Abstract
Aim
To evaluate the diagnostic and prognostic performance of alternative diagnostic strategies to oral glucose tolerance tests, including random plasma glucose, fasting plasma glucose and HbA1c, during the COVID‐19 pandemic.
Methods
Retrospective service data (Cambridge, UK; 17 736 consecutive singleton pregnancies, 2004–2008; 826 consecutive gestational diabetes pregnancies, 2014–2019) and 361 women with ≥1 gestational diabetes risk factor (OPHELIA prospective observational study, UK) were included. Pregnancy outcomes included gestational diabetes (National Institute of Health and Clinical Excellence or International Association of Diabetes and Pregnancy Study Groups criteria), diabetes in pregnancy (WHO criteria), Caesarean section, large‐for‐gestational age infant, neonatal hypoglycaemia and neonatal intensive care unit admission. Receiver‐operating characteristic curves and unadjusted logistic regression were used to compare random plasma glucose, fasting plasma glucose and HbA1c performance.
Results
Gestational diabetes diagnosis was significantly associated with random plasma glucose at 12 weeks [area under the receiver‐operating characteristic curve for both criteria 0.81 (95% CI 0.79–0.83)], fasting plasma glucose [National Institute of Health and Clinical Excellence: area under the receiver‐operating characteristic curve 0.75 (95% CI 0.65–0.85); International Association of Diabetes and Pregnancy Study Groups: area under the receiver‐operating characteristic curve 0.92 (95% CI 0.85–0.98)] and HbA1c at 28 weeks' gestation [National Institute of Health and Clinical Excellence: 0.83 (95% CI 0.75–0.90); International Association of Diabetes and Pregnancy Study Groups: 0.84 (95% CI 0.77–0.91)]. Each measure predicts some, but not all, pregnancy outcomes studied. At 12 weeks, ~5% of women would be identified using random plasma glucose ≥8.5 mmol/l (sensitivity 42%; specificity 96%) and at 28 weeks using HbA1c ≥39 mmol/mol (sensitivity 26%; specificity 96%) or fasting plasma glucose ≥5.2–5.4 mmol/l (sensitivity 18–41%; specificity 97–98%).
Conclusions
Random plasma glucose at 12 weeks, and fasting plasma glucose or HbA1c at 28 weeks identify women with hyperglycaemia at risk of suboptimal pregnancy outcomes. These opportunistic laboratory tests perform adequately for risk stratification when oral glucose tolerance testing is not available.
What's new?
Screening procedures for hyperglycaemia in pregnancy have temporarily changed during the COVID‐19 pandemic as oral glucose tolerance tests are challenging to conduct with social distancing measures in place, public transport restrictions and reduced clinical capacity.
National recommendations for Australia, Canada, New Zealand and the UK propose alternative opportunistic screening strategies using HbA1c, random plasma glucose and fasting plasma glucose (FPG), performed with routine antenatal bloods at booking and 28 weeks.
We identified that random plasma glucose, HbA1c and FPG were all associated with gestational diabetes diagnosis and that all three tests can predict obstetric and neonatal outcomes but lack the evidence base and sensitivity of an oral glucose tolerance test.
Future work should prioritize inclusive screening strategies which identify women at highest risk of materno‐fetal complications and systematic procedures for longer‐term cardio‐metabolic follow‐up.
