Dear Editors,
Immune thrombocytopenic purpura (ITP) is an acquired disease characterized by thrombocytopenia secondary to autoantibodies against platelet antigens. Secondary ITP is common after viral infections 1 . We here report a case of ITP in a patient with COVID‐19 pneumonia.
An 89‐year‐old man with congestive heart failure, essential hypertension, type 2 diabetes, chronic kidney disease, and atrial fibrillation on anticoagulation (AC) presented to the emergency department in March 2020 with a 3‐day history of shortness of breath, dry cough, and diarrhea. On admission, he was stable and required 2 L oxygen to keep his oxygen saturation 90%. Physical examination was remarkable for bibasilar crackles.
Laboratory tests on admission showed a normal white cell count, hemoglobin (Hb) of 12.0 g/d, and platelet count (175 000/mm3). C‐reactive protein (CRP) and D‐dimer were elevated at 10.9 mg/dL and 970 ng/mL. A nasopharyngeal swab was positive for COVID‐19. Chest X‐ray showed bilateral lung infiltrates.
The patient was admitted to the regular medical floor and started on hydroxychloroquine, intravenous piperacillin/tazobactam, and azithromycin. On day 13, the patient’s oxygen requirements increased and he was transferred to the intensive care unit (ICU) for monitoring. On the same time, his platelet count dropped acutely to less than 2,000/mm3 (Figure 1). At the same day, his Hb was 10.4 g/dL. D‐dimer and fibrinogen were elevated at 13 180 ng/mL and 446 mg/dL. PT, partial thromboplastin time, and INR were 21.9 seconds, 40.5 seconds, and 2.0 respectively. Peripheral blood smear did not show any schistocytes. The international society on thrombosis and hemostasis (ISTH) DIC score was 7. The 4T score for possible heparin‐induced thrombocytopenia (HIT) was 4 (intermediate probability), and antiplatelet factor 4 antibody and antinuclear antibodies were negative. Drug‐dependent platelet antibodies were negative for tazobactam IgG or IgM antibodies; however, the test was positive for non‐drug‐related IgG antiplatelet antibodies. Ultrasound of the lower extremities on day 13 showed acute left tibial deep vein thrombosis (DVT). Computed tomography of the chest was negative for pulmonary embolism.
Figure 1.
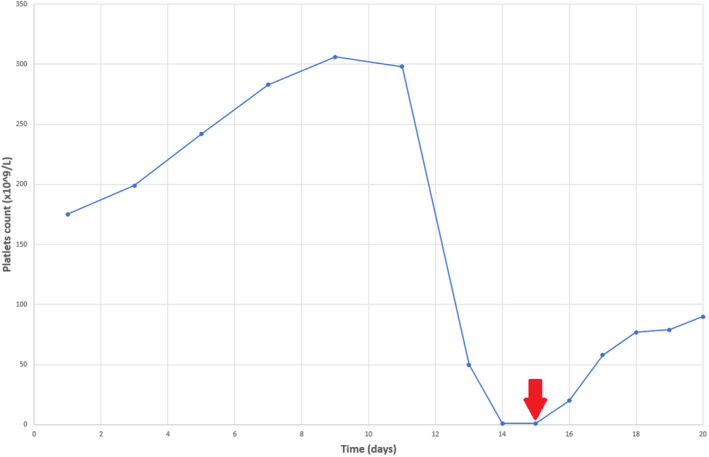
Changes in platelet count level during admission. Day 1 (baseline) represents the day of admission. Red arrow indicates the date of treatment initiation with dexamethasone and intravenous immunoglobulins
Since INR was subtherapeutic on the day of admission (INR = 1.1), oral warfarin was started. On day 9, INR was 3.3 and warfarin was held. The patient received a single dose of prophylactic enoxaparin the next day, 3 days before the acute drop in platelet count. Argatroban was started for possible HIT (although unlikely) and then stopped when HIT excluded. Three units of platelets were transfused, and platelet count continued to be less than 2000/mm3; however, no bleeding developed at any point. On day 15, the patient was started on dexamethasone 40 mg daily (received 4 doses) and 1 g/kg intravenous immunoglobulin (IVIG) daily for 2 days. By the end of the treatment course, his platelet count was 79 000/mm3 and he was restarted on systemic heparin. The patient required endotracheal intubation and family decided to go with comfort care. Patient passed away after 20 days of admission.
