltrates > 50% of the lung field within 24‐48 hours, whereas critical pneumonia was defined as respiratory failure, septic shock and/or multiple organ dysfunction/failure and critical pneumonia as respiratory failure, septic shock and/or multiple organ dysfunction/failure.
2.3. Data extraction and study quality assessment
Data were collected by two independent reviewers (RM and JB) and entered into a predesigned data extraction form. Differences between the two reviewers regarding study eligibility were resolved by consensus.
Study and population characteristics were extracted from each included study. Regarding study characteristics, the name of the first author, the date of publication, the study type, the number of centres and countries contributing to the research and possible funding were extracted. Also, total population, mean age, percentage of male participants, number of patients taking ACEI and/or ARB, number of patients taking other antihypertensives or no treatment, type and dose of antihypertensive drug, number of patients experiencing the primary outcome in each treatment group and details for possible matched characteristics were recorded.
The Newcastle‐Ottawa Scale (NOS) was used to assess the quality of studies. 15 In this scale, a 'star system' is used, in which a study is judged on three aspects: selection of the study groups; comparability of the groups; and ascertainment of either the exposure or outcome of interest for case‐control or cohort studies, respectively. A study can be awarded a maximum of one star for each numbered item within the selection and exposure categories. A maximum of two stars can be given for comparability. In this systematic review, studies with scores above 6 were considered as high quality, 3‐6 as moderate and those with scores below than 3 as low quality. 16 The NOS was applied independently by the two reviewers (RM and JB). Differences between them were resolved by consensus.
2.4. Data synthesis and statistical analysis
Pooled analysis was performed to estimate the risk ratio (RR) and 95% confidence interval (95% CI) comparing the occurrence of the composite outcome in COVID‐19–infected hypertensive patients under treatment with ACEI/ARB vs no treatment or treatment other than ACEI/ARB. All analyses were conducted using RevMan software version 5.3. Fixed‐effect models or random‐effect models were used depending on the heterogeneity between estimates. We used the I2 statistics to assess the magnitude of heterogeneity. The fixed‐effects model was used if I2 < 50%, and the random‐effects model was used if I2 ≥ 50%.
Publication bias was examined by the use of a funnel plot of each study's effect size against the precision (1/SE).
We planned to conduct two subgroup analyses: (a) including only high‐quality studies and (b) including only peer‐reviewed published studies.
3. RESULTS AND DISCUSSION
3.1. Results
3.1.1. Search results and study characteristics
Our comprehensive search initially identified 1207 articles. The flow chart diagram in Figure 1 shows our literature search, study selection and the number of studies included. After removing duplicates, reviews, opinion letters, guidelines or consensus, and studies not addressing the research question, 46 full‐text articles were assessed for eligibility. Among them, 18 studies that satisfied the eligibility criteria were included in this systematic review and meta‐analysis, totalling 17 311 COVID‐19–infected hypertensive patients. Among them, 8328 were taking ACEI or ARB and 8983 were under other or no treatment.
Figure 1.
