Abstract
Aims
To systematically review the currently available evidence investigating the association between olfactory dysfunction (OD) and the novel coronavirus (COVID‐19). To analyse the prevalence of OD in patients who have tested positive on polymerase chain reaction (PCR) for COVID‐19. To perform a meta‐analysis of patients presenting with olfactory dysfunction, during the pandemic, and to investigate the positive predictive value for a COVID‐19‐positive result in this population. To assess whether olfactory dysfunction could be used as a diagnostic marker for COVID‐19 positivity and aid public health approaches in tackling the current outbreak.
Methods
We systematically searched MedLine (PubMed), Embase, Health Management Information Consortium (HMIC), Medrxiv, the Cochrane Library, the Cochrane COVID‐19 Study Register, NIHR Dissemination centre, Clinical Evidence, National Health Service Evidence and the National Institute of Clinical Excellence to identify the current published evidence which associates coronaviridae or similar RNA viruses with anosmia. The initial search identified 157 articles. A total of 145 papers were excluded following application of our exclusion criteria. The 12 remaining articles that presented evidence on the association between COVID‐19 and olfactory dysfunction were critically analysed.
Results
Olfactory dysfunction has been shown to be the strongest predictor of COVID‐19 positivity when compared to other symptoms in logistic regression analysis. In patients who had tested positive for COVID‐19, there was a prevalence of 62% of OD. In populations of patients who are currently reporting OD, there is a positive predictive value of 61% for a positive COVID‐19 result.
Conclusion
Our review has shown that there is already significant evidence which demonstrates an association between OD and the novel coronavirus—COVID‐19. It is unclear if this finding is unique to this coronavirus as individual viral phenotypes rarely present in such concentrated large numbers. We have demonstrated that OD is comparatively more predictive for COVID‐19 positivity compared to other associated symptoms. We recommend that people who develop OD during the pandemic should be self‐isolate and this guidance should be adopted internationally to prevent transmission.
Keywords: 2019 novel coronavirus disease, Anosmia, COVID‐19, olfaction disorders, SARS coronavirus, SARS virus, smell
Key points.
The majority of studies reviewed were either cross‐sectional questionnaires or case series.
Olfactory dysfunction was shown to be the strongest predictor of COVID‐19 when compared to other symptoms in regression analysis.
In patients who have tested positive for COVID‐19, there was a 62% prevalence of olfactory dysfunction.
In patients with anosmia, assessed during the current COVID‐19 outbreak, there was a positive predictive value of 61%.
The association between olfactory dysfunction should aid public health bodies in adapting their guidance to identify cases and prevent spread of COVID‐19.
1. INTRODUCTION
There was already a wealth of anecdotal evidence that suggested olfactory dysfunction(OD) was an important symptom in patients who had contracted the novel SARS‐CoV‐2 coronavirus (COVID‐19) prior to the main outbreak in the United Kingdom. Initial reports made in newspapers from Germany indicated that as many as two thirds of cases of COVID‐19 reported loss of smell whilst in South Korea, 15.3% of patients who have tested positive had perceived disturbance of smell or taste. 1 Since these initial reports, a number of studies have demonstrated a clear association between OD and COVID‐19. This is the first worldwide pandemic where reporting of symptoms, aided by social media and telecommunication systems, has been shared so widely. High‐profile public figures have reported both symptoms which have led to widespread interest in the symptoms across both the press and the public. 2
It has previously been demonstrated that the genetically similar SARS‐CoV virus can spread via a synapse‐connected route to the medullary cardiorespiratory centre. 3 Coronaviral RNA has been identified post‐mortem concentrated in the brainstem of human patients during the previous SARS‐CoV pandemic, and studies in mice have shown that previously described corona viruses can invade intracranially when administered intranasally indicating that the virus may travel via the olfactory nerves. Helms et al present a series of patients infected during the current COVID‐19 outbreak and demonstrate numerous neurological sequelae and abnormalities on cross‐sectional imaging of the brain. 4