1. INTRODUCTION
Gestational diabetes mellitus (GDM) affects approximately 5% of pregnant women in the UK and is associated with perinatal morbidity, including large‐for‐gestational‐age (LGA) infants, complicated deliveries and neonatal hypoglycaemia. 1 The oral glucose tolerance test (OGTT) is currently the recommended approach to the diagnosis of GDM in the UK and internationally. 2 , 3 Early in 2020, a novel virus, SARS‐CoV‐2, reached pandemic levels of worldwide infection. 4 In the UK, pregnant women have been advised to remain in self‐isolation for at least 12 weeks except for essential excursions for food, healthcare and health reasons (including outdoor daily exercise), with similar restrictions internationally. This, alongside public transport limitations, especially during peak hours, social distancing and laboratory requirements, has made it challenging for healthcare providers to implement routine OGTTs. Furthermore, with staff shortages due to self‐isolation, illness or redeployment, the clinical capacity for managing large numbers of pregnant women with milder forms of hyperglycaemia has been reduced. An alternative approach to screening for severe forms of maternal hyperglycaemia during the COVID‐19 pandemic was required for urgent implementation. 5 The intention was not aimed at identifying an equivalent group of women as those detected by OGTT, but rather to identify pregnant women with the highest glucose levels for whom specialist management remained essential throughout the pandemic. The alternative strategy recommends glucose testing during other hospital or community appointments to minimize additional clinical contacts. 5
The OGTT has well recognized limitations in terms of test reproducibility, tolerability and seasonal influences, 6 , 7 but remains the most commonly recommended diagnostic strategy for GDM. 2 , 3 Excess fetal growth acceleration is detectable from 20 weeks’ gestation, predating diagnosis of GDM. 8 However, approximately half of women with identified risk factors for GDM, do not have an OGTT performed in routine care settings. Importantly, among women with stillbirths, an OGTT was only performed in 38% of women with GDM risk factors. 9 A nationwide UK audit confirmed that OGTT testing was least likely to be performed in obese women and those from higher‐risk ethnic groups. 10 Although some women may refuse OGTT testing, it is clear that screening for GDM is not consistently implemented in accordance with guideline recommendations. 6 , 10
A recent case–control study identified a 44% greater risk of stillbirth in women with risk factors when an OGTT was not performed. 9 Likewise, women with raised fasting plasma glucose (FPG) levels who were not diagnosed with GDM experienced a fourfold greater risk of stillbirth. 9 Women who were appropriately screened and treated had no increased risk of stillbirth. 9 This failure to effectively implement OGTT in routine clinical care settings even prior to the pandemic leaves women exposed to potentially modifiable risks for stillbirth.
Relatively little research has focused on identifying a suitable alternative to the OGTT. Some novel technologies and biomarkers show promise in small studies, but do not have proven diagnostic performance or wide availability. Performing an OGTT at home or replacing it with glucometer or continuous glucose monitoring readings was not feasible for widespread implementation during the pandemic.
The interim testing strategy for hyperglycaemia in pregnancy aimed to: (1) identify women with the most severe hyperglycaemia for prioritization of resources towards those at highest risk of suboptimal obstetric and neonatal outcomes; (2) fit around women’s routine antenatal visits at 12, 20 and 28 weeks (for blood tests or ultrasound scans), avoiding additional visits to healthcare environments; (3) use established laboratory methods with good analytical performance and wide availability; (4) avoid increasing clinical workload during staff shortage (prioritizing test specificity over sensitivity); (5) be simple to understand (by non‐specialists) and easy to rapidly implement across healthcare environments; and (6) use an existing evidence base to support it and to enable audit after the pandemic.
Several diagnostic strategies for hyperglycaemia in pregnancy have been suggested for use during the COVID‐19 pandemic (Table 1). The UK approach advises measuring HbA1c and random plasma glucose in all women with risk factors for GDM (previous GDM, BMI >30 kg/m2, high‐risk ethnic groups, family history of diabetes, previous macrosomic baby >4.5 kg) at the first antenatal visit (Table 4). Women with HbA1c levels ≥48 mmol/mol or random plasma glucose levels ≥11.1 mmol/l are managed as having pre‐gestational, most commonly type 2 diabetes. Women with HbA1c levels 41–47 mmol/mol or random plasma glucose levels 9–11.0 mmol/l are managed as having early‐onset GDM. Women with HbA1c <41 mmol/mol and random plasma glucose <9 mmol/l are retested at 28 weeks, with a repeat HbA1c and FPG (if possible) or random plasma glucose performed. Women with FPG ≥5.6 mmol/l, HbA1c ≥39 mmol/mol or random plasma glucose ≥9 mmol/l are managed as having GDM. The pandemic testing procedures do not exclude GDM and further testing should be performed, at any gestational age, in women with glycosuria, symptoms of diabetes, or ultrasound features of LGA infant or polyhydramnios. 5 Similar strategies were recommended in Australia, Canada and New Zealand (Table 1). 11 , 12 , 13
Table 1.