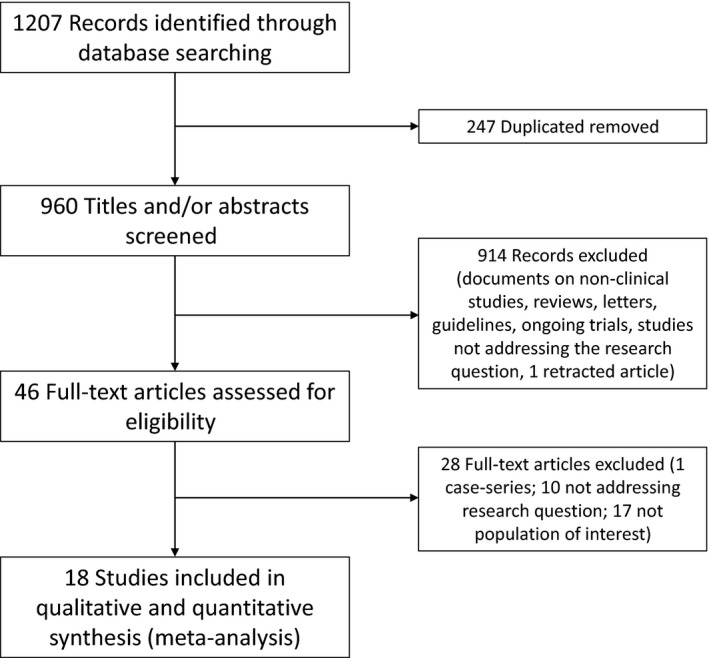
Flow chart depicting literature review and study selection
All of the selected studies were published in 2020 and were all of observational nature. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 In seven studies, the outcome that matched our research question was death 17 , 20 , 21 , 26 , 27 , 28 , 31 ; in two studies, severe pneumonia 23 , 34 ; in three studies, critical or severe pneumonia 18 , 19 , 32 ; in one study, death or critical or severe pneumonia 33 ; in one study, severe pneumonia or death 24 ; and in one study, death, admission to ICU or mechanical ventilation requirement. 25 In three of the studies where the outcomes death and severe pneumonia, 22 , 30 or critical pneumonia 29 were discriminated, we chose mortality as the worst possible outcome for our analysis since the data necessary to evaluate the outcome as a composite were not available. Table 1 summarizes the study characteristics. Quality assessment through the Newcastle‐Ottawa Scale showed scores between 5 and 9 points, indicating high‐quality studies, except for three studies that scored 5 or 6 points (moderate quality).
Table 1.
Description of the included studies
| Reference | Country | Study type | Number of patients included in the analysis of interest | Population in the analysis of interest | Age (y, mean or median*) | Male sex (%) | n with ACEI and/or ARB/n without ACEI and/or ARB | NOS total score |
|---|---|---|---|---|---|---|---|---|
| Bravi et al 33 | Italy | CC | 543 | COVID‐19–infected hypertensive patients | 58 | 47.3 | 450/93 | 8 |
| Feng et al 32 | China | RC | 113 | COVID‐19–infected hypertensive patients | 53* | 56.9 | 33/80 | 8 |
| Feng et al 34 | China | RC | 65 | COVID‐19–infected hypertensive patients under treatment | 47* | 50.4 | 16/49 | 7 |
| Gao et al 17 | China | RC | 710 | Hospitalized COVID‐19–infected hypertensive patients under treatment | 64.3 | 52.1 | 183/527 | 9 |
| Hu et al 18 | China | PC | 149 | COVID‐19–infected hypertensive patients | 57* | 59.1 | 65/84 | 5 |
| Huang et al 19 | China | PC | 50 | COVID‐19–infected hypertensive patients | 60.3 | 54 | 20/30 | 6 |
| Ip et al 20 | USA | RC | 1584 | Hospitalized COVID‐19–infected hypertensive patients | N/A | N/A | 646/938 | 7 |
| Khera et al 21 | USA | RC | 7933 | Hospitalized COVID‐19–infected hypertensive patients under treatment | 77* | 45.4 | 4587/3346 | 8 |
| Li et al 22 | China | RC | 362 | COVID‐19–infected hypertensive patients under treatment | 66* | 52.2 | 115/247 | 7 |
| Liu et al 23 | China | RC | 78 | COVID‐19–infected hypertensive patients | 65.2 | 55.1 | 22/56 | 6 |
| Meng et al 24 | China | RC | 42 | COVID‐19–infected hypertensive patients under treatment | 64.5* | 57.1 | 17/25 | 7 |
| Reynolds et al 25 | USA | RC | 2573 | COVID‐19–infected hypertensive patients | 64* | 50.8 | 1293/1280 | 9 |
| Richardson et al 26 | USA | RC | 1366 | COVID‐19–infected hypertensive patients | 63* | 60.3 | 413/953 | 7 |
| Tan et al 27 | China | RC | 100 | COVID‐19–infected hypertensive patients | 67* | N/E | 31/69 | 7 |
| Tedeschi et al 28 | Italy | PC | 311 | COVID‐19–infected hypertensive patients | 76* | 72 | 175/136 | 8 |
| Yang et al 29 | China | RC | 126 | COVID‐19–infected hypertensive patients | 66* | 49.2 | 43/83 | 7 |
| Zeng et al 30 | China | RC | 75 | COVID‐19–infected hypertensive patients | 67 | 55 | 28/47 | 7 |
| Zhang et al 31 | China | RC | 1128 | COVID‐19–infected hypertensive patients | 64* | 53 | 188/940 | 9 |
All studies were conducted in 2020.