Brann et al (in a paper made available prior to peer review) have identified non‐neuronal cell types, such as sustentacular and olfactory stem cells and horizontal basal cells are the potential target of COVID‐19 in the human olfactory epithelium via the ACE2 receptor and the spike protein protease TMPRSS2. This presents three main theories for potential loss of smell in COVID‐19: firstly, a local inflammatory response affecting sensory function, secondly damage to supporting cells and finally escalating damage to the architectural structure of the entire olfactory epithelium, due to damage to sustentacular cells and Bowman's glands. 5
Viral upper respiratory tract infection (URTI) is one of the known major identifiable causes of olfactory dysfunction (OD) due to the degeneration of olfactory epithelium. 6 Due to the widespread and insidious nature of viral URTI, there are no data relating to the incidence of post‐viral OD for specific viruses but post‐viral cases typically account for 11% of all cases of OD in the community 7 with cases presenting to specialist clinics typically representing 20% of cases. 8 This group is often represented as a higher proportion in online surveys and patient fora at around 30%. 9 , 10 Patients often present to the otolaryngologist in persistent cases, but those that resolve soon after the infective process has subsided are likely rarely reviewed or reported. 11
BMJ best practice has recently published an update on coronavirus and the range of symptoms that are associated with this. They quote the anecdotal evidence published by ENT UK 1 and the American Academy of Otolaryngology 12 regarding the link between anosmia and coronavirus. Both these international bodies have recommended self‐isolation for patients who develop these symptoms. 13 Fortunately following lobbying by ENT UK and the British Rhinological Society (BRS), OD has now been incorporated into national public health policy with Public Health England (PHE) following the WHO in recognising loss of smell and taste as a key symptom of COVID‐19 infection. 2
The aim of this systematic review and meta‐analysis is to identify the currently available evidence for the relationship between COVID‐19 and self‐reported loss of smell. This will include assessing the potential for OD as a diagnostic marker in COVID‐19, outlining the current peer‐reviewed evidence relating to this relationship and how it can be utilised going forwards in clinical practice.
We decided to focus on OD and not include loss of taste in this review. OD will lead to reduced retronasal olfaction and subsequently impact the perception of taste in these patients. Flavour perception involves input from ortho‐ and retronasal olfaction and gustation, complemented by trigeminal stimulation through touch and pain fibres. Patients typically find it difficult to isolate true gustatory sensations from retronasal olfaction without objective gustatory testing. 14 Given the difficulties in interpreting this symptom, in the absence of more detailed questions regarding taste perception, we decided to solely review OD.
2. METHODS
We systematically searched MedLine (PubMed), Embase, the Health Management Information Consortium (HMIC), Medrxiv, the Cochrane Library, the Cochrane COVID‐19 Study Register, NIHR Dissemination centre, Clinical Evidence, National Health Service Evidence and the National Institute of Clinical Excellence to identify the current published evidence which associates coronaviridae or similar RNA viruses with anosmia. The search strategy for MedLine and Embase is demonstrated in Appendix 1. The final search was undertaken in 18th April 2020. We included all years and all languages in the search.
The initial search identified 157 articles. A total of 145 articles were excluded as they did not investigate a link between the current coronavirus outbreak and OD, were conference abstracts, isolated case reports or did not have an English version available. The literature search is presented in the PRISMA flow diagram (Figure 1). One case series presented loss of smell and taste in combination, where patients were included if they had experienced either symptom. As such, we were unable to isolate olfactory dysfunction in their population. Reporting related but independent symptoms in this way prevents formal analysis of their individual epidemiological factors and impact on patient outcomes.
FIGURE 1.
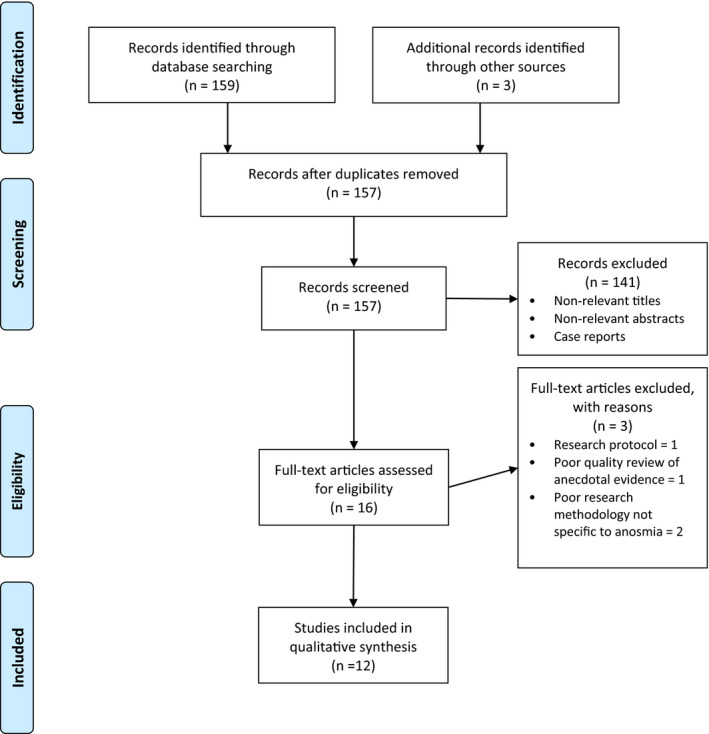
PRISMA flow diagram: olfactory dysfunction in COVID‐19
We used the ROBINS‐E (Risk of Bias in Non‐randomised Studies—of Exposures) tool to assess the studies for bias. The articles were assessed across 7 parameters: confounding factors, selection of participants, classification of exposures, departures from intended exposures, missing data, measurement of outcomes and the reported result. All the studies were assessed at “serious” risk of bias due these common themes: lack of adjustment for confounding variables, differences in follow‐up and the start of exposure, variation in reporting, and numerous different subgroups reported.
