Abstract
Purpose
The aim of this study was to evaluate the applicability of bedside ultrasonography for the diagnosis of deep venous thrombosis (DVT) in patients infected with corona virus disease 2019 (COVID‐19) with and without treatment with low molecular weight heparin (LMWH).
Methods
We retrospectively analyzed the records of deceased and surviving patients in whom ultrasonography detected or not a DVT, and in whom LMWH was or not prescribed.
Results
The incidence of DVT is higher in the deceased (33/35) than in the surviving (22/46) patients. LMWH was administered in a larger proportion of surviving (18/22) than of deceased (18/33) patients. D‐dimer concentrations decreased in patients who received LMWH in both groups.
Conclusions
There was a high incidence of DVT in patients who succumbed to COVID‐19. Bedside ultrasonography can detect the presence of DVT as early as possible and help assessing the risk of venous thromboembolism, allowing early and reasonable use of LMWH.
Keywords: bedside ultrasonography, COVID‐19, deep venous thrombosis, low molecular weight heparin
1. INTRODUCTION
Corona virus disease 2019 (COVID‐19), which was first reported in Wuhan, China, in late December 2019, is currently spreading to other provinces in mainland China and to other countries around the globe, with major public health and economic consequences. 1
Bedside ultrasonography can detect deep venous thrombosis (DVT) in a timely and accurate manner, which helps assessing the risk of venous thromboembolism. Bedside checks can be repeated in a short time span because there is no radiation, and can provide safe and important diagnostic information for patients with COVID‐19.
Based on existing studies, the clinical manifestations and pathological results of COVID‐19 infection are associated with acute respiratory distress syndrome (ARDS), 2 characterized by respiratory distress and difficulty to correct hypoxemia, with a high mortality rate, 3 due to excessive intra‐alveolar fibrin deposition. The imbalance between activation of coagulation and inhibition of fibrinolysis in patients with ARDS leads to fibrin formation, which occurs both systemically and in the lungs and airspace. 4 Nearly 20% of COVID‐19 patients present severe coagulation abnormalities, which may occur in almost all of the severe and critical ill COVID‐19 cases. 5 The large amount of thrombin can induce the expression of E‐selectin, P‐selectin, and platelet‐activating factors, promote the aggregation and adhesion of platelets and activated neutrophils, and strengthen the interaction between endothelial cells and neutrophils, 6 ultimately promoting the further aggravation of inflammation. Therefore, the pathological setting of ARDS is the mutual promotion between hypercoagulability and inflammation. In the present study, we used bedside ultrasonographic examination to detect DVT and to assess the effect of low molecular weight heparin (LMWH), which possesses dual anticoagulant and anti‐inflammatory effects.
2. MATERIALS AND METHODS
2.1. Patients
Our study was conducted in accordance with the principles of the Declaration of Helsinki. The locally appointed ethics committee has approved the research protocol, and informed consent has been obtained from all subjects.
Diagnosis of COVID‐19 was based on a positive nucleic acid test of SARE‐CoV‐2, according to the Diagnosis and Treatment Plan of Novel Coronavirus Pneumonia (trial version 7th) issued by the National Health Commission of the People's Republic of China. 7 COVID‐19 severity was assessed according to the following clinical classification: (a) light type: mild clinical symptoms, no pneumonia on imaging; (b) common type: fever, respiratory symptoms, pneumonia on imaging; (c) severe type: respiratory distress with respiratory rate ≥30/min and/or oxygen saturation ≤93% at rest and/or partial arterial pressure of oxygen/fraction of inspired oxygen ≤300 mm Hg (40 kPa); and (d) critical type: respiratory failure requiring mechanical ventilation and/or shock and/or other organ failure and admission to intensive care unit (ICU).
We performed the retrospective analysis of 81 severe and critical COVID‐19 patients who had had an ultrasonographic examination performed in the ICU of Wuhan Jinyintan Hospital from January 1, 2020 to March 5, 2020. The total number of patients on ICU during the same time was 108. At the end‐point date of March 5, 2020, the patients were divided into two groups: those who had succumbed to COVID‐19, and the surviving patients. We did not report underlying diseases, since they have been reported to be absent in 47% of deceased patients 8 .
