Abstract
Drug repositioning represents an effective way to control the current COVID‐19 pandemic. Previously, we identified 24 FDA‐approved drugs which exhibited substantial antiviral effect against severe acute respiratory syndrome coronavirus 2 in Vero cells. Since antiviral efficacy could be altered in different cell lines, we developed an antiviral screening assay with human lung cells, which is more appropriate than Vero cell. The comparative analysis of antiviral activities revealed that nafamostat is the most potent drug in human lung cells (IC50 = 0.0022 µM).
Keywords: COVID‐19, drug repositioning, FDA‐approved drug, SARS‐CoV‐2
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is an emerging infectious disease caused by a coronavirus. 1 The causative virus was named as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) because it is very similar to SARS‐CoV (79.5%) and this virus belongs to the Betacoronavirus genus within the Coronaviridae family. 2 Both SARS‐CoV and Middle East respiratory syndrome coronavirus (MERS‐CoV) also belong to the same Betacoronavirus genus.
Neither vaccine nor therapeutic has been developed for SARS‐ and MERS‐CoV and the current standard of care for the patients with COVID‐19 is just supportive care. However, numerous clinical trials are ongoing globally with Food and Drug Administration (FDA)‐approved drugs as drug repositioning programs (https://www.covid-trials.org/). Among these drugs, (hydroxy)chloroquine, lopinavir/ritonavir, and remdesivir are those that are the most frequently being tested worldwide due to the well‐known in vitro antiviral effects on both MERS‐ and SARS‐CoV and even on SARS‐CoV‐2. 3
Previously, we identified a total of 24 potential antiviral drug candidates from FDA‐approved drugs using Vero cells. 4 Since antiviral efficacy could be altered in different cell lines, we developed a new image‐based antiviral screening assay with Calu‐3 cells, a well‐known human lung cell line, 5 and compared the antiviral efficacy of the antiviral candidates in between Vero and Calu‐3 cells.
2. MATERIALS AND METHODS
2.1. Virus and cells
Calu‐3 used in this study is a clonal isolate, which shows higher growth rate compared with the parental Calu‐3 obtained from the American Type Culture Collection (ATCCHTB‐55). Calu‐3 was maintained at 37°C with 5% CO2 in Eagle's Minimum Essential Medium (EMEM, ATCC), supplemented with 10% heat‐inactivated fetal bovine serum (FBS) and 1X Antibiotic‐Antimycotic solution (Gibco). SARS‐CoV‐2 (βCoV/KOR/KCDC03/2020) was provided by Korea Centers for Disease Control and Prevention (KCDC), and was propagated in Vero cells. Viral titers were determined by plaque assays in Vero cells. All experiments using SARS‐CoV‐2 were performed at Institut Pasteur Korea in compliance with the guidelines of the KNIH, using enhanced biosafety level 3 (BSL‐3) containment procedures in laboratories approved for use by the KCDC.
2.2. Reagents
Chloroquine diphosphate (CQ; C6628) was purchased from Sigma‐Aldrich (St. Louis, MO), lopinavir (LPV; S1380) was purchased from SelleckChem (Houston, TX), and remdesivir (HY‐104077) was purchased from MedChemExpress (Monmouth Junction, NJ). Chloroquine was dissolved in Dulbecco's Phosphate‐Buffered Saline (DPBS; Welgene), and all other reagents were dissolved in dimethyl sulfoxide (DMSO) for the screening. Anti‐SARS‐CoV‐2 N protein antibody was purchased from Sino Biological Inc (Beijing, China). Alexa Fluor 488 goat anti‐rabbit IgG (H + L) secondary antibody and Hoechst 33342 were purchased from Molecular Probes. Paraformaldehyde (PFA) (32% aqueous solution) and normal goat serum were purchased from Electron Microscopy Sciences (Hatfield, PA) and Vector Laboratories, Inc (Burlingame, CA), respectively.