Although COVID‐19 is a respiratory tract disease, multiple systems can be affected including hematopoietic and lymphatic systems among others. Thrombocytopenia has been reported by multiple studies and was linked to disease mortality 2 . ITP induced by COVID‐19 is rare and has been reported in few cases 3 , 4 , 5 . Our case presented with viral pneumonia secondary to COVID‐19 and developed secondary ITP.
Immune thrombocytopenic purpura is an acquired hemorrhagic disease characterized by thrombocytopenia and autoantibodies against platelet antigens. Clinically patients with ITP may be asymptomatic or can present with bleeding. ITP is a diagnosis of exclusion; it can be diagnosed after excluding all possible causes of thrombocytopenia 1 . In a recently published case report, COVID‐19 patient developed acute thrombocytopenia, skin purpura, and epistaxis on day 4 after admission, other possible causes of thrombocytopenia were excluded, and ITP was concluded to be the most probable diagnosis 3 . In another case series, three COVID‐19 patients developed ITP, two of the three patients presented with skin purpura and mucosal bleeding. The third patient developed acute transfusion‐resistant thrombocytopenia and died after intracerebral hemorrhage 4 . The patient in our case developed acute thrombocytopenia, and possible causes such as DIC, HIT, thrombotic thrombocytopenic purpura, and drug‐induced thrombocytopenia have been excluded. Although the patient had acute DVT that may contribute to consumptive thrombocytopenia, the timing, magnitude, and acuity of thrombocytopenia are unlikely to be due to DVT alone. Also, the patient was found to have positive IgG antibodies against the platelets and did not respond to platelet transfusion which makes ITP the most likely diagnosis. Our patient did not experience any bleeding events although he had severe thrombocytopenia, this may be explained by the fact that diagnoses and management were established in a timely manner.
Immune thrombocytopenic purpura treatment consists of systemic steroids and IVIG as first line. Second‐line treatment options include splenectomy, rituximab, immunosuppressive therapy, and thrombopoietin receptor agonists (TRAs). More than 70% of the patients respond to conventional therapy. TRAs are reserved for patients resistant to first‐ and second‐line therapies and are considered safe and effective 6 . Recent guidelines published by Pavord S et al recommended steroids as a first‐line therapy for ITP secondary to COVID‐19. TRA may be associated with increased risk of thrombosis and elevated liver enzymes and are better to be avoided in COVID‐19 patients. IVIG was recommended to be used when immediate platelet count elevation is needed 7 . In the case report by Abrar‐Ahmad Zulfiqar et al, 3 the patient was treated with IVIG, steroids, platelet transfusion, and eltrombopag. The patient had delayed response to treatment, and the course was complicated by intracerebral hemorrhage. Our patient was treated with a combination of dexamethasone and IVIG because he had severe thrombocytopenia that required immediate elevation of platelet count and responded well to therapy and did not develop any bleeding complications.
Since COVID‐19 patients are at increased risk of hypercoagulability and VTEs, it is extremely important to monitor platelet count on daily basis in severe patients admitted to ICU. Acute thrombocytopenia may be the only sign of an evolving ITP or DIC and requires immediate action to prevent further complications such as major bleeding, especially in patients receiving systemic AC.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
TK, FA, LS, HD, and AD contributed to the conception and design of study. TK, FA, and AD interpreted the data. TK wrote the first draft of the report. All authors contributed to article revision and approved the submitted version.
CONSENT
Cleveland Clinic Committee on Human Research approved the study. Written informed consent was obtained.
REFERENCES
- 1. Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346(13):995‐1008. [DOI] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A Novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zulfiqar AA, Lorenzo‐Villalba N, Hassler P, Andres E. Immune thrombocytopenic purpura in a patient with Covid‐19. N Engl J Med. 2020;382(18):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bomhof G, Mutsaers P, Leebeek FWG, et al. COVID‐19‐associated immune thrombocytopenia. Br J Haematol. 2020;190(2):e16850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amgalan A, Othman M. Hemostatic laboratory derangements in COVID‐19 with a focus on platelet count. Platelets. 2020:1‐6. 10.1080/09537104.2020.1768523 [DOI] [PubMed] [Google Scholar]
- 6. Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780‐3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pavord S, Thachil J, Hunt B, et al. Practical guidance for the management of adults with Immune Thrombocytopenia during the COVID‐19 pandemic. Br J Haematol. 2020;189(6):1038‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]