3. RESULTS
3.1. Methodology
There were 12 articles that have investigated the association between COVID‐19 and OD. The studies vary in their methodology and in the patient populations that they target. A summary of the studies evaluated, their study methodology and the quality of the evidence is presented below in Table 1. Several of the studies had not completed the peer review process, and this status is also demonstrated in the table.
TABLE 1.
Summary of papers in review
| Article Title | Primary Author | Methodology | Peer review completed |
|---|---|---|---|
| Real‐time tracking of self‐reported symptoms to predict potential COVID‐19 | C Menni | Cross‐sectional questionnaire (via symptom reporting app) | Yes |
| Olfactory and gustatory dysfunction as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease: a multi‐centre European Study | J Lechian | Cross‐sectional questionnaire | Yes |
| Self‐reported olfactory and taste disorder sin SARS‐CoV‐2 patients: a cross‐sectional study | A Giacomeli | Cross‐sectional questionnaire | Yes |
| Coincidence of COVID‐19 Epidemic and olfactory dysfunction outbreak | S Bagheri | Cross‐sectional questionnaire | Yes |
| Presentation of new onset anosmia during the COVID‐19 pandemic | C Hopkins | Cross‐sectional questionnaire | Yes |
| Association of Chemosensory Dysfunction in COVID‐19 Patients Presenting with Influenza‐like Symptoms | C Yan | Cross‐sectional questionnaire | Yes |
| COVID‐19 Anosmia Reporting Tool: Initial Findings | R Kaye | Clinician‐Reporting Tool/ Cross‐sectional questionnaire | Yes |
| Isolated sudden onset anosmia in COVID‐19 infection. A novel syndrome | S Gane | Case series | Yes |
| Neurological Manifestations of Hospitalised Patients with COVID‐19 in Wuhan, China: a retrospective case series study | L Mao | Case series | Yes |
| Sinonasal pathophysiology of SARS‐CoV‐2 and COVID‐19: a systematic review of the current evidence | I Gengler | Case series reported within SR | Systematic review published but case series not peer‐reviewed |
| The Use of Google Trends to Investigate the loss of smell related searches during the COVID‐19 outbreak | A Walker | Search term analysis | Yes |
| Smell dysfunction: A Biomarker for COVID‐19 | ST Moein | Case‐control study | Yes |
3.2. Description of studies
3.2.1. Cross‐sectional questionnaire
The most common methodology for assessment in the articles searched was a cross‐sectional questionnaire which was conducted over a variety of mediums, that is face to face, mobile application or web‐based forms. The cohort of patients targeted also varied between inpatient and outpatient populations and in their geographical location (Tables 2 and 3). Whilst there are clear limitations to this approach, it is not possible to demonstrate a direct causal relationship between COVID‐19 and OD but they are able to present associations in the symptomatology of this pandemic.
TABLE 2.
Meta‐analysis of patients with COVID‐19‐positive PCR result and prevalence of olfactory dysfunction
| Lead Author | n COVID‐19 positive | N with OD | Percentage with OD | Average age with OD | Proportion Female | Setting | Location |
|---|---|---|---|---|---|---|---|
| C Menni | 579 | 344 | 59% | 41 | 69% | Outpatient based | UK based |
| J Lechian | 417 | 357 | 86% | No data | No data | Inpatient and Outpatient | Belgium, Spain, France, Italy |
| C Yan | 59 | 40 | 68% | No data | No data | Outpatient based | USA |
| ST Moein | 60 | 58 | 97% | 47 | 33% | Inpatient | Iran |
| L Mao | 214 | 11 | 5% | No data | No data | Inpatient | China |
| Totals | 1329 | 819 | 62% prevalence of OD in COVID + ve population | ||||
Abbreviation: OD, olfactory dysfunction.
