Abstract
The COVID‐19 disease caused by the SARS‐CoV‐2 has emerged as a worldwide pandemic and caused huge damage to the lives and economy of more than hundred countries. As on May 10, 2020, more than 4,153,300 people stand infected from the virus due to an unprecedented rate of transmission and 282,700 have lost their lives because of the disease. In this context, medicinal plants may provide a way to treat the disease by targeting specific essential proteins of the virus. We screened about 51 medicinal plants and found that Tea (Camellia sinensis) and Haritaki (Terminalia chebula) has potential against SARS‐COV‐2 3CLpro, with an IC50 for Green Tea as 8.9 ± 0.5 μg/ml and Haritaki 8.8 ± 0.5 μg/ml. The in‐silico studies suggested that Tea component Thearubigins binds to the cysteine 145 of protease active site and could be a pharmacoactive molecule. We predict that the inhibition in protease activity may be able to halt the SARS‐CoV‐2 replication cycle and therefore, we propose Green Tea, Black Tea, and Haritaki plant extracts as potential therapeutic candidates for SARS‐CoV‐2 infection. Further investigation on role of bioactive constituents of extracts is needed to establish the molecular basis of inhibition and towards expedited drug discovery.
Keywords: 3CLpro , Haritaki, medicinal plants, SARS‐CoV‐2, tea
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) originated from Wuhan, China, has recently emerged as a global pandemic and a serious blow to human life and economy. The etiological agent of the disease is a positive‐sense single‐stranded RNA virus that belongs to the Coronaviridae family. Phylogenetically, the virus relates to the SARS‐CoV that emerged in 2002 and hence been named as the SARS‐CoV‐2 (Gorbalenya et al., 2020). The COVID‐19 is characterized by the serious illness such as pneumonia and even respiratory failure in extreme cases (Chan et al., 2020) among other multiple effects which are being deciphered with each passing day. The disease transmission occurs via droplet means and upon infection it displays higher fatality rate in elderly and immunocompromised individuals. These group of sufferers have more chances of disease progression (Liu, Chen, Lin, & Han, 2020). The disease leaves lesions on lungs and has effects liver as well. These pathological manifestations effects the post‐infection physiology of internal organs (Xu, Liu, Lu, Yang, & Zheng, 2020).
The virus comprises 26 different proteins, which include 10 major structural proteins, namely the envelope (E), membrane (M), nucleocapsid (N), and the trimeric spike (S) proteins that are vital to the completion of its replication cycle. Further, the viral genome encodes two proteases‐ the papain‐like protease (PLpro); and the 3‐chymotrypsin‐like protease (3CLpro) also known as the main protease (Mpro) of the virus (Anand, Ziebuhr, Wadhwani, Mesters, & Hilgenfeld, 2003). Both of the proteases are essential for the processing of polyproteins PP1A and PP1B, translated from the RNA of the virus (Hilgenfeld, 2014) and the 3CLpro enzyme has emerged as an interesting premise for the development of drugs targeting the virus. The 3CLpro is very important for virus to replicate and propagate and its inhibitors may halt the disease at an early stage of replication. It recognises and cleaves the virus non‐structural polyprotein at 12 sites. One of the site on the polyprotein includes Leu‐Gln*(Ser, Ala, Gly) (* denotes the cleavage site). To impede virus replication, multiple strategies are being employed. Medicinal plants could be harnessed as one of the safest means of medication and have been used traditionally for various manifestations. The anti‐viral activities of several plants have been elucidated so far (Newman & Cragg, 2007). The role of plant lectins as anti‐SARS‐CoV‐2 has been proposed (Capell et al., 2020). We have shortlisted the medicinal plants which are reported to possess the anti‐viral, anti‐oxidant, and anti‐inflammatory properties. Further, the aqueous extracts were screened for 3CLpro inhibition, in‐vitro. We found that tea (Camellia sinensis) and Haritaki (Terminalia chebula) as potential inhibitors of 3CLpro activity of SARS‐CoV‐2. Additonally, we compared the activity inhibition by different types of Teas and found that Green Tea and Black Tea displayed comparable potencies to inhibit the 3CLpro activity of SARS‐CoV‐2.
The pharmacoactive molecules like catechins, present in Green Tea, have been shown to possess multiple health‐boosting activities like hypoglycemic, hypotensive, anti‐tumor, anti‐oxidative, hypolipidemic, anti‐bacterial, and anti‐viral effects (Cabrera, Artacho, & Giménez, 2006; Cooper, Morré, & Morré, 2005a, 2005b; Mahmood et al., 2016). The (−)‐Epigallocatechin gallate (EGCG) present in Green Tea has a virucidal effect on the Zika virus (Carneiro, Batista, Braga, Nogueira, & Rahal, 2016). Similarly, the Prodelphinidin B‐2 3′‐O‐Gallate molecule has shown an anti‐viral effect against the Herpes Simplex Virus type 2 (HSV‐2; Cheng, Lin, & Lin, 2002). Further, the Green Tea extract has also been demonstrated as a safe personal hygiene option against viral infections like human influenza A/H1N1 virus (Lee, Jang, Kim, Kim, & Seong, 2018). Interestingly, Theaflavin‐3, 3′‐digallate (TF3) which is produced from polymerization and oxidization of the epicatechin gallate and EGCG during fermentation of fresh Tea leaves, displayed 3CLpro inhibition (Chen et al., 2005). Similarly, Haritaki is also believed to possess anti‐bacterial and anti‐viral activities (Lee et al., 2011). Furthermore, it has been reported beneficial against viruses like HSV‐2 (Kesharwani et al., 2017) and Influenza A virus (Li et al., 2020). Epigallocathechin‐3‐gallate present in the C. sinensis inhibited the replication of hepatitis‐B virus (Karamese, Aydogdu, Karamese, Altoparlak, & Gundogdu, 2015). We assessed the proteolytic activity of the 3CLpro protein and found that the extracts of Tea (Green Tea and Black Tea) and Haritaki displayed a significant reduction in proteolytic activity.
