Myoclonus has been reported as a possible manifestation of coronavirus disease 2019 (COVID‐19), yet its neurophysiology and pathogenesis were poorly investigated. 1 , 2 , 3 , 4 We describe a middle‐aged man with COVID‐19 who underwent extensive examinations for his disabling myoclonus.
CASE REPORT
A 58‐year‐old hypertensive man with a 1‐week history of fever and cough presented to the emergency department with dyspnea. A nasopharyngeal swab tested positive for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The patient was admitted to the intensive care unit after 1 week and placed on invasive mechanical ventilation as a result of respiratory distress. He was treated with hydroxychloroquine, tocilizumab, and remdesivir. Respiratory status quickly improved, thus he was extubated after 5 days, and oxygen therapy was progressively weaned off. Two days after discharge from the intensive care unit, he became markedly agitated. His mental status normalized in 48 hours; however, at this point he developed multifocal myoclonus elicited by action and tactile stimuli, predominant in the right proximal inferior limb muscles, preventing his ability to stand (Video SS1). Cognitive deficits were not observed.
Electrolytes and renal and liver function tests were unremarkable. Cerebrospinal fluid (CSF) analysis, performed 8 days after myoclonus onset, demonstrated 5 leukocytes/μL, elevated protein levels (75 mg/dL) and CSF/serum albumin ratio (13.1), and negative SARS‐CoV‐2 reverse‐transcription polymerase chain reaction. Cytokine analyses revealed interleukin‐6 at 11.6 pg/mL in CSF (29.3 pg/mL in serum, reference < 5.9) and interleukin‐8 at 38 pg/mL in CSF (11 pg/mL in serum, reference < 70). A serologic panel of autoantibodies against neuronal intracellular and cell surface antigens was negative. Brain magnetic resonance imaging showed cerebral small‐vessel disease of moderate severity. Electroencephalogram (EEG) was unremarkable. Polymyography confirmed the presence of multifocal positive myoclonus with a burst duration of 140 to 220 milliseconds. Back‐averaging analysis did not show EEG time‐locked discharges (Fig. 1). The patient was treated with clonazepam and levetiracetam, resulting in marked amelioration of the myoclonus within 5 days.
FIG 1.
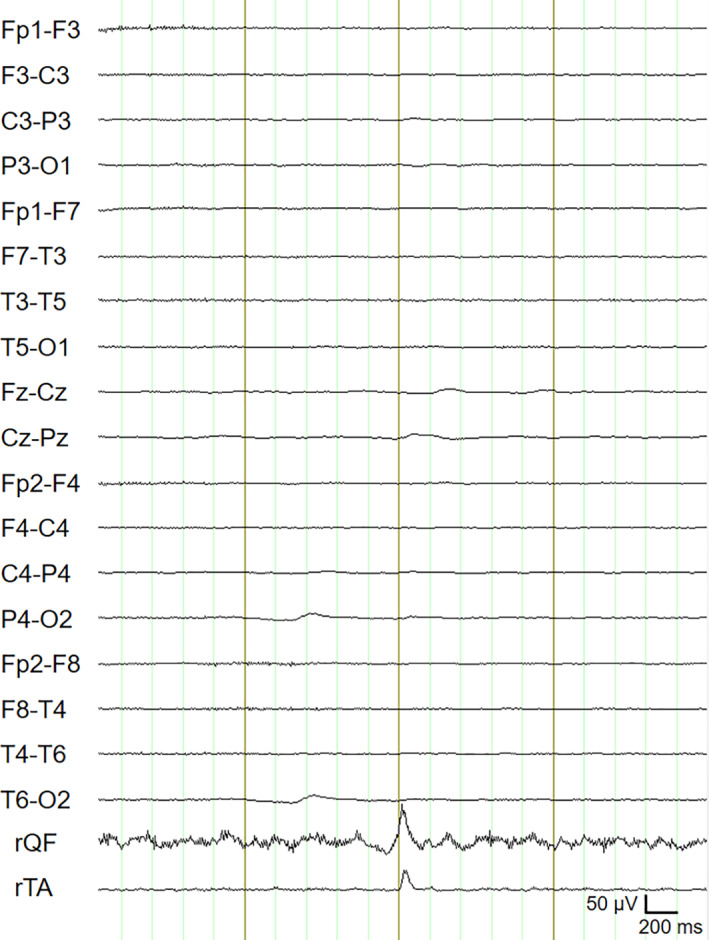
Electroencephalogram jerk‐locked back‐averaging. A total of 52 myoclonia were collected in a bipolar electromyogram from right tibialis anterior (rTA) during voluntary contraction of the right quadriceps femori (rQF). The electromyogram traces were first filtered (high‐pass filter, 23 Hz; low‐pass filter, 300 Hz) and then rectified. Myoclonia were marked at the onset. The 4‐second electroencephalogram and electromyogram epochs (mark ± 2 seconds) were selected. Averaging of the 52 jerks did not show any electroencephalogram correlates.
DISCUSSION
This case report confirms that myoclonus can occur in the context of diffuse inflammation related to COVID‐19. In previously published reports, myoclonus has been described as spontaneous or action‐induced, multifocal or generalized, with a nonspecific distribution. 1 , 2 , 3 , 4
In our patient, the prominent involvement of axial and proximal limb muscles, myoclonus stimulus sensitivity, the absence of cortical discharges at EEG jerk‐locked back‐averaging, and the long duration of myoclonic bursts are consistent with subcortical myoclonus, possibly secondary to brainstem involvement.