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; CC, case‐control study; CV, cardiovascular; N/A, not available; N/E, not extractable; NOS, Newcastle‐Ottawa Scale; PC, prospective cohort; RC, retrospective cohort.
means the median is reported instead of the mean.
In a qualitative synthesis, seven studies reported a lower risk of the outcome with the use of ACEI/ARB 20 , 23 , 27 , 31 , 32 , 33 , 34 and eleven studies found no association. 17 , 18 , 19 , 21 , 22 , 24 , 25 , 26 , 28 , 29 , 30
3.2. Meta‐analysis
The results of our pooled analysis for the 18 identified studies are presented in Figure 2. The use of ACEI or ARB was found to significantly decrease the risk of the composite outcome (death, admission to ICU, mechanical ventilation requirement or progression to severe or critical pneumonia): RR: 0.84 (95% CI: 0.73‐0.95), test for overall effect: Z = 2.68, P = .007. A random‐effects model was used since significant heterogeneity was observed in the analysis: I2 = 65%, χ2 = 48.91, df = 17, P < .001.
Figure 2.
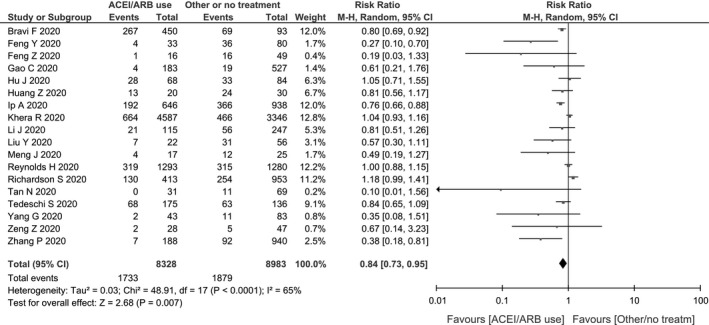
Forest plot showing risk ratios (RRs) of the composite outcome (death, admission to intensive care unit (ICU), mechanical ventilation requirement or progression to severe or critical pneumonia)
In our subgroup analyses, no significant differences were found when excluding studies with a NOS score below 7: RR: 0.83 (95% CI: 0.72‐0.96, P = .01, I2 = 70%). When excluding preprints, the protective association between RAS inhibitors use and the primary outcome remained marginally significant: RR: 0.82 (95% CI: 0.67, 1.00, P = .05, I2 = 61%) (Figure 3).
Figure 3.
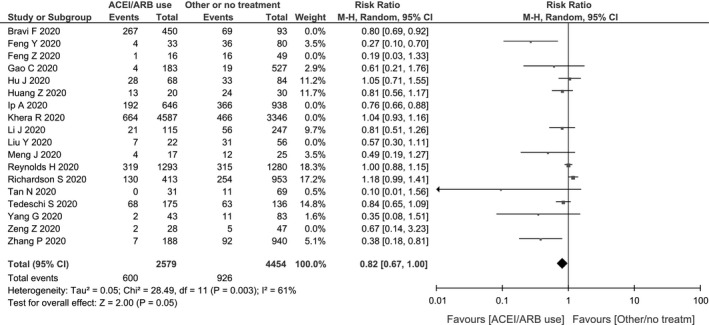
Sensitivity analysis excluding preprints
Finally, the funnel plot indicated some degree of publication bias, with a lack of small studies showing increased risk of ACEI/ARB use (Figure 4).