2. MATERIALS AND METHODS
2.1. Cloning and expression of main protease 3CLpro
The 3CLpro gene (Wuhan‐Hu‐1, NC_045512.2, region 10,055–10,972) which is 918 base pair synthesized in pEXA2 vector was procured from Eurofins Genomics, Japan and was used for sub cloning in protein expression vector since it has been designed with BamHI and XhoI restriction enzyme sites. The digested desired gene insert was purified and ligated to a modified pET‐28a vector between BamHI and XhoI sites. This modified vector contains the sequences coding for PreScission, flag tag, and hexahistidine. The cloned gene in the pET‐28a vector was subjected to protein expression. After analyzing the soluble expressions from various expressing strains of Escherichia coli, we chose BL‐21DE3 Rosetta RIL cells for expressing the protein. The 3CLpro protein tagged with 6‐histidines at N‐terminal end was expressed using an auto‐induction method of protein production with appropriate antibiotics kanamycin (50 μg/ml) and chloramphenicol (34 μg/ml; Studier, 2005). The cells were first cultured for 2 hr at +37°C, further grown at +18°C for 18 hr. The cells were harvested by centrifugation, and the pellet was re‐suspended in buffer A (50 mM Tris pH 7.5, 500 mM NaCl, 10% glycerol, 10 mM Imidazole) containing protease inhibitors 1 mM DTT, 0.5 mM PMSF and DNAseI 0.001 mg/ml with 5 mM MgCl2. The cells were lysed by adding lysozyme having final concentration 1 mg/ml and disrupted by sonication on ice with 30 s ON and 30 s OFF for 10 min, and the lysate was clarified by centrifugation at 10,000g for 30 min. The clear lysate was applied onto a Ni‐NTA column pre‐equilibrated with buffer A on ÄKTA™ start FPLC system (GE Healthcare). The column was washed with 100 mL of Buffer A, and 3CLpro protein was eluted with gradient elution 0–500 mM imidazole in buffer A. The fractions were analyzed on SDS‐PAGE for purity. The eluted fractions were pooled, dialyzed, and purified through QFF anion exchange chromatography with a linear gradient of NaCl (0–500 mM). The purified protein was homogeneous with about >95% purity and confirmed with western blot analysis. Fractions containing 3CLpro protein were pooled and cleaved with recombinant Precision protease at +4°C overnight. The overnight cleaved protein was reapplied on the Ni‐NTA column and the unbound sample has been collected. It was concentrated, buffer exchanged (20 mM Tris HCl pH 7.5, 150 mM NaCl, 10% Glycerol, and 1 mM DTT), flash frozen and stored at −80°C for all biochemical and biophysical studies.
2.2. Screening and preparation of plant extracts
The extensive review of scientific and Ayurvedic literature provided the basis of selection of plants having anti‐oxidant, anti‐inflammatory, anti‐viral, and other fortifying characteristics. All plants used in the study were supplied by an Ayurvedic physician at Morarji Desai National Institute of Yoga, New Delhi, India. Two grams of crude plants were powdered and dissolved in 10 mL de‐ionized water. The extraction of pharmacoactive compounds was done by keeping the aqueous extracts at 50°C for 12 hr. The resultant suspension was centrifuged at 4,000g for 10 min, the supernatant was collected and kept for drying in petridish at 42°C for 24 hr. The dried plant extracts powder was weighed and re‐dissolved at a concentration of 100 mg/ml in de‐ionized water and 10 μL aliquots (1 mg per tube) was stored in −20°C (Raghavendhar, Tripati, Ray, & Patel, 2019). In each experiments, the dilutions were freshly made as desired concentration of 1 mg/ml and used only once.
2.3. Establishment of protease activity using purified 3CLpro and casein substrate
The protease activity of the purified 3CLpro was tested using a modified protocol from previously reported protocol using casein as a substrate (Banik, Biswas, & Karmakar, 2018; Cupp‐Enyard, 2008). The 3CLpro enzyme is non‐specific as it cleaves at 12 different locations in SARS‐CoV‐2. Casein substrate contains peptide sequences that can be cleaved by 3CLpro of SARS‐CoV‐2. Hence, we have used the casein substrate for activity measurements. The substrate stock of 0.65% (274 μM) was prepared in phosphate buffer pH 7.4 and the enzyme (3CLpro) was taken in varying concentrations. The 3CLpro enzyme (10 μM) and varying casein substrate (0–257 μM) were mixed to a final volume of 400 μl and incubated at 37°C for an hour. After the incubation period reaction was stopped by adding 200 μl of 110 mM trichloroacetic acid (TCA) for 10 min. The final reaction mixture was then centrifuged for 10 min at 15,000g and absorbance at OD280 was measured using a spectrometer (Beckman Coulter DU 800 spectrophotometer) and values of reactions were subtracted with that of the buffer. The absorbance at 280 nm is the measurement of l‐tyrosine produced by the protease activity. The standard plot of l‐tyrosine was plotted for the velocity calculations. The maximum velocity of the enzymatic reaction was calculated using substrate concentration versus velocity plot and fitted with Michaelis–Menten equations.