TABLE 3.
Meta‐analysis of patients with new onset olfactory dysfunction and prevalence of COVID‐19 positivity
| Lead Author | N with OD | N COVID + ve test | Percentage COVID + ve |
Average Age |
Female | Setting | Location |
|---|---|---|---|---|---|---|---|
| S Bagheri | 10 069 | No data | No data | 32.5 | 71% | Outpatient based | Iran |
| S Gane | 11 | No data | No data | 37.6 | 27% | Outpatient | UK |
| C Hopkins | 2428 | No data | No data | 30‐39 | 73% | Outpatient based | UK |
| I Gengler a | 55 | 52 | 94% | No data | No data | No data | France |
| C Yan | 73 | 40 | 55% | No data | No data | Outpatient based | USA |
| C Menni | 557 | 345 | 62% | No data | No data | Outpatient based | UK |
| Bold values where patients with olfactory dysfunction were PCR tested for COVID‐19 and included in meta‐analysis below (Yan et al, Menni et al): | |||||||
| Total | 630 | 385 | 61% PPV for COVID + ve test in OD | ||||
Abbreviation: OD, olfactory dysfunction.
Awaiting peer review.
3.2.2. Case series
The majority of the remaining studies present case series. They present similar cross‐sectional evidence to the questionnaire designed studies with a common aim of investigating the symptomatology of anosmia in the COVID‐19 era. This approach should contribute to limiting the level of bias in their results when compared to an outcome‐based case series, but they are similarly only able to demonstrate association and not causal effect. The complementary data output, between the questionnaire and case‐series approaches, allows us to compare the two approaches concurrently in a meta‐analysis.
3.2.3. Search term analysis
Walker et al were unique in their approach and used Google Trends to track search terms related to loss of smell. They demonstrate statistically significant association of the Google search terms and the incidence of COVID‐19 cases and deaths. The previous figures for the same time period in 2019 and the H1N1 pandemic were used as controls. This correlation was present across numerous countries including Italy, Spain, the United Kingdom (UK), the United States of America (USA), Germany, France, Iran and the Netherlands. They propose that this technique could be used to track disease hot spots internationally where targeted control measures could then be implemented. For this to be effective, there need to be clear data on the positive predictive value of new onset anosmia and COVID‐19 positivity. 15
3.2.4. Case‐control study
In the only study so far to institute validated quantitative olfactory testing, Moein et al, in Iran, evaluated 60 patients who had tested positive for COVID‐19. Their control group were selected from a group of 141 controls from a previously conducted study. They handpicked age‐ and sex‐matched individuals from this cohort in an attempt to mirror their COVID‐19‐positive group. COVID‐19 patients completed the Persian version of the 40‐odorant University of Pennsylvania Smell Identification Test (UPSIT) assisted by a trained examiner, they do not explain how they administered the test in their previously investigated control group. Ninety‐eight per cent of their COVID‐19 group had some level of OD with 25% of these subjects being completely anosmic. There was a statistically significant reduction in scores, in all 40 stimuli, within the COVID‐19 group. There were no differences in demographics between the two groups, but the way the control group was matched will have affected this data. 16
3.3. Risk of bias and limitations
When analysing data related to COVID‐19 positivity, it is important to recognise the sensitivity of the test is variable. Bronchoalveolar lavage is the most sensitive test (93%) whilst nasal swabs (63%) and pharyngeal swabs (46%) have lower positive rates. 14 Moein et al, who conducted the UPSIT, case‐control study, and Mao et al were the only authors to report the technique and anatomical location of their polymerase chain reaction (PCR) analysis of COVID‐19 status. Moien et al used nasal aspirates or washes, and Mao et al's group used throat swabs. 16 , 17 Due to the relatively low sensitivity of the test used, there will be a proportion of false negatives that will falsely lower the incidence of COVID‐19 positivity in the OD groups and will therefore also impact on the calculation of sensitivity and specificity.