2.2. Instruments and methods
Bedside ultrasonographic examination in search of DVT was performed in patients showing lower limb swelling and/or increased D‐dimer values, using a M9 ultrasound system (Mindray Medical International, Shenzhen, China) and a 3.0 to 13.0 MHz linear transducer. Patient's both lower limbs were examined above and under the knee, and B‐mode images were obtained in transverse and longitudinal sections. The extent of thrombosis was recorded and detailed in the patients' medical records. The ultrasonic criteria of acute DVT were an enlarged vein, the presence of a hypoechoic intraluminal mass (Figure 1), absent or incomplete vein collapse at the compression test (Figure 2), and absence of detectable blood flow signal on color Doppler flow imaging (Figure 3). Patients with DVT were followed‐up every 3 days, and administration of LMWH as well as D‐dimer levels was recorded.
FIGURE 1.
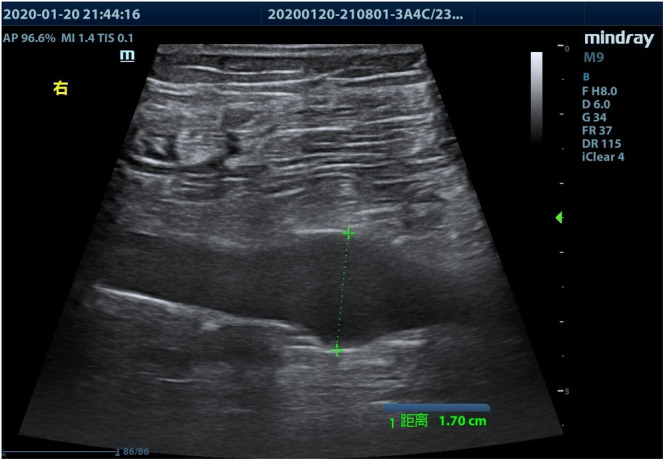
Ultrasonographic image of acute deep venous thrombosis showing locally enlarged vein
FIGURE 2.
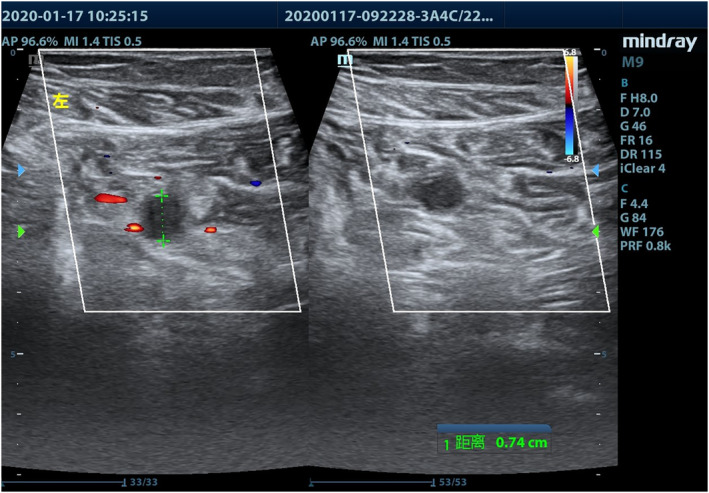
Deep venous thrombosis at the calf, showing incompressible vein because of thrombosis. Left panel: without compression; right panel: during compression with the ultrasound probe
FIGURE 3.
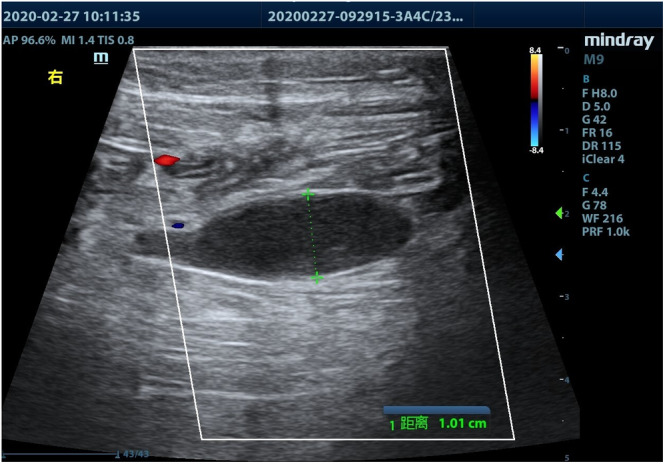
Enlarged vein with thrombosis: no blood flow signal can be detected by color Doppler flow imaging
2.3. Statistical analysis
SPSS 20.0 statistical software (IBM, New York, NY) was used for statistical analysis. Categorical data were expressed as number (percentage) and χ2 test was used for comparisons. Continuous variables were expressed as mean ± SD and compared with paired student's t‐test. A P value <.05 was accepted as statistically significant.