2.4. Evaluation of protease inhibition by plant extracts
The plants aqueous extracts were evaluated for their potential to inhibit the enzyme activity. The 10 μM of 3CLpro enzyme and 137 μM of casein substrate were used to study the inhibition effects of extracts. Different concentrations (0–450 μg/ml) of extracts were screened to assess their inhibitory potential. Two sets of reactions were prepared, one for reaction with extracts having 3CLpro and another without 3CLpro, as control. The rate of inhibition and potential efficacy was calculated by standard kinetics curve.
2.5. Biomolecular interaction studies using an intrinsic fluorescence assay
Fluorescence quenching was performed to analyze the effect of plants extracts on the 3CLpro structural dynamics. The tertiary structure perturbations post‐interaction to ligands present in the extracts were assessed using intrinsic fluorescence of 3CLpro. Ten μM of 3CLpro protein was incubated with or without extracts at different concentrations (0–80 μg/ml), fluorescence intensity was monitored on Cary Eclipse fluorescence spectrophotometer (Agilent technologies). The parameters used for excitation wavelength is 295 nm and emission scan between 310 and 400 nm with the slit width for excitation (5 nm) and emission (10 nm). The experiments were performed at controlled temperature 25°C. The change in intensity or wavelength was used for the protein ligand interaction analysis.
2.6. Comparative protease inhibition study of Green Tea, Black Tea, and Haritaki
To decipher the difference in efficacies to inhibit the viral protease by universally used Tea, we compared the enzymatic inhibition for Green Tea as wells as Black Tea mentioned above using the 10 μM of 3CLpro at varying concentrations of Tea. Moreover, we have analyzed the effective protease inhibition by both tea with Haritaki. All the parameters like substrate, enzyme, and inhibitor concentrations were kept constant during the reactions.
2.7. Docking studies of abundantly present active molecules
The crystal structure PDB 6LU7 of the 3CLpro was taken from the RCSB databank. The active site prediction was performed using the metaPocket2.0 server and the best hit near to catalytic site having His41 and Cys145 was fixed. This was used for site‐specific docking using ParDock software. The 3‐dimensional structures of active compounds present in the medicinal plants were taken from the PubChem database. The docking results were analyzed based on binding energy and binding poses.
3. RESULTS
3.1. Cloning and expression of 3CLpro
The gene encoding 3CLpro was cloned in pET‐28a vector having 6x His, FLAG (DYKDDDDK) sequence and PreScission cleavage (LEVLFQGP) sequence. The cloning was successfully done, the double digested product of the expected size of nearly 5.4 kb and 918 bp for vector and insert, respectively (Figure 1A).
FIGURE 1.
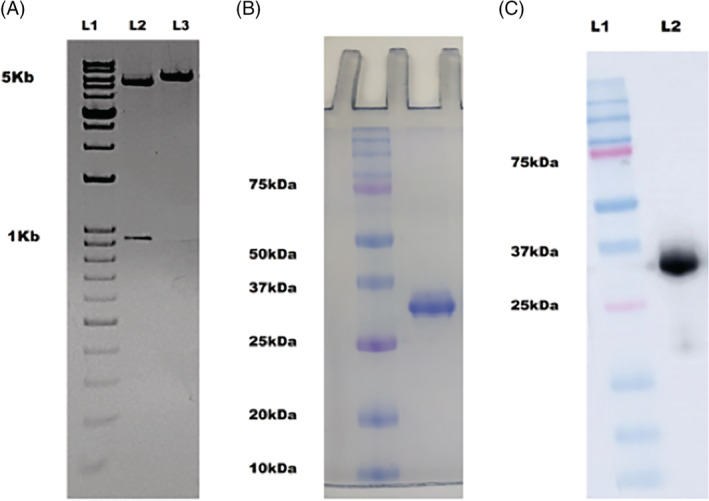
Cloning and purification of 3CLpro protein. (A) 1% agarose gel showing the bands of the double digested plasmid with vector 5.4 kb and insert around 918 bases (L2), the cloned plasmid showing band at 6.4 kb (L3). (B) SDS‐PAGE on 12% acrylamide gel, depicting homogenous protein at ~34 kDa (C) The western blot of purified 3CLpro using anti‐His antibody with mol wt ~34 kDa (L2) [Colour figure can be viewed at wileyonlinelibrary.com]
The 3CLpro was expressed in E. coli expression system and purified to homogeneity with affinity and anion exchange chromatography (Figure 1B). The confirmation of the protein was done using western blot analysis using anti‐His antibody (Figure 1C).
4. ESTIMATION OF ENZYME PARAMETERS BY CASEIN‐BASED PROTEASE ASSAY
The activity assay to validate the enzymatic property of recombinantly purified 3CLpro was established by using the casein substrate. The calculation of K m for casein was performed by the varying casein substrate and it was obtained as 137 μM (Figure 2).
FIGURE 2.