Figure 4.
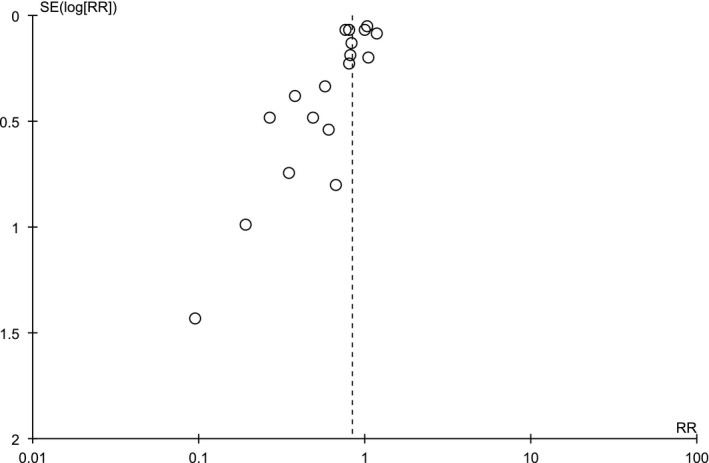
Funnel plot of the comparison of the composite outcome between patients under ACEI/ARB and not under ACEI/ARB
3.3. Discussion
In this meta‐analysis, we found that the use of ACEI/ARB was not associated with an increased risk of death, admission to intensive care unit (ICU), mechanical ventilation requirement or progression to severe or critical pneumonia in COVID‐19–infected hypertensive patients. In fact, we observed a modest but significant 16% reduction in the risk of this primary outcome.
Controversy has arisen in the scientific community regarding the use of RAS inhibitors in the context of COVID‐19: some experts hypothesized that these drugs might increase susceptibility to the virus by increasing the expression of ACE2, the SARS‐CoV‐2 receptor for host cell entry. 9 , 10 On the other hand, scientific societies recommend not to discontinue RAS inhibitors because of insufficient evidence of their potential harm and overwhelming evidence on their benefits 35 , 36 , 37 , 38 , 39 and even some researchers advocate for the use of RAS inhibitors based on the fact that, through increasing the expression of ACE2, they counterbalance the reduction in pulmonary ACE2 provoked by the virus either through internalization with viral entry and/or downregulation of ACE2 enzyme during this process, which would lower the production of inflammatory cytokines, 40 thus exerting a protective role in lung injury. 41 Remarkably, RAS inhibitors have been shown to be associated with reduced mortality in patients with sepsis. 42 A study conducted by Henry C et al 43 have found a beneficial effect of ACEI/ARB in patients admitted with viral pneumonia, as they significantly reduced the pulmonary inflammatory response and cytokine release caused by virus infection. Moreover, other studies have shown that these drugs would attenuate the inflammatory response in COVID‐19–infected patients, possibly through the inhibition of IL‐6 levels, 24 C‐reactive protein and procalcitonin. 29 On the other hand, recent large‐scale studies have found that the use of ACEI/ARB is not significantly associated with incident COVID‐19 in hypertensive patients. 44
Recent evidence has consistently shown that hypertension and other cardiovascular morbidities are more frequent in COVID‐19–infected patients who carry the worst prognosis. 2 , 3 , 45 Of note, most of these studies did not make adjustments for important covariates such as age, although more recent studies are starting to show that this association holds even after such adjustments. 46 , 47 As many subjects with cardiovascular morbidities, particularly hypertensives, are under treatment with ACEI/ARB, it has been speculated that the use of these drugs could be the underlying cause of the relation between these morbidities and a poorer COVID‐19 prognosis. If this was the case, we would have found a greater risk of severe forms of COVID‐19 in subjects under treatment with ACEI/ARB, but we have not. In fact, the evidence presented in this meta‐analysis supports the idea that ACEI/ARB should not be discontinued when treating COVID‐19 patients with hypertension.