The majority of the responses to questionnaires were received remotely using electronic response forms of mobile‐based applications which will cause selection bias. Younger more technologically interactive cohort are more likely to interact, and this subgroup seems to be less affected by COVID‐19 when compared to older age groups who have a higher morbidity and mortality. 18 For example, Menni et al, who used a mobile‐based application, report an average age of 41.48 (CI = 13.77) for those in their non‐PCR‐tested group, including over 1.5 million people. 19 Hospitalised populations are also less likely to interact with these methods due to their disease severity, Internet connection or associated interventional treatments.
Cross‐sectional questionnaires and case series are prone to bias due to influence of confounding variables, assessment of patients at different time points relative to their exposure and reporting bias. In case series, specifically consecutive patients often missed in data collection. In these studies, however the researchers are simply presenting patient factors and associated symptoms rather than treatments or interventions and their subsequent effects or outcomes and this observational nature could help to reduce observer bias. In studies that were conducted requiring historical data from the patients, there is a risk of recall bias and under‐reporting or inaccuracies of symptoms specifically where onset and duration of symptoms is involved.
3.4. Comparing COVID‐positive and olfactory dysfunction populations
Two distinct populations have been assessed in the literature. The first group were those patients who had received testing and were confirmed positive COVID‐19 patients; the prevalence of OD was then analysed. The second were people who had experienced OD and the prevalence of COVID‐19 within this cohort. Menni et al and Yan et al report data from both groups concurrently and presented data for both populations in their results. 19 , 20
3.4.1. Prevalence of olfactory dysfunction in COVID‐19‐positive patients
Menni et al used the “COVID RADAR” symptom tracker app to extract a cohort of patients who had tested positive for COVID‐19 and their associated symptomatology. Nearly 2.5 million people reported symptoms on this app, but only 15 638 were tested and 6452 tested positive. This small proportion of their total population, and limited case definition used for access to testing at the time of the study, and risk of false negatives were the main limitations of this study. They then analysed the COVID‐19‐positive and negative groups for prevalence of symptoms. In the COVID‐19‐positive group, 64.76% had experienced loss of smell compared to 22.68% in the negative group. For patients reporting loss of smell, they report an odds ratio of 6.40 for a positive COVID‐19 when compared to a negative result, after adjusting for age, sex and body mass index. In their model, loss of smell and taste was the strongest predictor of a COVID‐19‐positive result. 19
Lechian et al's multi‐centre study analysed patient and volunteer healthcare professionals who had a PCR‐positive result for COVID‐19 with a questionnaire. Patients in the intensive care unit, patients with previous OD and those without a COVID‐19 PCR result were excluded from analysis. The impact of OD was evaluated using a quality of life tool (sQOD‐NS). 85.6% of their cohort of 417 patients reported OD. The majority self‐rated as anosmic (79.6%), but others experienced hyposmia, phantosmia and parosmia. Anosmic patients were found to have a significantly lower sQOD‐NS score compared with the hyposmic and normosmic individuals. This OD was not significantly associated with rhinorrhoea or nasal obstruction, but a significant association was found with females being proportionally more affected than males. In the subgroup of patients who had clinically resolved infection, the OD persisted in 63% of cases. 21
Yan et al sent an email invitation to complete a survey to 1480 patients who had undergone COVID‐19 testing. They had a 58% response from COVID‐19‐positive patients and a 15% response from the negative group. Their survey evaluated patient‐reported symptoms with a focus on smell and taste. Sixty‐eight per cent of the COVID‐19‐positive group reported OD, and similarly to Menni et al, they found that loss of sense of smell (and taste) showed the largest magnitudes of association to COVID‐19 positivity when compared with other symptoms. Seventy‐two per cent of the COVID‐19‐positive patients with OD reported improvement at the time of the survey. 20
Mao et al were one of the first groups to present the symptomatology of patients presenting with a positive COVID‐19 swab result. 5.1% of this group of 214 patients had experienced hyposmia. This was a retrospective analysis of electronic patient data, and as such, there is a risk that OD was not a symptom explored or documented in individual consultations within this cohort of patients. According to “diagnostic criteria” that are not described, they divided their patients into severe and non‐severe groups. Of the 11 patients who had reported hyposmia, 3 were non‐severe and 8 were severe. 17
Giacomelli et al interviewed 59 of 88 inpatients with COVID‐19 demonstrated on PCR, there were 29 non‐respondents due to receiving ventilation, dementia and linguistic barriers. They report combined rates of smell or taste disturbance, and as such, comparative incidence rates solely for OD were not possible to produce from the data presented. In their cohort, the olfactory and gustatory disorders occurred in proportionally younger and more commonly female subjects and no patients had recovered at the time of interview. No data relating to time of interview following onset of symptoms are reported. 22
In the studies that investigated olfactory symptoms independently, we present the prevalence rates in Table 2 to form a meta‐analysis for the prevalence of OD in the population of patients that have tested positive.