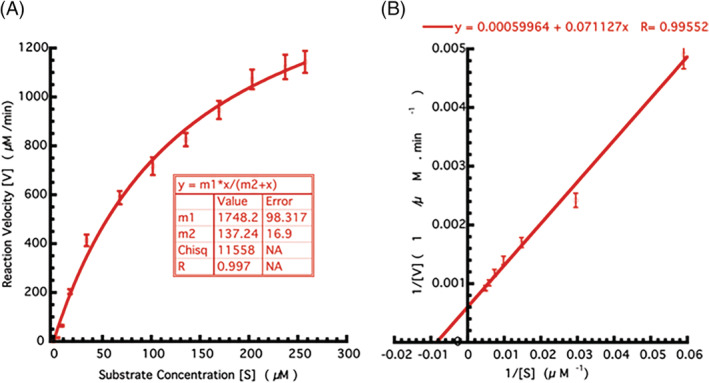
Enzyme kinetic studies of 3CLpro. (A) Evaluation of kinetic parameters in Michaelis–Menten fit. The varying concentration of the casein substrate (x‐axis) plotted against the velocity (y‐axis). (B) Inverse substrate versus inverse velocity plot (Lineweaver–Burk plot) depicting the casein substrate cleavage by the action of 3CLpro. All the experiments were performed in triplicates [Colour figure can be viewed at wileyonlinelibrary.com]
4.1. Inhibition of 3CLpro enzyme by plant extracts
The plant extracts were screened for protease activity and inhibition potential is shown in Table 1. Results shown a significant reduction in protease activity of 3CLpro by Green Tea (C. sinensis) and Haritaki (T. chebula).
TABLE 1.
List of medicinal plants screened for SARS‐CoV‐2 main protease 3CLpro inhibition
| S.no | Plants common name | Plants scientific name | Part used | % activity inhibition at 100 μg/ml of aqueous extract |
|---|---|---|---|---|
| 1 | Aloe vera | Aloe barbadensis miller | Leaf | 0 |
| 2 | Amala | Phyllanthus emblica | Fruit | 10% |
| 3 | Anise | Pimpinella anisum | Fruit | 0 |
| 4 | Antmul | Tylophora indica | Leaf | 0 |
| 5 | Bael | Aegle marmelos | Pulp of fruit | 0 |
| 6 | Ber | Ziziphus mauritiana | Fruit | 0 |
| 7 | Bhilawa | Semecarpusana cardium | Fruit | 0 |
| 8 | Bhojpatra | Betula utilis | Leaf | 0 |
| 9 | Black tea | Camellia sinensis | Leaf | 100% |
| 10 | Chirata | Swertia chirayita | Leaf and stem | 0 |
| 11 | Chopachini | Smilax glabra | Rhizome | 0 |
| 12 | Cinnamon | Cinnamomum verum | Bark | 0 |
| 13 | Coconut | Cocos nucifera | Husk fiber | 0 |
| 14 | Daaruharidra | Berberi saristata | Stem | 0 |
| 15 | Deodar | Cedrus deodara | Bark | 0 |
| 16 | Elaichi | Elettaria cardamomum | Fruit | 0 |
| 17 | Fennel | Foeniculum vulgare | Fruit | 0 |
| 18 | Fenugreek | Trigonella foenum‐graecum | Fruit | 0 |
| 19 | Garlic | Allium sativum | Clove | 0 |
| 20 | Giloy | Tinospora cordifolia | Stem | 13% |
| 21 | Ginger | Zingiber officinale | Rhizome | 0 |
| 22 | Gojihva | Onosma bracteatum | Leaf and stem | 0 |
| 23 | Green tea | Camellia sinensis | Leaf | 100% |
| 24 | Guggul | Commiphora wightii | Resin | 8% |
| 25 | Haridra | Curcuma longa | Rhizome | 5% |
| 26 | Haritaki | Terminali achebula | Fruit | 100% |
| 27 | Kachur | Curcuma zedoaria | Rhizome | 0 |
| 28 | Kalmegh | Andrographis paniculata | Leaf | 0 |
| 29 | Kantakari | Solanum surratense | Fruit | 0 |
| 30 | Lemon | Citrus Limon | Fruit | 0 |
| 31 | Mamira | Coptis teeta | Leaf | 0 |
| 32 | Maricha | Piper nigrum | Fruit | 0 |
| 33 | Mulethi | Glycyrrhiza glabra | Root | 40% |
| 34 | Musta | Cyperus rotundus | Rhizome | 0 |
| 35 | Nimba | Azadirachta indica | Leaf | 15% |
| 36 | Nirgundi | Vitex negundo | Leaf | 0 |
| 37 | Pippali | Piper longum | Fruit | 0 |
| 38 | Puskarmool | Inulara cemosa | Root | 0 |
| 39 | Rasna | Pluchea lanceolate | Leaf, stem | 0 |
| 40 | Sallaki | Boswellia serrata | Resin | 12% |
| 41 | Shalparni | Desmodium gangeticum | Root | 0 |
| 42 | Shatavari | Asparagus racemosus | Rhizome | 0 |
| 43 | Shleshmatak | Cordia dichotoma | Fruit | 0 |
| 44 | Spirulina | Arthrospira platensis | Entire plant | 0 |
| 45 | Sunthi | Zingiber officinale | Rhizome | 10% |
| 46 | Surajmukhi beej | Helianthus annuus | Seed | 5% |
| 47 | Suranjaan | Colchicum luteum | Rhizome | 0 |
| 48 | Talispatra | Abies webbiana | Leaf | 0 |
| 49 | Tilpushpi | Digitalis purpurea | Leaf | 0 |
| 50 | Tulsi | Ocimum sanctum | Leaf | 20% |
| 51 | Vasaka | Adhatoda vasica | Leaf | 0 |
The IC50 values of Green Tea and Haritaki was found to be 8.9 ± 0.5 and 8.8 ± 0.5 μg/ml, respectively (Figure 3). The aqueous extracts of plants Mulethi (Glycyrrhiza glabra), Tulsi (Ocimum tenuiflorum), Giloy (Tinospora cordifolia), and Neem (Azadirachta indica) have less efficiency in inhibiting the protease activity of 3CLpro. These plants could not inhibit the 3CLpro protease activity to 50% even with 450 μg/ml of the plant extracts.