Recommendations from Australia, Canada, New Zealand and the UK for identification of hyperglycaemia in pregnancy during the COVID‐19 pandemic [5,11–13]
| Australia | Canada | New Zealand | UK | |
|---|---|---|---|---|
| Glucose measures at 12 weeks | HbA1c | HbA1c or FPG | HbA1c | HbA1c and random plasma glucose |
| Interpretation of early pregnancy glucose measures | HbA1c >41 mmol/mol diagnosed with GDM | HbA1c before 20 weeks to identify overt diabetes in high‐risk women only | HbA1c >40 mmol/mol: refer to specialist clinic |
HbA1c 41–47 mmol/mol or random plasma glucose 9–11 mmol/l: manage as early GDM. HbA1c ≥ 48 mmol/mol or random plasma glucose >11.1 mmol/l: manage as likely type 2 diabetes. |
| Glucose measures at 28 weeks | OGTT or FPG |
Standard two‐step protocol or HbA1c and random plasma glucose |
Standard 2‐h OGTT or FPG if HbA1c at 12 weeks <41 mmol/mol |
HbA1c and random plasma glucose or FPG (FPG if possible according to clinical capacity) |
| Interpretation of glucose measures at 28 weeks |
Standard OGTT (fasting ≥ 5.1 mmol/l; 1‐h ≥ 10 mmol/l or 2‐h ≥ 8.5 mmol/l ) or FPG alone ≥ 5.1 mmol/l |
HbA1c ≥5.7% (39 mmol/mol) or random plasma glucose ≥11.1 mmol/l: diagnosed with GDM |
FPG ≥5.0 mmol/l: treat as GDM. FPG 4.5–5.0 mmol/l: SMBG for 2 weeks and dietetic support, especially if risk factors for GDM |
HbA1c ≥ 39 mmol/mol or random plasma glucose >9 mmol/l or FPG ≥ 5.6 mmol/l: diagnosed with GDM. |
| Women with previous GDM | Can have standard testing schedule or be assumed to have GDM and started on self‐monitoring at home | Not specifically mentioned. Standard testing pathway applies | Start SMBG from 12 weeks | Treat as GDM from 12 weeks if HbA1c 41–47 mmol/mol or random plasma glucose 9–11 mmol/l. If HbA1c <41 mmol/mol and random plasma glucose <9 mmol/l: standard testing at 28 weeks applies |
| Provisions for testing at other times | Clinicians to use clinical judgement about suitability of testing | Testing can be repeated in later pregnancy is there is a high clinical suspicion of diabetes | Not mentioned |
Test in the presence of heavy glycosuria (2+ or above), diabetes symptoms or according to scan features (LGA fetus or polyhydramnios) |
| Postpartum testing | With OGTT to be delayed by 6–12 months. For women at high risk of type 2 diabetes, consider self‐monitoring at home or HbA1c 4–6 months | Defer until after the pandemic is over | Not mentioned | HbA1c at 3–6 months after birth |
FPG, fasting plasma glucose; GDM, gestional diabetes; LGA, large‐for‐gestational‐age; OGTT, oral glucose tolerance test; SMBG, self‐monitoring of blood glucose.
Table 4.