On the other hand, RAS inhibitors have established benefits in protecting the cardiovascular system. 48 , 49 Their discontinuation may increase the chance of decompensation in high‐risk patients as the benefits that are specific to these drugs may not be offset by other antihypertensive agents. For instance, it has been shown that the withdrawal of ACEI/ARB during heart failure hospitalization is associated with higher rates of post‐discharge mortality. 50 Besides, antihypertensive medication changes would require frequent dose adjustment and management of adverse effects, increasing the need for patients to visit their doctors and therefore the exposure to COVID‐19 and risk of infection.
Clearly, more research is needed to elucidate the multifaceted role of the RAS in connection with SARS‐CoV‐2 infection. The evidence regarding the use of RAS inhibitors in patients with COVID‐19 infection is still emerging. Currently, in hypertensive patients who previously used ACEI/ARB, these drugs may not need to be discontinued in order to prevent COVID‐19 complications.
Finally, our findings must be interpreted in the context of the meta‐analysis limitations. First, we could not make a distinction between the effects of ACEI and ARB separately, since most studies evaluated the two drug classes together. Second, the studies included in the meta‐analysis were observational and some were preprints. Although preprints are usually considered a priori very low‐quality studies because of the lack of peer review, in the context of the pandemia, with vertiginous generation of new information and the urgent need of real‐time data, they have become a source of consultation for many experts. We could not find any randomized clinical trials that already showed results addressing our research question. On the other hand, in planning future trials, randomizing subjects to discontinue drugs with proven benefits would probably raise ethical concerns. Third, most of the included observational studies were retrospective cohorts and potential selection bias of patients is an indisputable concern. Fourth, residual confounders may be present in these observational studies, and even when measured, many studies did not make adjustments for such potential confounders. It must be noted, however, that not adjusting for age and cardiovascular comorbidities would shift the results towards a higher risk since these variables are positively associated with both the use of ACEI/ARB and COVID‐19 poorer outcomes. In the same line, it is possible for some of the included studies to have actually included a few non‐hypertensive subjects but instead some patients with cardiovascular comorbidities under treatment with ACEI/ARB, such as heart failure or acute coronary syndrome. It must be mentioned, however, that these comorbidities would have acted as positive confounders, being positively associated both with ACEI/ARB use and a worse COVID‐19 prognosis. In that case, the result would have been an association between ACEI/ARB use and a worse COVID‐19 evolution. However, even under the effect of those potential confounders, ACEI/ARB were still found to be protective.
4. WHAT IS NEW AND CONCLUSION
According to our findings, the use of ACEI/ARB is associated with a modest but significant reduction in the risk of death, admission to intensive care unit (ICU), mechanical ventilation requirement or progression to severe or critical pneumonia in COVID‐19–infected hypertensive patients. Large prospective studies are required to confirm these results and to explore the mechanisms for a possible protective role of RAS inhibitors in the context of COVID‐19. In the meantime, these early results suggest that patients on ACEI/ARB should continue their treatment during COVID‐19 illness, supporting current recommendations from multiple scientific societies.
CONFLICT OF INTEREST
Rocío Martínez and Jessica Barochiner declare that they have no conflict of interest.
AUTHORS’ CONTRIBUTION
Jessica Barochiner conceived and designed the study, acquired the data, analysed and interpreted the data, drafted the article, revised the article critically for important intellectual content and finally approved the submitted version. Rocío Martínez acquired the data, interpreted the data, revised the article critically for important intellectual content and finally approved the submitted version.
ETHICAL APPROVAL
This is a systematic review and meta‐analysis of the literature. No human subjects or animals were involved in this research.
CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Ms Erika Barochiner for language editing and to Prof. Javier Medolla for translation of articles in Chinese.