FIGURE 3.
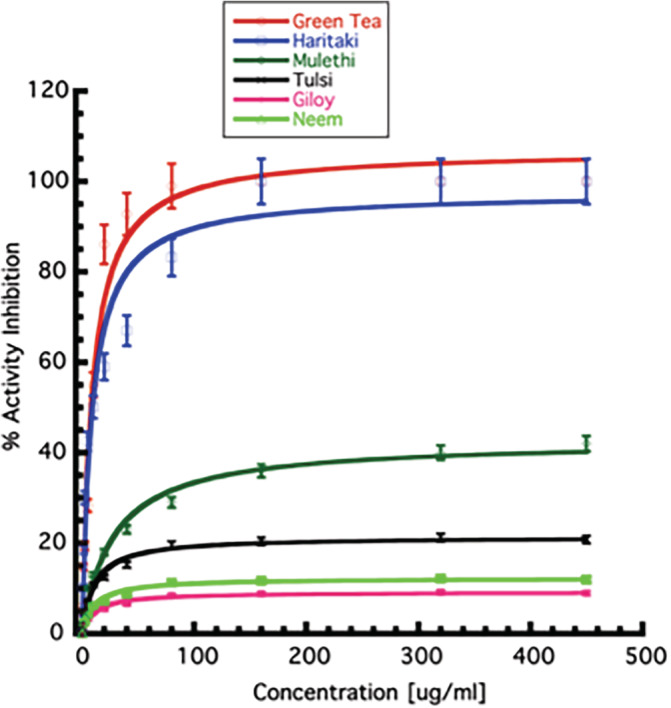
Determination of inhibitory concentration of plant extracts. The inhibition of percentage enzyme activity in the presence of varying concentrations (0–450 μg/ml) of plant extracts are shown. The value at which the 50% enzyme activity‐reduction observed is taken as IC50 of individual extracts. All the experiments were performed in triplicates. Error bars represent SEM [Colour figure can be viewed at wileyonlinelibrary.com]
4.2. Evaluation of tertiary structure changes in 3CLpro post‐interaction to plant extracts
The interactions of the active moieties present in the extracts interacted with the 3CLpro protein and resulted in the tertiary structure perturbation. The fluorescence quenching was observed as depicted by the decrease in the intensity of the fluorescence spectra (Figure 4).
FIGURE 4.
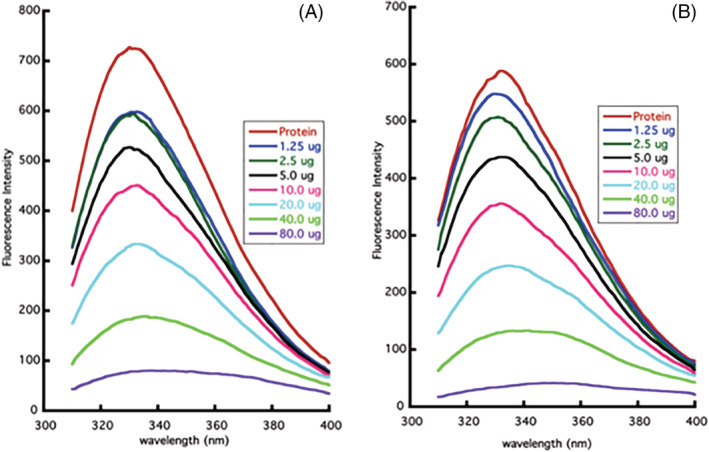
Estimation of tertiary structure perturbation in the presence of plant extracts. (A) Titration of 3CLpro with varying concentrations of Green Tea (Camellia sinensis). (B) Titration of 3CLpro with varying concentrations of Haritaki (Terminalia chebula) [Colour figure can be viewed at wileyonlinelibrary.com]
4.3. Comparative protease inhibition activity of Green Tea, Black Tea, and Haritaki
The comparison in the 3CLpro protease inhibition efficacies of Green Tea, Black Tea, and Haritaki were studied. Both Tea (Green Tea and Black tea) and Haritaki displayed comparable inhibition to protease activity; 50% inhibition at 10 μg/ml and more than 90% inhibition beyond 40 μg/ml. The results suggest that either form of Tea and Haritaki can be a potential anti‐viral agent against the SARS‐CoV‐2 (Figure 5).
FIGURE 5.