2.3. Dose‐response curve analysis by immunofluorescence
Ten‐point dose‐response curves (DRCs) were generated for each drug. Calu‐3 cells were seeded at 2.0 × 104 cells per well in EMEM, supplemented with 10% FBS and 1X Antibiotic‐Antimycotic solution (Gibco) in black, 384‐well, μClear plates (Greiner Bio‐One), 24 hours before the experiment. Ten‐point DRCs were generated, with compound concentrations ranging from 0.1 to 50 μM. For viral infection, plates were transferred into the BSL‐3 containment facility and SARS‐CoV‐2 was added at a multiplicity of infection of 0.1. The cells were fixed at 24 hpi with 4% PFA and analyzed by immunofluorescence. The acquired images were analyzed using in‐house software to quantify cell numbers and infection ratios, and antiviral activity was normalized to positive (mock) and negative (0.5% DMSO) controls in each assay plate. DRCs were generated in Prism7 (GraphPad) software, with dose‐response‐inhibition nonlinear regression analysis. Half maximal Inhibitory concentration (IC50) and half maximal cytotoxic concentration (CC50) values were obtained with the identical analysis method. Mean values of independent duplicate experiments were used for analysis. Each assay was controlled by Z'‐factor and the coefficient of variation in percent (%CV).
3. RESULTS AND DISCUSSION
In our previous drug repositioning study, we identified a total of 24 potential antiviral drug candidates from FDA‐approved drugs. 4 These drugs showed very potent antiviral efficacy against SARS‐CoV‐2 (0.1 µM < IC50 < 10 µM) in the experiments using Vero cells. Although Vero cells are commonly used for virus infection and propagation, they were originally isolated from the African green monkey kidney, thus do not represent the respiratory cells from the human lung, which is the main target tissue for SARS‐CoV‐2 infection. In this study, we compared the antiviral efficacy of the 24 potential antiviral drug candidates against SARS‐CoV‐2 using Calu‐3 human lung cells. Calu‐3 was originally isolated from human lung adenocarcinoma and is a well‐characterized epithelial cell line. 5
In order to conduct the DRC analysis with drugs, Calu‐3 cells were treated with each drug candidate 1 hour before SARS‐CoV‐2 infection. The infected cells were incubated for 24 hours and then fixed for immunofluorescence. Both viral N protein and host cell nucleus were stained by immunofluorescence and the quantitative analysis to measure the inhibition of virus infection and the cell viability due to drug treatment was conducted using our in‐house Image Mining software.
The DRC analysis of the reference drugs (ie, chloroquine, lopinavir, and remdesivir) (Figure 1) showed differences in IC50 in between Vero and Calu‐3 cells. While the IC50 values of both chloroquine and lopinavir increased by approcimately 10 folds and 2 folds, respectively (Table 1), the IC50 of remdesivir rather decreased by 10 folds compared with that with Vero cells, perhaps due to the low metabolic capacity or prodrug activation rate in Vero cells 6 (Table 2). These discrepancies might in part account for the different outcomes from numerous clinical trials using chloroquine, lopinavir, and remdesivir. So far, the treatment with (hydroxy)chloroquine or lopinavir/ritonavir did not show any promising results concerning the COVID‐19 treatment 7 , 8 , 9 ; however remdesivir seems to be effective for treatment of patients with COVID‐19 in certain clinical settings. 10
Figure 1.
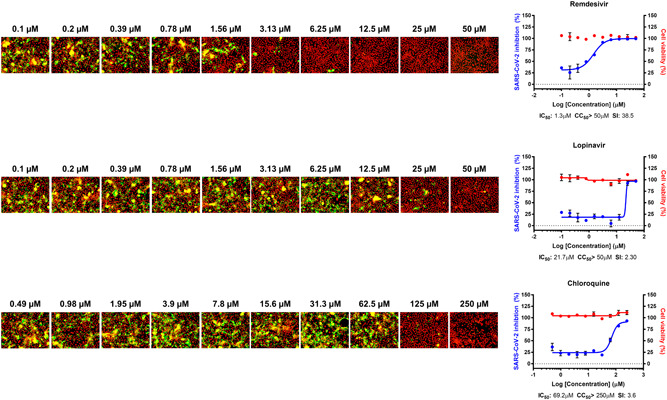
Dose‐response curve (DRC) of reference drugs. Three reference drugs—remdesivir, lopinavir, and chloroquine—were serially diluted by two folds to generate a 10‐point DRC. Graphs are shown on the right and representative images at each point are shown on the left. The red line indicates cell viability, and the blue line indicates inhibition of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. SARS‐CoV‐2 infectivity was measured by immunofluorescence of SARS‐CoV‐2 N protein. Each point is a mean of duplicate experiments ± standard deviation. Half maximal inhibitory concentration (IC50), half maximal cytotoxic concentration (CC50), and selective index (SI) are noted below each graph. Nucleus is shown in red, and viral N protein is shown in green
Table 1.