3. RESULTS
There was no difference in clinical data between deceased and surviving patients (Table 1). There were deceased 35 patients, among whom 33 had DVT of whom 18 received LMWH. There were 46 surviving patients, among whom 22 had DVT of whom 18 received LMWH. The incidence of DVT was higher in deceased than in surviving patients (P < .001). The proportion of DVT patients who did not receive LMWH was greater among deceased than among surviving patients (P < .034). DVT was detected 2.5 ± 1.5 days after admission and LMWH administrating started 4.5 ± 1.5 days after admission in the whole population sample.
TABLE 1.
Patients' clinical characteristics
| N | Gender | Range of deep venous thrombosis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Comorbidities | I | II | III | ||||||||||
| n | % | n | % | Age (years) | Body mass index | n | % | n | % | n | % | n | % | ||
| Group A | 35 | 25 | 71.4 | 10 | 28.6 | 62.4 ± 10.2 | 22.3 ± 1.9 | 26 | 72.3 | ||||||
| Group B | 46 | 29 | 63.0 | 17 | 37.0 | 61.1 ± 14.1 | 21.8 ± 2.0 | 32 | 69.5 | ||||||
| Group A+ | 33 | 23 | 70.0 | 10 | 30.0 | 62.8 ± 10.3 | 22.6 ± 1.7 | 24 | 72.7 | 24 | 72.7 | 7 | 21.3 | 2 | 6.0 |
| Group B+ | 22 | 14 | 63.6 | 8 | 36.4 | 61.3 ± 13.3 | 22.0 ± 1.6 | 15 | 68.2 | 16 | 72.7 | 5 | 22.7 | 1 | 4.6 |
Note: Comorbidities—hypertension, hyperglycemia, coronary atherosclerotic heart disease, cerebral infarction, cerebral hemorrhage, malignant tumor, chronic obstructive pulmonary disease, etc. I: Intermuscular vein; II: Intermuscular vein, peroneal vein and posterior tibial vein; III: Intermuscular vein, peroneal vein, posterior tibial vein, popliteal vein and above.
D‐dimer values were normally and similarly distributed in both groups. The concentration of D‐dimer in the group of deceased patients with DVT treated with LMWH was 36.2 ± 4.5 and 23.9 ± 3.6 mg/L, respectively, before and after treatment (P < .05). In the group of surviving patients with DVT treated with LMWH, it was 28.4 ± 5.6 and 14.7 ± 3.0 mg/L, respectively, before and after treatment (P < .05) (Table 2).
TABLE 2.
Deep venous thrombosis (DVT) in groups A and B
| Groups | Without DVT (n) | With DVT (n) |
|---|---|---|
| Group A (n = 35) | 2 | 33 * |
| Group B (n = 46) | 24 | 22 |
P < .05.
4. DISCUSSION
The main clinical manifestations of COVID‐19 are fever, fatigue, and dry cough. Pneumonia of varying degrees may appear if the condition deteriorates. A considerable number of patients suffer from respiratory distress, with some requiring admission to the ICU due to respiratory or another other organ failure. Some patients suffer from a sudden deterioration, with significant increases in D‐dimer values, and an increased risk of sudden death. Therefore, a rapid clinical alarm regarding the risk of venous thromboembolism using bedside ultrasonography is essential. In the present study, DVT was present in a notably greater number of patients who succumbed to COVID‐19 than patients who survived. Most COVID‐19 patients (98%) have fever, 9 which can lead to hemoconcentration and increased blood viscosity. Additionally, patients with severe disease end up restricted to their beds, often with multiple organ failure and blood coagulation dysfunction.