Barochiner J, Martínez R. Use of inhibitors of the renin‐angiotensin system in hypertensive patients and COVID‐19 severity: A systematic review and meta‐analysis. J Clin Pharm Ther. 2020;45:1244–1252. 10.1111/jcpt.13246
This author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
DATA AVAILABILITY STATEMENT
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. WHO Coronavirus Disease (COVID‐19) . Dashboard [Internet]. https://covid19.who.int/?gclid=EAIaIQobChMIhLKYx5Lu6QIVxIGRCh1WeA3JEAAYASAAEgIAxvD_BwE. [Cited 2020 Jun 6]
- 2. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sommerstein R, Kochen MM, Messerli FH, Gräni C. Coronavirus disease 2019 (COVID‐19): do angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc. 2020;9(7):e016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi S, Qin MU, Shen BO, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS‐CoV‐2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111(20):2605‐2610. [DOI] [PubMed] [Google Scholar]
- 9. Esler M, Esler D. Can angiotensin receptor‐blocking drugs perhaps be harmful in the COVID‐19 pandemic? J Hypertens. 2020;38(5):781‐782. [DOI] [PubMed] [Google Scholar]
- 10. Diaz JH. Hypothesis: angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID‐19. J Travel Med. 2020;27(3):1‐7. 10.1093/jtm/taaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11(8):875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006‐1012. [DOI] [PubMed] [Google Scholar]
- 14. Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19) [Internet]. https://www.who.int/docs/default‐source/coronaviruse/who‐china‐joint‐mission‐on‐covid‐19‐final‐report.pdf. [Cited 2020 Jun 6].
- 15. Ottawa Hospital Research Institute [Internet]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Cited 2020 Jun 6]
- 16. Behboudi‐Gandevani S, Amiri M, Bidhendi Yarandi R, Ramezani TF. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta‐analysis. Diabetol Metab Syndr. 2019;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao C, Cai Y, Zhang K, et al. Association of hypertension and antihypertensive treatment with COVID‐19 mortality: a retrospective observational study. Eur Heart J. 2020;41(22):2058‐2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu J, Zhang X, Zhang X, et al. COVID‐19 patients with hypertension have more severity condition, and ACEI/ARB treatment have no infulence on the clinical severity and outcome. J Infect. 2020. 10.1016/j.jinf.2020.05.056. [Epub ahead of print]. [DOI] [Google Scholar]
- 19. Huang Z, Cao J, Yao Y, et al. The effect of RAS blockers on the clinical characteristics of COVID‐19 patients with hypertension. Ann Transl Med. 2020;8(7):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ip A, Parikh K, Parrillo JE, et al. Hypertension and renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. medRxiv. 2020. Apr 29;2020.04.24.20077388. [Google Scholar]
- 21. Khera R, Clark C, Lu Y, et al. Association of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with coronavirus disease‐19. medRxiv. 2020. May 19;2020.05.17.20104943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y, Huang F, Xu J, et al. Anti‐hypertensive angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID‐19 patients. medRxiv. 2020. Mar 27;2020.03.20.20039586. [Google Scholar]
- 24. Meng J, Xiao G, Zhang J, et al. Renin‐angiotensin system inhibitors improve the clinical outcomes of COVID‐19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid‐19. N Engl J Med. 2020;382(25):2441‐2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323(20):2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan N‐D, Qiu Y, Xing X‐B, et al. Associations between angiotensin converting enzyme inhibitors and angiotensin II receptor blocker use, gastrointestinal symptoms, and mortality among patients with COVID‐19. Gastroenterology. 2020. 10.1053/j.gastro.2020.05.034. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tedeschi S, Giannella M, Bartoletti M, et al. Clinical impact of renin‐angiotensin system inhibitors on in‐hospital mortality of patients with hypertension hospitalized for COVID‐19. Clin Infect Dis. 2020. Apr 27. 10.1093/cid/ciaa492. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang G, Tan Z, Zhou L, et al. Effects of ARBs and ACEIs on virus infection, inflammatory status and clinical outcomes in COVID‐19 patients with hypertension: a single center retrospective study. Hypertension. 2020. Apr 29. 10.1161/HYPERTENSIONAHA.120.15143 [DOI] [PubMed] [Google Scholar]
- 30. Zeng Z, Sha T, Zhang Y, et al. Hypertension in patients hospitalized with COVID‐19 in Wuhan, China: a single‐center retrospective observational study. medRxiv. 2020 Apr 11;2020.04.06.20054825. [Google Scholar]
- 31. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126(12):1671‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feng Y, Ling Y, Bai T, et al. COVID‐19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bravi F, Flacco ME, Carradori T, et al. Predictors of severe or lethal COVID‐19, including angiotensin converting enzyme inhibitors and angiotensin II receptor blockers, in a sample of infected Italian citizens. medRxiv. 2020. May 21; 2020.05.21.20109082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feng Z, Li J, Yao S, et al. The use of adjuvant therapy in preventing progression to severe pneumonia in patients with coronavirus disease 2019. A multicenter data analysis. medRxiv. 2020. Apr 10;2020.04.08.20057539. [Google Scholar]
- 35. ESH STATEMENT ON COVID‐19 | European Society of Hypertension [Internet]. https://www.eshonline.org/spotlights/esh‐statement‐covid‐19/. [Cited 2020 Jun 7].