3.4.2. Prevalence of COVID‐19 in new onset olfactory dysfunction cohorts
Due to the differences in public health approaches and the availability of testing, it is difficult to demonstrate clear associations between new onset OD and COVID‐19 positivity. There were however three studies that did have PCR results for patients presenting with OD since the start of the COVID‐19 pandemic. In Table 2, we demonstrate that there is a high prevalence of COVID‐19 positivity in patients currently presenting with OD.
The American Academy of Otolaryngology‐Head and Neck Surgery (AAO‐HNS) developed a COVID‐19 Anosmia reporting tool for clinicians. Responses were collected from clinicians around the world relating to the association of COVID‐19 and anosmia. They do not clearly state if all submitted patients had PCR testing performed. A total of 237 entries were analysed; anosmia was the initial symptom in more than 25% of cases, 27% had noticed some improvement and in 40% was the symptom that led to a test being performed. 23
Bagheri et al conducted a widely completed online survey, of the general population in Iran, to identify patients with OD since the inception of the outbreak in their country. They demonstrated high numbers of people who had experienced OD in their cohort. Their respondents were commonly female (71%) and experienced sudden onset in their OD (76%). Only 1.1% were admitted to hospital for treatment indicating a largely mild disease when OD was experienced. 24 In a similar online questionnaire study, conducted in the UK, by Hopkins et al the demographic features were replicated. The majority of this British population with OD reported complete loss of smell (74.4%), and in 16% of cases, it was their only symptom. A proportion of these patients did report receiving a PCR test with a 74% positive rate in this subgroup. 25
Gane et al present a case series of 11 patients presenting with sudden onset anosmia during the epidemic in the United Kingdom. In 5 of these patients, it was an isolated symptom and just one of these patients was self‐isolating. 26 Gengler et al present findings (in an unpublished paper made available before peer review) from a French case series, not currently published, which demonstrated a positive COVID‐19 nasal PCR swab in 94% of their 55‐patient series. 27
Table 3 demonstrates the average ages and gender proportions of the six studies with OD cohorts.
4. DISCUSSION
Our review has shown that there is already significant evidence which demonstrates an association between OD and the novel coronavirus—COVID‐19. It is unclear if this finding is unique to this coronavirus as individual viral phenotypes rarely present in such concentrated large numbers. Classically, patients present with persistent symptoms following a viral illness many weeks or months after. The symptomatology during the infective phase of the virus has not previously been studied, and therefore, it is not possible to draw direct comparison between other similar viruses. Walker et al have however demonstrated trends between increasing cases of COVID‐19 and the increase in positive novel coronavirus cases that was not mirrored during the previous H1N1 pandemic in 2009. 15
Due to the rapid spread of COVID‐19 in this pandemic, it is understandable that there is a lack of studies using objective measures and rigorous controls. The most common methodologies used were cross‐sectional questionnaires and case series. These approaches are at risk of bias, and we can only discuss associations as a result. Further research will be required to demonstrate clearer links between OD and COVID‐19 going forwards.
When we assessed patients who had experienced OD during the outbreak, there were several studies that demonstrated an increase in the prevalence of loss of smell in their populations when compared to previous estimates. 19 , 24 , 25 The largest data sets, conducted predominantly in the outpatient setting, by Hopkins and Bagheri et al indicated a female preponderance in their cohorts (73% and 71% respectively). These two studies also demonstrated an average affected age between 30 and 40. It has been demonstrated that both advanced age and the male sex are risk factors for the severe form of the disease and an increased rate of mortality. 18 It could be that this cohort of patients was not targeted by this study due to the more elderly populations not interacting with web‐based surveys or being within the inpatient population due to their disease severity. Moein et al demonstrated in their study of inpatients that OD was a common finding in this population too when they applied objective UPSIT testing to confirmed cases. 16 Further research is needed to identify whether the incidence of OD varies between different ages and genders and as such whether particular disease phenotypes for COVID‐19 can give clinicians prognostic information.