List of drugs with increased IC50 in Calu‐3 cells
| Drug name | IC50 in Vero, µMa | IC50 in Calu‐3, µMb | Fold change |
|---|---|---|---|
| Fold change >4 | |||
| Tetrandrine | 3 | 13.5 | 4.50 |
| Berbamine hydrochloride | 7.87 | >50 | 6.35 |
| Abemaciclib | 6.62 | 43.7 | 6.60 |
| Cepharanthine | 4.47 | 30 | 6.71 |
| Gilteritinib | 6.76 | >50 | 7.40 |
| Chloroquine | 7.28 | 69.2 | 9.51 |
| Amodiaquine dihydrochloride | 5.15 | >50 | 9.71 |
| Mefloquine | 4.33 | >50 | 11.55 |
| Fold change >2 | |||
| Salinomycin sodium | 0.24 | 0.5 | 2.08 |
| Lopinavir | 9.12 | 21.7 | 2.38 |
| Ciclesonide | 4.33 | 10.64 | 2.46 |
| Proscillaridin | 2.04 | 5.95 | 2.92 |
| Niclosamide | 0.28 | 0.84 | 3.00 |
| Anidulafungin | 4.64 | 17.23 | 3.71 |
| Digoxin | 0.19 | 0.72 | 3.79 |
| Bazedoxifene | 3.44 | 12.63 | 3.67 |
Abbreviation: IC50, half maximal inhibitory concentration.
IC50 was determined by Jeon et al. 4
IC50 was determined in this study.
Table 2.
List of drugs with decreased IC50 in Calu‐3 cells
| Drug name | IC50 in Vero, µMa | IC50 in Calu‐3, µMb | Fold change |
|---|---|---|---|
| Nafamostat mesylate | 13.88 | 0.0022 | 0.00016 |
| Camostat mesylate | >50 | 0.187 | 0.00374 |
| Remdesivir | 11.41 | 1.3 | 0.11 |
| Hydroxyprogesterone caproate | 6.3 | 3.87 | 0.61 |
| Digitoxin | 0.23 | 0.16 | 0.70 |
| Cyclosporine | 5.82 | 4.69 | 0.81 |
Abbreviation: IC50, half maximal inhibitory concentration.
IC50 was determined by Jeon et al 4 except for nafamostat mesylate.
IC50 was determined in this study.
Interestingly, the IC50 values of most drugs in our study increased in varying degrees in Calu‐3 cells (Figures 2A,C) (Tables 1 and 3). Only six drugs showed decreases in IC50 (Figure 2B) (Table 2): nafamostat mesylate, camostat mesylate, remdesivir, hydroxyprogesterone caproate, digitoxin, and cyclosporine. Although nafamostat mesylate and camostat mesylate were not selected as potent antiviral drug candidates in our earlier study, we compared the antiviral efficacy of these drugs at this time in between Vero and Calu‐3 cells following the discovery that TMPRSS2, a host protease necessary for priming viral spike glycoprotein, could be a target for COVID‐19 antiviral development. 11 The discrepancy in IC50 was specifically remarkable with nafamostat mesylate; the IC50 decreased by approximately 6000 folds when the drug was used in the SARS‐CoV‐2‐infected Calu‐3 cells perhaps due to the dominant role of TMPRSS2‐dependent viral entry in the Calu‐3 human lung epithelial cells. 12 , 13 In addition, the IC50 of nafamostat mesylate was exceptionally low (0.0022 µM), which indicates that nafamostat mesylate is approximately 600‐fold more potent than remdesivir in Calu‐3 cells. It became more apparent that blood clotting is one of the complicating manifestations in patients with COVID‐19, 14 , 15 and nafamostat mesylate may play dual roles not only as an antiviral to block viral entry but also as an anticoagulant to remove blood clots frequently associated with acute respiratory distress syndrome. A recent case report on the treatment of three patients with COVID‐19 with nafamostat 16 and other in vitro studies 17 , 18 corroborated our findings. However, nafamostat has a short half‐life in the serum, thus requires continuous intravenous injection, which disables convenient administration for a large group of patients. Solving this disadvantage will increase the accessibility of more COVID‐19 patients to nafamostat treatment.