If D‐dimer levels increase and a DVT is detected, medical therapy is prescribed according to the patient's coagulation status and other organ functions. LMWH usage is routine at a dosage of 5000u subcutaneous injection every 12 hours, adjusted using dynamic assessment. We observed a higher proportion of patients who did not receive LMWH among those who deceased (Tables 3). These patients were mostly in critical condition and anticoagulants might have been contraindicated due to hepatic, renal, cardiac, or coagulation dysfunction. If DVT was detected early by bedside ultrasonography, that is, before deterioration that could contraindicate anticoagulation therapy occurred, LMWH might have reduced mortality, since the clinical manifestations and pathological abnormalities of COVID‐19 are associated with ARDS. 2 When ARDS occurs, it induces an inflammatory reaction, which causes a cascade reaction in the coagulation system and damage to the fibrinolysis system, resulting in the deposition of fibrin in the alveoli and pulmonary microcirculation vessels, with an obviously hypercoagulable status. 10 Autopsy of ARDS patients has shown that thrombi in the pulmonary arteriole can occur before pulmonary congestion, edema, hemorrhage, and hyaline membrane formation. When ARDS does occur, the alveolar‐capillary permeability increases first, then alveolar epithelial damage is caused by micro‐thrombotic embolism, 11 promoting the aggregation and adhesion of neutrophils, 12 and finally, pulmonary fibrosis develops. Therefore, the pathological setting of ARDS is the mutual promotion between a hypercoagulable status and an inflammatory reaction.
TABLE 3.
Low molecular weight heparin (LMWH) usage of groups M and N
| Groups | Receive LMWH (n) | With LMWH (n) |
|---|---|---|
| Group M (n = 33) | 18 | 15 * |
| Group N (n = 22) | 18 | 4 |
P < .05.
LMWH has the advantages of long half‐life, high bioavailability, strong anticoagulant effect, and few adverse reactions, and is safer and more effective than unfractionated heparin. On the one hand, LMWH plays an antithrombotic role through a significant anti‐Xa and anti‐IIa factors, improves coagulation disorders, reduces the damage to vascular endothelial cells caused by thrombosis, 13 , 14 promotes the synthesis of endothelial cells and release of tissue factor pathway inhibitors, and enhances fibrinolysis, while having little impact on platelet function and rarely causing bleeding. On the other hand, LMWH also blocks the NF‐κB pathway and inhibits the activation of the p38 MARK signal transduction pathway through factor X, thus blocking the release of inflammatory mediators and inhibiting neutrophil chemotaxis and phagocytosis. 15 , 16 , 17 Therefore, LMWH has both anticoagulant and anti‐inflammatory effects.
The Diagnosis and Treatment Plan of Novel Coronavirus Pneumonia (trial version 7th) issued by the National Health Commission of the People's Republic of China 7 summarizes the pathological results of autopsies of domestic patients with COVID‐9, demonstrating hyaline thrombosis in the alveolar septal vessels, and micro‐thrombosis in the hepatic portal area and renal interstitial vessels. It has also been reported that SARS‐CoV‐2 infection can induce a cytokine “storm.” 18 Therefore, anticoagulation and anti‐inflammatory therapies are essential. In our study, most patients who deceased had contraindications to anticoagulant therapy due to their critical condition. D‐dimer concentrations decreased to varying degrees under LMWH therapy in patients who received LMWH in both groups (Table 4). Early and reasonable use of LMWH appears feasible for COVID‐9 treatment.
TABLE 4.
Changes of D‐dimer before and after low molecular weight heparin (LMWH) treatment in groups A+ and B+
| Groups | Before (mg/L) | After (mg/L) | Percentual decrease (%) |
|---|---|---|---|
| The D‐dimer of patients in group A+ treated with LMWH (n = 18) | 36.21 ± 4.48 | 23.87 ± 3.55 * | 36.73 ± 8.24 |
| The D‐dimer of patients in group B+ treated with LMWH (n = 18) | 28.35 ± 5.59 | 14.68 ± 2.97 * | 43.15 ± 9.58 |
P < .05 compared with before treatment.