In areas where testing has not been adopted widely, tracking of this OD could be vital in identifying hot spots where population‐based management strategies can then be targeted. Tracking OD using mobile‐based applications, such as the one developed by Menni et al, will allow real‐time data tracking for aid models in the prediction of national or regional COVID‐19 cases. 19 This approach could lead to specific social distancing measures being implemented in areas where OD is widespread and will also help in modelling when these measures could be relaxed as most patients seem to recover their sense of smell following the illness.
5. CONCLUSION
Our meta‐analysis has demonstrated that the prevalence of OD in patients who have a positive PCR test for COVID‐19 is 62%. OD was demonstrated to be the most strongly associated symptom, for a positive test, when compared to fever, cough, fatigue, dyspnoea and diarrhoea. 19 , 20 In people who reported OD and had received PCR swab, there was a positive predictive value of 61% for a positive result. The evidence to support an association between OD and COVID‐19 continues to grow. The symptom has now been recognised by the World Health Organization and Public Health England. 28 This change in approach should mean an increase in the number of positive COVID‐19 cases self‐isolating and a subsequent reduction in the chance of spread with benefits for public health and containment of the pandemic.
CONFLICTS OF INTEREST
The authors declare that they have no relevant or material financial interests that relate to the research described in this paper.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Appendix 1. Search strategy—Loss of smell in COVID‐19
| 1 | EMBASE | exp ADULT/ | View results (8 180 639) |
| 2 | EMBASE | exp CHILD/ | View results (2 571 562) |
| 3 | EMBASE | (1 OR 2) | View results (10 062 017) |
| 4 | EMBASE | exp CORONAVIRIDAE/ OR CORONAVIRUS/ OR "CORONAVIRUS INFECTION"/ | View results (61 685) |
| OR "CORONAVIRUS INFECTIONS"/ OR VIRUS/ OR "(COVID‐19).ti,ab" OR "(COVID19).ti,ab" | |||
| 5 | EMBASE | "SARS‐RELATED CORONAVIRUS"/ OR "RNA VIRUS"/ | View results (9106) |
| 6 | EMBASE | (4 OR 5) | View results (69 724) |
| 7 | EMBASE | "SMELLING DISORDER"/ OR exp ANOSMIA/ OR exp HYPOSMIA/ | View results (9313) |
| 8 | EMBASE | exp "SMELLING DISORDER"/ OR "NEUROLOGIC DISEASE"/ | View results (136 148) |
| 9 | EMBASE | ANOSMIA/ OR DYSOSMIA/ OR HYPOSMIA/ OR "OLFACTORY HALLUCINATION"/ | View results (14 313) |
| OR PAROSMIA/ OR "TASTE ABNORMALITY"/ OR "TASTE ANOMALY"/ OR "TASTE ABSENCE"/ | |||
| 10 | EMBASE | (7 OR 8 OR 9) | View results (143 896) |
| 11 | EMBASE | (3 AND 6 AND 10) | View results (141) |
| 12 | Medline | exp ADULT/ | View results (7 081 727) |
| 13 | Medline | exp CHILD/ | View results (1 882 783) |
| 14 | Medline | (12 OR 13) | View results (8 276 487) |
| 15 | Medline | CORONAVIRUS/ OR exp CORONAVIRIDAE/ OR "RNA VIRUSES"/ OR "(COVID19).ti,ab" OR "(COVID‐19).ti,ab" | View results (20 713) |
| 16 | Medline | ALPHACORONAVIRUS/ OR BETACORONAVIRUS/ OR GAMMACORONAVIRUS/ | View results (555) |
| 17 | Medline | (15 OR 16) | View results (20 713) |
Rocke J, Hopkins C, Philpott C, Kumar N. Is loss of sense of smell a diagnostic marker in COVID‐19: A systematic review and meta‐analysis. Clin Otolaryngol. 2020;45:914–922. 10.1111/coa.13620
REFERENCES
- 1. Hopkins C, Kumar N.Loss of sense of smell as a marker of COVID‐19 infection. https://www.entuk.org/. Accessed July 3, 2020.
- 2. Barr S.Why are loss of smell and taste reportedly symptoms of COVID‐19? The Independent. https://www.independent.co.uk/life‐style/. Accessed July 3, 2020.