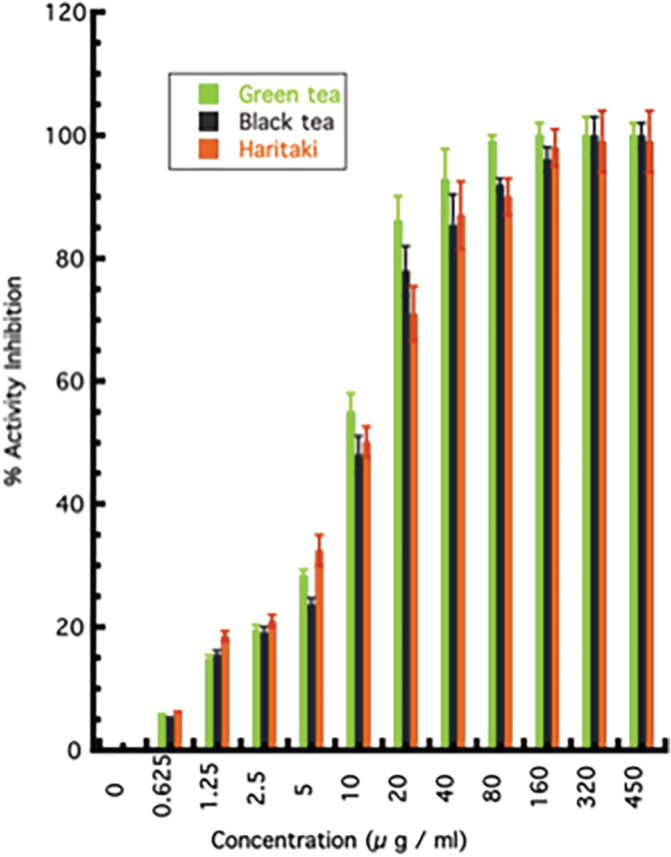
The comparison of inhibitory potential between Green Tea, Black Tea, and Haritaki towards 3CLpro activity inhibition. The graph represents the activity inhibition by Green Tea, Black Tea, and Haritaki. All the experiments were performed in triplicates and error bars represent SEM [Colour figure can be viewed at wileyonlinelibrary.com]
4.4. Deciphering molecular insights through an in‐silico approach
The key residues of the active site are histidine 41 and cysteine 145. The active site‐specific docking revealed the binding of thearubigin, a compound present in Tea. The binding energy of the molecule was found to be −8.53 kcal/mol. It displayed direct interaction with the cysteine 145 of the active site. Another molecule, quercetin‐3‐O‐rutinoside present in Tea, shown to form extensive hydrogen bonding with the residues of the active site pocket. The amino acid residues involved in the bi‐molecular interactions include leucine 141, asparagine 142, glycine 143, and glutamine 166. The docking energy for this interaction was found to be −7.73 kcal/mol. Within the range of 4 Å, the hesperidin displayed hydrophobic interaction with methionine 165 and glutamine 189 and hydrogen bonding with asparagine 142 and glycine 146. The binding for this interaction was −7.46 kcal/mol.
5. DISCUSSION
The COVID‐19 disease has garnered immediate attention from scientists and medical experts worldwide. The hunt for an effective therapy and potential intervention against SARS‐CoV‐2 has become a primary goal. In this context, we report medicinal plant extracts from Tea (Green Tea and Black Tea) and Haritaki as potential therapeutic options for the management of infection. The Ab‐Initio drug discovery programs require huge capital investment and some undesirous events like immunogenicity, toxicity, and efficacy remain a matter of concern in the clinical trials (Corsello et al., 2017). We harnessed principle of medicinal plants to tackle the challenge of COVID‐19. The present study screened 51 plants having anti‐viral potential against the main protease of SARS‐CoV‐ 2. The study reveals the inhibitory potential of Tea and Haritaki against 3CLpro; a crucial enzymatic protein of SARS‐CoV‐2 (Anand et al., 2003). The halt in this enzymatic activity can potentially block the replication of the virus inside the cells and may confer a protective effect in COVID‐19. The infection of the SARS‐CoV‐2 produces a wave of inflammatory mediators and manifests severe oxidative damage (Varga et al., 2020). The inflammatory and oxidative damage to the cells conferred by the virus can be countered by the anti‐inflammatory and anti‐oxidative properties of these plants (Cabrera et al., 2006; Chan, Lim, & Chew, 2007; Karamese et al., 2015; Lee et al., 2018). Also, as these plants are routinely used for treatment in different kinds of diseases and disorders, the risks of cytotoxic effects are also relatively low (Cheng et al., 2002; Karamese et al., 2015). The anti‐viral properties of Tea have been demonstrated against the influenza virus, zika virus, hepatitis‐B virus, and SARS virus (Carneiro et al., 2016; Mahmood et al., 2016).
We found that components present in the aqueous extract of Tea and Haritaki inhibited the 3CLpro of SARS‐CoV‐2 (Figure 3). However, the anti‐viral effect of these extracts and bioactive constituents needs to be validated in the in‐vivo studies. The activity data (Figure 5) suggests that both Tea and Haritaki might be good source for the design of potential inhibitors for the 3CLpro of SARS‐CoV‐2. We have tried to investigate the potential and probable molecules that are abundantly present in Tea. Among the virtually screened molecules, thearubigin, quercetin‐3‐O‐rutinoside, and hesperidin displayed interaction with the active site of the protease (Figure 6). To our best, this is the first in‐vitro reporting and validation of the protease inhibition by Green Tea, Black Tea, and Haritaki extracts against 3CLpro of SARS‐CoV‐2. These findings lays the foundation for further analysis of active molecules and their validation in higher validation systems.