Usually, ICU doctors request a bedside ultrasonographic examination when observing swelling of the patients' lower limbs and/or increased D‐dimer values. Some thrombosis, especially isolated distal DVT, including thrombosis of the anterior tibial, posterior tibial, fibular, and muscular veins below the knee 19 are characterized by variable symptoms and often occult onset. 20 Bedside ultrasonography can be quite beneficial, especially for immobile patients. We suggest that the patients should undergo regular ultrasonographic examinations to detect DVT as early as possible. Moreover, early and reasonable use of LMWH is recommended.
A limitation of this study lies in its relatively short duration. Some surviving patients may worsen or even die at later time points. In addition, we did not specify whether the patients had previous comorbidities, since subgroup analysis would not have been possible due to the small sample size. Further studies, with a larger sample size and extended observation period are required.
5. CONCLUSION
The prevention and early detection of DVP are particularly important for the comprehensive management of COVID‐19 patients. Bedside ultrasonography can detect DVT early and help assessing the risk of pulmonary embolism. On this basis, prophylactic or therapeutic LMWH treatment should be administered generously.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Zhang P, Qu Y, Tu J, et al. Applicability of bedside ultrasonography for the diagnosis of deep venous thrombosis in patients with COVID‐19 and treatment with low molecular weight heparin. J Clin Ultrasound. 2020;48:522–526. 10.1002/jcu.22898
Pu Zhang and Yali Qu contributed equally to this study.
REFERENCES
- 1. Bogoch II, Watts A, Thomas‐Bachli A, et al. Potential for global spread of a novel coronavirus from China. J Travel Med. 2020;27:taaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bersten AD, Edibam C, Hunt T, et al. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian State. Am J Respir Crit Care Med. 2002;165:443. [DOI] [PubMed] [Google Scholar]
- 4. Sebag SC, Bastarache JA, Ware LB. Therapeutic modulation of coagulation and fibrinolysis in acute lung injury and the acute respiratory distress syndrome. Curr Pharm Biotechnol. 2011;12:1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhai Z, Li C, Chen Y, et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vincent JL. New therapeutic implications of anticoagulation mediator replacement in sepsis and acute respiratory distress syndrome. Crit Care Med. 2000;28:S83. [DOI] [PubMed] [Google Scholar]
- 7. National Health Commission of the PRC . Diagnosis and Treatment Plan of Novel Coronavirus Pneumonia (trial version 7th). (in Chinese). Accessed June 29, 2020.
- 8. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ware LB, Matthay MA, Parsons PE, et al. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35:1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skiba CI, Rogowski F. Adhesion molecules and their role in pathogenesis of ARDS. Przeql Lek. 1996;53:627. [PubMed] [Google Scholar]
- 12. Hofstra JJ, Vlaar AP, Knape P, et al. Pulmonary activation of coagulation and inhibition of fibrinolysis after burn injuries and inhalation trauma. Trauma. 2011;70:1389. [DOI] [PubMed] [Google Scholar]
- 13. Christensen TD, Vad H, Pedersen S, et al. Coagulation profile in patients undergoing video‐assisted thoracoscopic lobectomy: a randomized, controlled trial. PLoS One. 2017;12:e0171809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sadowski R, Gadzala‐Kopciuch R, Buszewski B. Recent developments in separation of low molecular weight heparin anticoagulants. Curr Med Chem. 2019;269:166. [DOI] [PubMed] [Google Scholar]
- 15. Li LF, Yang CT, Huang CC, et al. Low‐molecular‐weight heparin reduces hyperoxia‐augmented ventilator‐induced lung injury via serine/threonine kinase‐protein kinase B. Respir Res. 2011;12:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hagiwara S, Iwasaka H, Hidaka S, et al. Danaparoid sodium inhibits systemic inflammation and prevent endotoxin‐induced acute lung injury in rats. Crit Care. 2008;12:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morris TA. Low molecular weight heparins: background and pharmacology. Semin Respir Crit Care Med. 2000;21:537. [DOI] [PubMed] [Google Scholar]
- 18. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palareti G, Schellong S. Isolated distal deep vein thrombosis: what we know and what we are doing. J Thromb Haemost. 2012;10:11. [DOI] [PubMed] [Google Scholar]
- 20. Robert‐Ebadi H, Righini M. Management of distal deep vein thrombosis. Thromb Res. 2017;149:48. [DOI] [PubMed] [Google Scholar]