- 3. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92(6):552‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med. 2020;382(23):2268‐2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brann D, Tskukahara T, Weinreb C, et al.Non‐neuronal expression of SARS‐CoV‐2 entry genes in the olfactory system suggests mechanisms underlying COVID‐19 associated anosmia. 2020. *Pre‐print, awaiting peer‐review PPR130125. 10.1101/2020.03.25.009084 [DOI] [PMC free article] [PubMed]
- 6. Choi R, Goldstein BJ. Olfactory epithelium: cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig Otolaryngol. 2018;3(1):35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Damm M, Temmel A, Welge‐Lussen A, et al. Olfactory dysfunctions. Epidemiology and therapy in Germany, Austria and Switzerland. HNO. 2004;52(2):112‐120. [DOI] [PubMed] [Google Scholar]
- 8. Philpott C. Smell and taste disorders in the UK: First experiences with a specialised smell and taste outpatient clinic. Roy Coll Surg Eng Bull. 2015;96:156‐159. [Google Scholar]
- 9. Keller A, Malaspina D. Hidden consequences of olfactory dysfunction: a patient report series. BMC Ear Nose Throat Disord. 2013;13(1):8‐6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Langstaff L, Pradhan N, Clark A, et al. Validation of the olfactory disorders questionnaire for English‐speaking patients with olfactory disorders. Clin Otolaryngol. 2019;44(5):715‐728. [DOI] [PubMed] [Google Scholar]
- 11. Miwa T, Ikeda K, Ishibashi T, et al. Clinical practice guidelines for the management of olfactory dysfunction – secondary publication. Auris Nasus Larynx. 2019;46(5):653‐662. [DOI] [PubMed] [Google Scholar]
- 12. Anosmia, Hyposmia, and Dysguesia Symptoms of Coronavirus Disease. AAO‐HNS 2020. https://www.entnet.org/. Accessed July 3, 2020.
- 13. Aksoy F, Yenigun A, Dogan R, Yilmaz F, Ozturan O, Yenigun VB. Investigation of the role of major respiratory viruses in the aetiology of nasal polyps using polymerase chain reaction technique. J LaryngolOtol. 2014;15:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bojanowski V, Hummel T. Retronasal perception of odors. Physiol Behav. 2012;107(4):484‐487. [DOI] [PubMed] [Google Scholar]
- 15. Walker A, Hopkins C, Surda P. The use of google trends to investigate the loss of smell related searches during COVID‐19 outbreak. Int Forum Allergy Rhinol. 2020;10(7):839‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moein ST, Hashemian SMR, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol. 2020;10(8):944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caramelo F, Ferreira N, Oliveiros B.Estimation of risk factors for COVID‐19 mortality – preliminary results. 2020.*Pre‐print, awaiting peer‐review PPR114369. 10.1101/2020.02.24.20027268 [DOI]
- 19. Menni C, Valdes AM, Freidin MB, et al. Real‐time tracking of self‐reported symptoms to predict potential COVID‐19. Nat Med. 2020;26(7):1037‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and Covid‐19 in patients presenting with influenza‐like symptoms. Int Forum Allergy Rhinol. 2020;10(7):806‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;06(227):2251‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giacomelli A, Pezzati L, Conti F, et al. Self‐reported olfactory and taste disorders in SARS‐CoV‐2 patients: a cross‐sectional study. Clin Infect Dis. 2020;71(15):889–890. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaye R, Chang D, Kazahaya K, Brereton J, Denneny J. COVID‐19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020;163(1):132‐134. [DOI] [PubMed] [Google Scholar]
- 24. Bagheri SH, Asghari A, Farhadi M, et al. Coincidence of COVID‐19 epidemic and olfactory dysfunction outbreak. Med J Islamic Rep Iran. 2020;34(1):446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID‐19 pandemic. Rhinology J. 2020;58(3):295‐298. [DOI] [PubMed] [Google Scholar]
- 26. Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID‐19 infection. A novel syndrome? Rhinol J. 2020;58(3):299‐301. [DOI] [PubMed] [Google Scholar]
- 27. Gengler I, Want JC, Speth MM, Sedaghat AR. Sinonasal pathophysiology of SARS CoV‐2 and COVID‐19: a systematic review of the current evidence. Laryng Investig Otolaryngol. 2020;5(3):354‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Atherton F, McBride M, Smith G, Whitty C.Statement from the UK Cheif Medical Officers on an update to coronavirus symptoms: 18 May 2020. https://www.gov.uk. Accessed July 3, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