FIGURE 6.
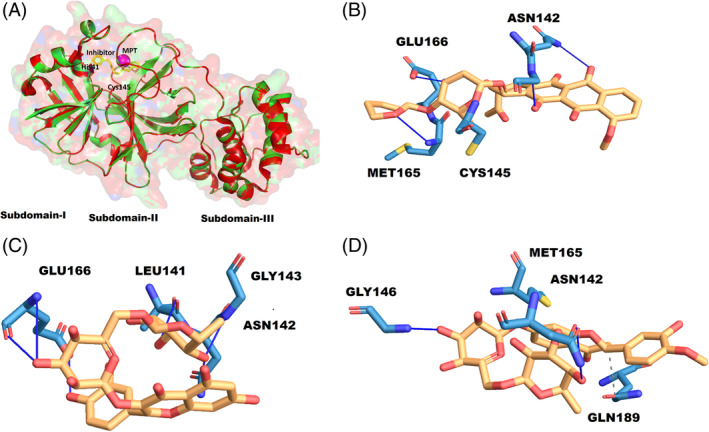
Active site details and binding of thearubigin. (A) Structure of native 6LU7 (green) and bounded (yellow), the active site space determined by metapocket2.0 (MPT in magenta), and the active site residues His41 and Cys145. (B) 2‐D interaction diagram of thearubigin with the residues of the active site. The interaction profile of quercetin‐3‐O‐rutinoside (C), and hesperidin (D) [Colour figure can be viewed at wileyonlinelibrary.com]
6. CONCLUSION
We present the prospective therapeutic potential of the aqueous extracts of Tea (C. sinensis) and Haritaki (T. chebula) against SARS‐CoV‐2 based on 3CLpro protease activity inhibition. These herb extracts contain a plethora of molecules researched to have anti‐viral, anti‐inflammatory, anti‐oxidant properties, and other beneficial health effects. In the current scenario of pandemic, an expedited evaluation of bioactive constituents is required to establish the anti‐SARS‐CoV‐2 property of these herbs. Moreover, larger randomized, double‐blind, placebo‐controlled multicenter clinical trials should be conducted before incorporation of these herbs as a prospective therapeutic option.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors thanks IIT Delhi and Kusuma Trust for the infrastructure support. They acknowledge the funding support from the Dean, Research and Development, IIT Delhi, New Delhi, India through Grant No MI02217G.
Upadhyay S, Tripathi PK, Singh M, Raghavendhar S, Bhardwaj M, Patel AK. Evaluation of medicinal herbs as a potential therapeutic option against SARS‐CoV‐2 targeting its main protease. Phytotherapy Research. 2020;34:3411–3419. 10.1002/ptr.6802
Saurabh Upadhyay, Praveen K. Tripathi, and Manju Singh authors are provided equal contribution.
Funding information Indian Institute of Technology Delhi, Grant/Award Number: MI02217G
REFERENCES
- Anand, K. , Ziebuhr, J. , Wadhwani, P. , Mesters, J. R. , & Hilgenfeld, R. (2003). Coronavirus main proteinase (3CLpro) structure: Basis for design of anti‐SARS drugs. Science, 300, 1763–1767. 10.1126/science.1085658 [DOI] [PubMed] [Google Scholar]
- Banik, S. , Biswas, S. , & Karmakar, S. (2018). Extraction, purification, and activity of protease from the leaves of Moringa oleifera . F1000Research, 7, 1151. 10.12688/f1000research.15642.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera, C. , Artacho, R. , & Giménez, R. (2006). Beneficial effects of green tea—A review. Journal of the American College of Nutrition, 25, 79–99. 10.1080/07315724.2006.10719518 [DOI] [PubMed] [Google Scholar]
- Capell, T. , Twyman, R. M. , Armario‐Najera, V. , Ma, J. K.‐C. , Schillberg, S. , & Christou, P. (2020). Potential applications of plant biotechnology against SARS‐CoV‐2. Trends in Plant Science, 0, 635–643. 10.1016/j.tplants.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro, B. M. , Batista, M. N. , Braga, A. C. S. , Nogueira, M. L. , & Rahal, P. (2016). The green tea molecule EGCG inhibits Zika virus entry. Virology, 496, 215–218. 10.1016/j.virol.2016.06.012 [DOI] [PubMed] [Google Scholar]
- Chan, E. W. C. , Lim, Y. Y. , & Chew, Y. L. (2007). Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chemistry, 102, 1214–1222. 10.1016/j.foodchem.2006.07.009 [DOI] [Google Scholar]
- Chan, J. F. W. , Yuan, S. , Kok, K. H. , To, K. K. W. , Chu, H. , Yang, J. , … Yuen, K. Y. (2020). A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: A study of a family cluster. The Lancet, 395, 514–523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.‐N. , Lin, C. P. C. , Huang, K.‐K. , Chen, W.‐C. , Hsieh, H.‐P. , Liang, P.‐H. , & Hsu, J. T.‐A. (2005). Inhibition of SARS‐CoV 3C‐like protease activity by Theaflavin‐3,3′‐digallate (TF3). Evidence‐Based Complementary and Alternative Medicine, 2, 209–215. 10.1093/ecam/neh081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H.‐Y. , Lin, C.‐C. , & Lin, T.‐C. (2002). Antiviral properties of Prodelphinidin B‐2 3′‐O‐Gallate from green tea leaf. Antiviral Chemistry & Chemotherapy, 13, 223–229. 10.1177/095632020201300403 [DOI] [PubMed] [Google Scholar]
- Cooper, R. , Morré, D. J. , & Morré, D. M. (2005a). Medicinal benefits of green tea: Part I. review of noncancer health benefits. Journal of Alternative and Complementary Medicine, 11, 521–528. 10.1089/acm.2005.11.521 [DOI] [PubMed] [Google Scholar]