- 36. Position Statement of the ESC Council on Hypertension on ACE‐Inhibitors and Angiotensin Receptor Blockers [Internet]. https://www.escardio.org/Councils/Council‐on‐Hypertension‐(CHT)/News/position‐statement‐of‐the‐esc‐council‐on‐hypertension‐on‐ace‐inhibitors‐and‐ang. [cited 2020 Jun 7].
- 37. COVID‐19 and concerns regarding use of ACEi/ARB/ARNi medications for heart failure or hypertension [Internet] . https://www.ccs.ca/images/Images_2020/CCS_CHFS_statement_regarding_COVID_EN.pdf. [cited 2020 Jun 7].
- 38. International Society of Hypertension . A statement from the International Society of Hypertension on COVID‐19 | The International Society of Hypertension [Internet]. https://ish‐world.com/news/a/A‐statement‐from‐the‐International‐Society‐of‐Hypertension‐on‐COVID‐19/. [cited 2020 Jun 7].
- 39. HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID‐19 [Internet]. https://professional.heart.org/professional/ScienceNews/UCM_505836_HFSAACCAHAstatement‐addresses‐concerns‐re‐using‐RAAS‐antagonists‐in‐COVID.jsp. [cited 2020 Jun 7]. [DOI] [PMC free article] [PubMed]
- 40. Ferrario CM, Strawn WB. Role of the renin‐angiotensin‐aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98(1):121‐128. [DOI] [PubMed] [Google Scholar]
- 41. Huang F, Guo J, Zou Z, et al. Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9‐infected patients. Nat Commun. 2014;5(1):1‐7. 10.1038/ncomms4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hsu W‐T, Galm BP, Schrank G, et al. Effect of renin‐angiotensin‐aldosterone system inhibitors on short‐term mortality after sepsis: a population‐based cohort study. Hypertension. 2020;75(2):483‐491. [DOI] [PubMed] [Google Scholar]
- 43. Henry C, Zaizafoun M, Stock E, Ghamande S, Arroliga AC, White HD. Impact of angiotensin‐converting enzyme inhibitors and statins on viral pneumonia. Baylor Univ Med Center Proc. 2018;31(4):419‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fosbøl EL, Butt JH, Østergaard L, et al. Association of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker use with COVID‐19 diagnosis and mortality. JAMA. 2020;324:e2011301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang J, Zheng YA, Gou XI, et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4‐week follow‐up. J Infect. 2020;80(6):639‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guan W‐J, Liang W‐H, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with COVID‐19. N Engl J Med. 2020;382(17):1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID‐19. Hypertens Res. 2020;43(7):648‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gilstrap LG, Fonarow GC, Desai AS, et al. Initiation, continuation, or withdrawal of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers and outcomes in patients hospitalized with heart failure with reduced ejection fraction. J Am Heart Assoc. 2017;6(2):1‐10. 10.1161/JAHA.116.004675 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