Sensitivity and specificity of various thresholds for prediction of National Institute of Health and Care Excellence‐ and International Association of Diabetes and Pregnancy Study Groups‐defined gestational diabetes [3,4]
|
HbA1c at 28 weeks vs OGTT at 28 weeks: OPHELIA data, n=340 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Threshold |
NICE‐GDM AUC 0.83 (95% CI 0.75, 0.90) |
IADPSG‐GDM AUC 0.84 (95% CI 0.77, 0.91) |
||||||
| Sensitivity, % | Specificity, % | n | % positive | Sensitivity, % | Specificity, % | n | % positive | |
| > 32 mmol/mol (5.1%) | 96 | 43 | 204 | 56 | 93 | 43 | 204 | 60 |
| > 33 mmol/mol (5.2%) | 93 | 56 | 162 | 45 | 87 | 56 | 162 | 48 |
| > 34 mmol/mol (5.3%) | 82 | 70 | 116 | 32 | 83 | 71 | 116 | 34 |
| > 35 mmol/mol (5.4%) | 70 | 79 | 85 | 24 | 67 | 79 | 85 | 25 |
| > 36 mmol/mol (5.4%) | 48 | 87 | 53 | 15 | 57 | 88 | 53 | 16 |
| > 37 mmol/mol (5.5%) | 41 | 92 | 35 | 9.7 | 47 | 93 | 35 | 10 |
| > 38 mmol/mol (5.6%) | 37 | 95 | 25 | 6.9 | 43 | 96 | 25 | 7.3 |
| > 39 mmol/mol (5.7%) | 26 | 96 | 19 | 5.3 | 30 | 97 | 19 | 5.6 |
| > 40 mmol/mol (5.8%) | 15 | 97 | 13 | 3.6 | 17 | 97 | 13 | 3.8 |
|
Random plasma glucose at 12 weeks vs OGTT at 28 weeks: older CUHFT data, n=17 736 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Threshold |
NICE‐GDM AUC 0.81 (95% CI 0.79, 0.83) |
IADPSG‐GDM AUC 0.81 (95% CI 0.79, 0.83) |
||||||
| Sensitivity | Specificity | n | % positive | Sensitivity | Specificity | n | % positive | |
| > 7.0 mmol/l | 73 | 83 | 3487 | 20 | 74 | 83 | 3487 | 20 |
| > 7.5 mmol/l | 69 | 89 | 2340 | 13 | 70 | 90 | 2340 | 13 |
| > 7.8 mmol/l | 65 | 92 | 1835 | 10 | 67 | 93 | 1835 | 10 |
| > 8.0 mmol/l | 60 | 94 | 1541 | 8.7 | 60 | 94 | 1541 | 8.7 |
| > 8.5 mmol/l | 42 | 96 | 947 | 5.3 | 43 | 97 | 947 | 5.3 |
| > 9.0 mmol/l | 29 | 98 | 576 | 3.2 | 30 | 98 | 576 | 3.2 |
| > 9.5 mmol/l | 19 | 99 | 340 | 1.9 | 20 | 99 | 340 | 1.9 |
| > 10.0 mmol/l | 11 | 100 | 171 | 1.0 | 12 | 100 | 171 | 1.0 |
|
Fasting plasma glucose at 28 weeks vs OGTT at 28 weeks: OPHELIA data, n = 360 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cutpoint |
NICE‐GDM AUC 0.75 (95% CI 0.65, 0.85) |
IADPSG‐GDM AUC 0.92 (95% CI 0.85, 0.98) |
||||||
| Sensitivity | Specificity | n | % positive | Sensitivity | Specificity | n | % positive | |
| > 4.5 mmol/l | 65 | 65 | 135 | 38 | 91 | 68 | 135 | 38 |
| > 5.0 mmol/l | 41 | 94 | 33 | 9.2 | 78 | 98 | 33 | 9.2 |
| > 5.1 mmol/l | 41 | 96 | 25 | 6.9 | 78 | 100 | 25 | 6.9 |
| > 5.2 mmol/l | 41 | 98 | 20 | 5.6 | 63 | 100 | 20 | 5.6 |
| > 5.3 mmol/l | 38 | 98 | 17 | 4.7 | 53 | 100 | 17 | 4.7 |
| > 5.4 mmol/l | 35 | 99 | 13 | 3.6 | 41 | 100 | 13 | 3.6 |
| > 5.5 mmol/l | 35 | 100 | 11 | 3.1 | 34 | 100 | 11 | 3.1 |
| > 5.6 mmol/l | 28 | 100 | 8 | 2.2 | 25 | 100 | 8 | 2.2 |
|
Fasting plasma glucose at 28 weeks vs OGTT at 28 weeks: older CUHFT data, n = 3832 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Threshold |
NICE‐GDM AUC 0.69 (95% CI 0.82, 0.85) |
IADPSG‐GDM AUC 0.79 (95% CI 0.77, 0.80) |
||||||
| Sensitivity, % | Specificity, % | n | % positive | Sensitivity, % | Specificity, % | n | % positive | |
| > 4.5 mmol/l | 65 | 61 | 1758 | 46 | 73 | 66 | 1758 | 46 |
| > 5.0 mmol/l | 33 | 91 | 597 | 16 | 44 | 97 | 597 | 16 |
| > 5.1 mmol/l | 29 | 94 | 473 | 12 | 40 | 100 | 473 | 12 |
| > 5.2 mmol/l | 26 | 96 | 370 | 9.7 | 31 | 100 | 370 | 9.7 |
| > 5.3 mmol/l | 23 | 98 | 299 | 7.8 | 25 | 100 | 299 | 7.8 |
| > 5.4 mmol/l | 18 | 99 | 229 | 6.0 | 19 | 100 | 229 | 6.0 |
| > 5.5 mmol/l | 16 | 100 | 182 | 4.7 | 15 | 100 | 182 | 4.7 |
| > 5.6 mmol/l | 14 | 100 | 148 | 3.9 | 13 | 100 | 148 | 3.9 |
AUC, area under the curve; CUHFT, Cambridge University Hospital NHS Foundation Trust; GDM, gestational diabetes; IADPSG, International Association of Diabetes and Pregnancy Study Groups; NICE, National Institute of Health and Care Excellence; IADPSG, International Association of Diabetes and Pregnancy Study Groups; OGTT, oral glucose tolerance test.