Figure 2.
Dose‐response curve (DRC) of the 24 drugs, nafamostat mesylate, and camostat mesylate. A, DRC of compounds with increased IC50 value compared to those with Vero cells (fold change above 2). B, DRC of compounds with decreased IC50 value compared to those with Vero cells (fold change less than 1). C, DRC of compounds with unchanged IC50 value compared to those with Vero cells (fold change approximately 1). The red line indicates cell viability, and the blue line indicates inhibition of SARS‐CoV‐2 infection. SARS‐CoV‐2 infectivity was measured by immunofluorescence of SARS‐CoV‐2 N protein. Each point is a mean of duplicate experiments ± standard deviation. IC50, CC50, and SI are noted below each graph. CC50, half maximal cytotoxic concentration; IC50, half maximal inhibitory concentration; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; SI, selective index
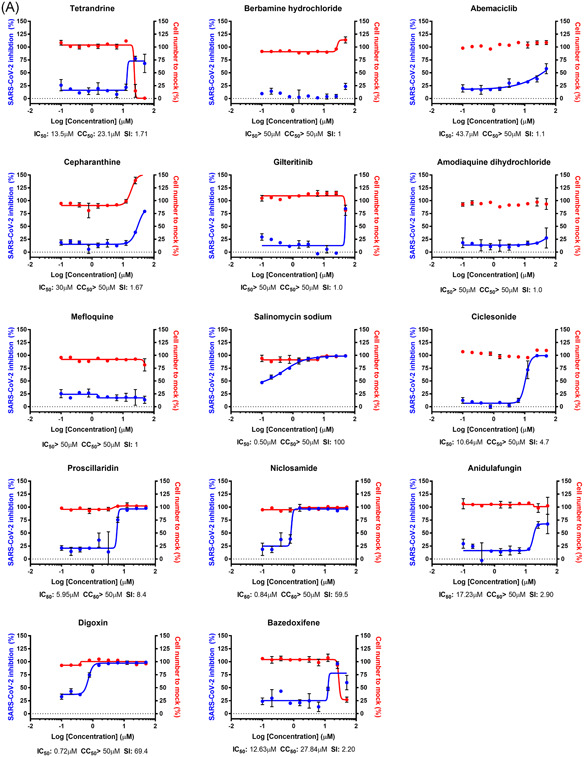
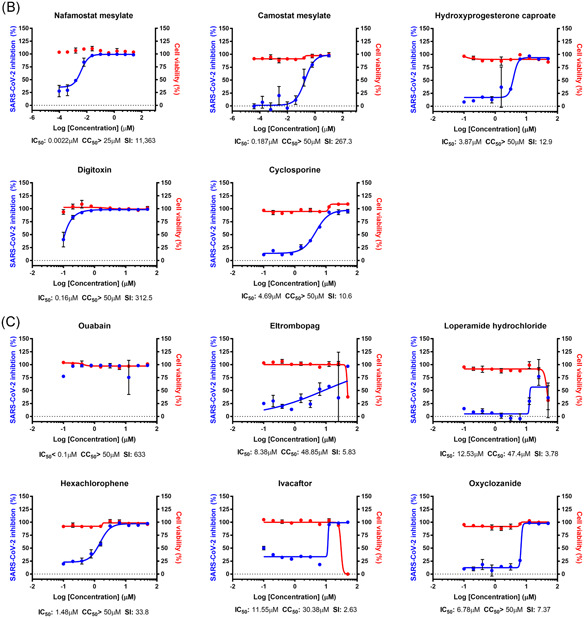
Table 3.
List of drugs with unchanged IC50 in Calu‐3 cells
| Drug name | IC50 in Vero, µMa | IC50 in Calu‐3, µMb | Fold change |
|---|---|---|---|
| Ouabain | <0.1 | <0.1 | 1.00 |
| Eltrombopag | 8.27 | 8.38 | 1.01 |
| Loperamide hydrochloride | 9.27 | 12.53 | 1.35 |
| Hexachlorophene | 0.9 | 1.48 | 1.64 |
| Ivacaftor | 6.57 | 11.55 | 1.76 |
| Oxyclozanide | 3.71 | 6.78 | 1.83 |
Abbreviation: IC50, half maximal inhibitory concentration.