- Cooper, R. , Morré, D. J. , & Morré, D. M. (2005b). Medicinal benefits of green tea: Part II. Review of anticancer properties. Journal of Alternative and Complementary Medicine, 11, 639–652. 10.1089/acm.2005.11.639 [DOI] [PubMed] [Google Scholar]
- Corsello, S. M. , Bittker, J. A. , Liu, Z. , Gould, J. , McCarren, P. , Hirschman, J. E. , … Golub, T. R. (2017). The drug repurposing hub: A next‐generation drug library and information resource. Nature Medicine, 23, 405–408. 10.1038/nm.4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupp‐Enyard, C. (2008). Sigma's non‐specific protease activity assay—Casein as a substrate. Journal of Visualized Experiments, (19), e899. 10.3791/899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya, A. E. , Baker, S. C. , Baric, R. S. , de Groot, R. J. , Drosten, C. , Gulyaeva, A. A. , … Ziebuhr, J. (2020). The species Severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nature Microbiology, 5, 536–544. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenfeld, R. (2014). From SARS to MERS: Crystallographic studies on coronaviral proteases enable antiviral drug design. The FEBS Journal, 281, 4085–4096. 10.1111/febs.12936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamese, M. , Aydogdu, S. , Karamese, S. A. , Altoparlak, U. , & Gundogdu, C. (2015). Preventive effects of a major component of green tea, epigallocathechin‐3‐gallate, on hepatitis‐B virus DNA replication. Asian Pacific Journal of Cancer Prevention, 16, 4199–4202. 10.7314/apjcp.2015.16.10.4199 [DOI] [PubMed] [Google Scholar]
- Kesharwani, A. , Polachira, S. K. , Nair, R. , Agarwal, A. , Mishra, N. N. , & Gupta, S. K. (2017). Anti‐HSV‐2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Complementary and Alternative Medicine, 17, 110. 10.1186/s12906-017-1620-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D. , Boo, K. H. , Woo, J. K. , Duan, F. , Lee, K. H. , Kwon, T. K. , … Lee, D. S. (2011). Anti‐bacterial and anti‐viral activities of extracts from Terminalia chebula barks. Journal of Applied Biological Chemistry, 54, 295–298. 10.3839/jksabc.2011.046 [DOI] [Google Scholar]
- Lee, Y. H. , Jang, Y. H. , Kim, Y. S. , Kim, J. , & Seong, B. L. (2018). Evaluation of green tea extract as a safe personal hygiene against viral infections. Journal of Biological Engineering, 12, 1. 10.1186/s13036-017-0092-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, P. , Du, R. , Wang, Y. , Hou, X. , Wang, L. , Zhao, X. , … Cui, Q. (2020). Identification of Chebulinic acid and Chebulagic acid as novel influenza viral neuraminidase inhibitors. Frontiers in Microbiology, 11, 182. 10.3389/fmicb.2020.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K. , Chen, Y. , Lin, R. , & Han, K. (2020). Clinical features of COVID‐19 in elderly patients: A comparison with young and middle‐aged patients. The Journal of Infection, 80, e14–e18. 10.1016/j.jinf.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood, M. S. , Mártinez, J. L. , Aslam, A. , Rafique, A. , Vinet, R. , Laurido, C. , … Ali, S. (2016). Antiviral effects of green tea (Camellia sinensis) against pathogenic viruses in human and animals (a mini‐review). African Journal of Traditional, Complementary, and Alternative Medicines, 13(2), 176–184. 10.4314/ajtcam.v13i2.21 [DOI] [Google Scholar]
- Newman, D. J. , & Cragg, G. M. (2007). Natural products as sources of new drugs over the last 25 years. Journal of Natural Products, 70, 461–477. 10.1021/np068054v [DOI] [PubMed] [Google Scholar]
- Raghavendhar, S. , Tripati, P. K. , Ray, P. , & Patel, A. K. (2019). Evaluation of medicinal herbs for anti‐CHIKV activity. Virology, 533, 45–49. 10.1016/j.virol.2019.04.007 [DOI] [PubMed] [Google Scholar]
- Studier, F. W. (2005). Protein production by auto‐induction in high density shaking cultures. Protein Expression and Purification, 41, 207–234. 10.1016/j.pep.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Varga, Z. , Flammer, A. J. , Steiger, P. , Haberecker, M. , Andermatt, R. , Zinkernagel, A. S. , … Moch, H. (2020). Endothelial cell infection and endotheliitis in COVID‐19. The Lancet, 395, 1417–1418. 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L. , Liu, J. , Lu, M. , Yang, D. , & Zheng, X. (2020). Liver injury during highly pathogenic human coronavirus infections. Liver International, 40, 998–1004. 10.1111/liv.14435 [DOI] [PMC free article] [PubMed] [Google Scholar]