Note that not all patients included underwent an OGTT.
The aim of the present study was to provide evidence‐based recommendations for a pragmatic diagnostic strategy for hyperglycaemia in pregnancy, applicable during the COVID‐19 pandemic.
2. METHODS
Data from complete and ongoing studies (Table 2; methodology and patient characteristics) were used to assess diagnostic performance of potential glucose measures including HbA1c, random plasma glucose, FPG and 1‐h and 2‐h plasma glucose after a 75‐g OGTT performed in a real‐world clinical setting.
Table 2.
Characteristics of women included in each dataset
| Study characteristics | Older CUHFT cohort | Recent CUHFT cohort | OPHELIA cohort |
|---|---|---|---|
| n = 17736 | n = 826 | n = 361 | |
| Time period covered | 2004–2008 | 2014–2019 | Oct 2019–ongoing |
| Study design | Retrospective service evaluation | Retrospective service evaluation | Prospective observational study |
| Population | All singleton pregnancies with liveborn infants | All singleton pregnancies with GDM diagnosed according to IADPSG criteria | Singleton pregnancies with ≥1 positive NICE risk factor |
| Centres | Single centre | Single centre | Four East of England centres |
| Who had a random plasma glucose at 12 weeks? | All women offered, results available in 72%† | Not applicable | Not applicable |
| Who had a 75‐g OGTT at 24–28 weeks? | Women with a 24‐week 50‐g glucose challenge result >7.7 mmol/l | Women with ≥1 GDM risk factor | Women with ≥1 GDM risk factor |
| GDM diagnostic criteria | 1998 WHO [31] | IADPSG [2] | NICE [3] |
| Treatment offered, n/N (%) | 776/17736 (4.4) | 826/826 (100) | 30/331 (8.3) |
| Maternal characteristics | |||
| Mean (sd) maternal age years | 30.9 (5.6) | 33.6 (5.4) | 31.7 (4.9) |
| Mean (sd) pre‐pregnancy BMI, kg/m2 | 24.8 (5.0) | 29.4 (7.5) | 33.0 (6.7) |
| Primiparous, n (%) | 9895 (56) | 283 (34) | 132 (37) |
| Ethnicity, n (%) | n=670 | n=345 | |
| White | 15934 (90) | 531 (79) | 315 (91) |
| Black | 258 (1.5) | 14 (2.1) | 29 (8.4) |
| Asian | 899 (5.1) | 111 (17) | 1 (0.3) |
| Other | 644 (3.6) | 14 (2.1) | 0 (0.0) |
| Maternal smoking, n (%) | 1643 (9.3) | 61/747 (8.2) | N/A |
| Mean (sd) random plasma glucose mmol/l at 12 weeks, mmol/l | 5.8 (1.4) | N/A | N/A |
| Mean (sd) HbA1c at 28 weeks, mmol/mol | N/A | 35.9 (4.9) | 32.5 (3.6) |
|
OGTT at 28 weeks |
n = 3848 |
n = 821–824 |
n = 359–360 |
| Mean (sd) Fasting glucose (OGTT time 0) | 4.5 (0.6) | 4.9 (0.7) | 4.4 (0.5) |
| Mean (sd) OGTT time 60 mins | 8.5 (1.9) | 10.6 (1.5) | N/A |
| Mean (sd) OGTT time 120 mins | 6.9 (1.6) | 7.6 (1.4) | 5.8 (1.4) |
|
Pregnancy outcomes |
n = 17001–17736 |
n = 817‐826 |
n = 278 |
| Mean (sd) estimated gestational age at birth, weeks | 39.2 (2.0) | 38.5 (1.4) | 39.4 (1.5) |
| LGA infant, n (%) | 2112 (1) | 134 (16) | N/A |
| Caesarean section, n (%) | 5005 (28) | 342 (41) | N/A |
| Neonatal hypoglycaemia, n (%) | N/A | 386 (47) | N/A |
| NICU admission, n (%) | 1071 (6.1) | 124 (15) | N/A |
CUHFT, Cambridge University Hospital NHS Foundation Trust; FPG, fasting plasma glucose; LGA, large‐for‐gestational‐age; NICU, neonatal intensive care unit.