IC50 was determined by Jeon et al. 4
IC50 was determined in this study.
In summary, we compared antiviral efficacy of the potential antiviral drug candidates against SARS‐CoV‐2 in between Vero and Calu‐3 cells and found that nafamostat mesylate is the most potent antiviral drug candidate in vitro. Importantly, nafamostat mesylate has been approved for human use in Japan and Korea for over a decade, thus it can be readily repurposed for COVID‐19 following phase II‐III clinical trials. Currently, a few clinical trials have been registered (https://clinicaltrials.gov/). According to our results, although in vivo animal models are preferred experimental systems for evaluating antiviral efficacy, in vitro testing using human lung cells is a viable option in addition to the commonly used Vero or VeroE6 cells for assessment of antiviral efficacy when the animal models are not readily available.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conception: WSR and SK. Study design: MK, WSR, and SK. Participation in experiments and data collection: MK and SJ. Data acquisition, writing manuscript, and statistical analysis: MK and SK. All authors reviewed the manuscript and approved the final version.
ACKNOWLEDGMENTS
The pathogen resource (NCCP43326) for this study was provided by the National Culture Collection for Pathogens. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF‐2017M3A9G6068245 and NRF‐2020M3E9A1041756).
Ko M, Jeon S, Ryu W‐S, Kim S. Comparative analysis of antiviral efficacy of FDA‐approved drugs against SARS‐CoV‐2 in human lung cells. J Med Virol. 2021;93:1403–1408. 10.1002/jmv.26397
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5(4):536‐544. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30(3):269‐271. 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeon S, Ko M, Lee J, et al. Identification of antiviral drug candidates against SARS‐CoV‐2 from FDA‐approved drugs. Antimicrob Agents Chemother. 2020;64(7). 10.1128/AAC.00819-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu Y, Chidekel A, Shaffer TH. Cultured human airway epithelial cells (Calu‐3): a model of human respiratory function, structure, and inflammatory responses. Crit Care Res Pract. 2010;2010:1‐8. 10.1155/2010/394578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pruijssers AJ, George AS, Schäfer A, et al. Remdesivir potently inhibits SARS‐CoV‐2 in human lung cells and chimeric SARS‐CoV expressing the SARS‐CoV‐2 RNA polymerase in mice. bioRxiv. 2020. 10.1101/2020.04.27.064279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with COVID‐19. N Engl J Med. 2020;382:2411‐2418. 10.1056/nejmoa2012410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. JAMA Netw Open. 2020;3(4):e208857. 10.1001/jamanetworkopen.2020.8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao B, Wang Y, Wen D, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe COVID‐19. N Engl J Med. 2020. 2020;382(19):1787‐1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID‐19—preliminary report. N Engl J Med. 2020. 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 11. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamamoto M, Matsuyama S, Li X, et al. Identification of nafamostat as a potent inhibitor of Middle East respiratory syndrome coronavirus S protein‐mediated membrane fusion using the split‐protein‐based cell‐cell fusion assay. Antimicrob Agents Chemother. 2016;60(11):6532‐6539. 10.1128/AAC.01043-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawase M, Shirato K, van der Hoek L, Taguchi F, Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012;86(12):6537‐6545. 10.1128/JVI.00094-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in COVID‐19 patients: awareness of an increased prevalence. Circulation. 2020;142:184‐186. 10.1161/CIRCULATIONAHA.120.047430 [DOI] [PubMed] [Google Scholar]
- 16. Jang S, Rhee J‐Y. Three cases of treatment with nafamostat in elderly patients with COVID‐19 pneumonia who need oxygen therapy. Int J Infect Dis. 2020;96:500‐502. 10.1016/j.ijid.2020.05.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffmann M, Schroeder S, Kleine‐Weber H, Müller MA, Drosten C, Pöhlmann S. Nafamostat mesylate blocks activation of SARS‐CoV‐2: new treatment option for COVID‐19. Antimicrob Agents Chemother. 2020;64(6). 10.1128/AAC.00754-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamamoto M, Kiso M, Sakai‐Tagawa Y, et al. The anticoagulant nafamostat potently inhibits SARS‐CoV‐2 S protein‐mediated fusion in a cell fusion assay system and viral infection in vitro in a cell‐type‐dependent manner. Viruses. 2020;12(6):629. 10.3390/v12060629 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