Defining the underlying pathophysiology is challenging. Myoclonus may present in the context of other viral infections, with concomitant encephalopathy/encephalitis or as an isolated postinfectious phenomenon. 5 , 6 SARS‐CoV‐2 may theoretically access subcortical structures involved in myoclonus generation via invasion of the olfactory bulb. 1 In our patient, however, clinical course (including the absence of hyposmia), magnetic resonance imaging, and CSF findings argue against a direct pathogenic role of central nervous system viral invasion. Although myoclonus appeared after a period of intubation, our patient did not suffer anoxic brain injury, thus excluding Lance‐Adams syndrome. Myoclonus onset timing and clinical course were also not consistent with an adverse drug reaction, a mechanism suggested in the form of serotonin syndrome in 2 patients treated with lopinavir/ritonavir. 2
Agitation and myoclonus were preceded by severe cytokine release syndrome, a distinctive feature of COVID‐19. 7 CSF analysis showed blood–brain barrier disruption, slightly elevated CSF interleukin‐6 levels, and an elevated interleukin‐8 CSF/blood ratio. These abnormalities may have been more pronounced if assessed at myoclonus onset. Interestingly, cytokine‐mediated neuroinflammation induced by SARS‐CoV‐2 has been implicated in steroid‐responsive COVID‐19‐associated encephalopathy. 8 In addition to marked agitation in our patient, previous reports also had clinical/instrumental findings suggestive of encephalopathy, including dysexecutive syndrome, delirium, somnolence, EEG slowing, elevated inflammatory markers, variable responses to immunotherapies, and a benign clinical course, 1 , 2 , 3 , 4 further suggesting an immune‐mediated/inflammatory pathogenesis.
In conclusion, subcortical myoclonus should be considered among the neurological manifestations associated with COVID‐19. The pathogenic role of cytokine‐mediated neuroinflammation should be addressed in future studies.
Author Roles
(1) Research Project: A. Study Concept and Design, B. Acquisition of Data, C. Analysis and Interpretation of Data, D. Supervision; (2) Manuscript Preparation: A. Writing of the First Draft, B. Review and Revision.
L.M.: 1A, 1B, 1C, 2A
F.R.: 1A, 1B, 1C, 2B
L.F.: 1A, 1C, 2B
G.R.: 1B, 1C
P.C.: 1C, 1D, 2B
M.G.: 1C, 1D, 2B
Disclosures
Ethical Compliance Statement
The authors confirm that approval of an institutional review board was not required for this work. Informed written consent for publication was obtained from the patient. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
The authors report no relevant disclosures or conflicts of interest for this manuscript.
Financial Disclosures for the Previous 12 Months
The authors report no financial disclosures for the previous 12 months other than employment from the respective affiliations.
Supporting information
Video S1 Rest: Single subtle myoclonic jerk of the left arm. Series of spontaneous small‐amplitude jerks of the right leg followed by a more prominent jerk. Posture: Maintenance of hip flexion elicits high‐amplitude myoclonic jerks at both inferior limbs, predominant proximally and on the right side; a spontaneous bilateral myoclonic jerk occurs following relaxation. Stimuli: Generalized myoclonus consistent with startle reflex evoked by sternum‐tap stimulus. Handclap elicits no response. Tactile stimulation of each foot evokes bilateral myoclonic jerks. Action: Heel–knee test is hindered by action‐induced myoclonus. Multifocal myoclonic jerks involving the trunk and the four limbs prevent the patient's ability to sit.
Acknowledgments
We thank Paolo Tinuper for helpful discussion on the case, the neurophysiology technologists Soraia Garrossi and Rita Signorelli for their quality work during this pandemic, and Olivia J. Henry for English‐language editing.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Rábano‐Suárez P, Bermejo‐Guerrero L, Méndez‐Guerrero A, et al. Generalized myoclonus in COVID‐19. Neurology 2020;95(6):e767–e772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Serrano M, Pérez‐Sánchez JR, Portela Sánchez S, et al. Serotonin syndrome in two COVID‐19 patients treated with lopinavir/ritonavir. J Neurol Sci 2020;415:116944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaumont H, San‐Galli A, Martino F, et al. Mixed central and peripheral nervous system disorders in severe SARS‐CoV‐2 infection. Journal of Neurology 2020. 10.1007/s00415-020-09986-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beach SR, Praschan NC, Hogan C, et al. Delirium in COVID‐19: a case series and exploration of potential mechanisms for central nervous system. Gen Hosp Psychiatry 2020;65:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carroll E, Sanchez‐Ramos J. Hyperkinetic movement disorders associated with HIV and other viral infections. Handb Clin Neurol 2011;100:323–334. [DOI] [PubMed] [Google Scholar]
- 6. Bhatia K, Thompson PD, Marsden CD. "Isolated" postinfectious myoclonus. J Neurol Neurosurg Psychiatry 1992;55(11):1089–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moore JB, June CH. Cytokine release syndrome in severe COVID‐19. Science 2020;368(6490):473–474. [DOI] [PubMed] [Google Scholar]
- 8. Pilotto A, Odolini S, Masciocchi S, et al. Steroid‐responsive encephalitis in coronavirus disease 2019. Annals of Neurology 2020;88(2):423–427. 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 Rest: Single subtle myoclonic jerk of the left arm. Series of spontaneous small‐amplitude jerks of the right leg followed by a more prominent jerk. Posture: Maintenance of hip flexion elicits high‐amplitude myoclonic jerks at both inferior limbs, predominant proximally and on the right side; a spontaneous bilateral myoclonic jerk occurs following relaxation. Stimuli: Generalized myoclonus consistent with startle reflex evoked by sternum‐tap stimulus. Handclap elicits no response. Tactile stimulation of each foot evokes bilateral myoclonic jerks. Action: Heel–knee test is hindered by action‐induced myoclonus. Multifocal myoclonic jerks involving the trunk and the four limbs prevent the patient's ability to sit.