Neonatal hypoglycaemia defined as neonatal glucose <2.6 mmol/l at least 4 h after birth. NICU admission defined as >24 h.
No difference between women with and without a random plasma glucose. 31
Gestational diabetes diagnosis was classified according to the criteria of the UK National Institute for Health and Care Excellence (NICE; 0 min ≥5.6 mmol/l; 120 min ≥7.8 mmol/l)3 and the International Association of Diabetes and Pregnancy Study Groups (IADPSG), adopted by the WHO (IADPSG/WHO; 0 min ≥5.1; 60 min ≥10.0; 120 min ≥8.5 mmol/l)2.
Older (2004–2008) and more recent data (2014–2019) from approved service evaluations at Cambridge University Hospital NHS Foundation Trust (CUHFT) used for this study have been previously described. 14 , 15 In brief, the older cohort includes 17 736 consecutive women with singleton pregnancies, with random plasma glucose performed at booking followed by a universal 50‐g glucose challenge test at 24 weeks. Women with a 50‐g glucose challenge result of >7.7 mmol/l had a 75‐g OGTT at 28 weeks’ gestation (n = 3848) and were offered treatment (776/17 736; 4.4%) in line with NICE guidance. 16 A minority (<5%) had an OGTT using capillary rather than venous blood. The more recent cohort included 826 consecutive women with GDM (risk factor screening; 75‐g OGTT 24–28 weeks using IADPSG/WHO criteria, 20 October 2014 to 31 January 2019), who received standard clinical management. Detailed information on pregnancy outcomes was gathered from electronic medical records as part of an ongoing service evaluation. 14
In addition, data were included from 361 women with one or more risk factors for GDM, 3 recruited from an ongoing multicentre prospective study, OPHELIA (Observational study of Pregnancy Hyperglycaemia, Endocrine causes, Lipids, Insulin and Autoimmunity; REC 18/LO/0477; researchregistry no.5528). Briefly, women with a singleton pregnancy and one or more GDM risk factors 3 were invited for a 24–28‐week 75‐g OGTT with measurement of HbA1c. A total of 8.3% of women had GDM (NICE criteria) and were offered treatment. Pregnancy outcome data from this ongoing study are not available.
Assessment of neonatal outcomes was performed using the older and recent CUHFT datasets. We chose outcomes which are directly related to hyperglycaemia, are consistently measured during GDM pregnancies, are potentially modifiable by standard clinical management, 1 , 17 and which have a defined impact on healthcare costs. 3
Large for gestational age was defined as having a birth weight >90th centile using locally derived standardized centiles adjusted for infant sex and gestational age. 7 Neonatal hypoglycaemia was defined as a capillary blood glucose level <2.6 mmol/l on more than one occasion at least 4 h after birth. Admission to the neonatal intensive care unit (NICU) was defined as admission for 24 h or longer.
The ability of each glucose‐related variable to predict GDM diagnosis was assessed using receiver‐operating characteristic (ROC) curves. Unadjusted logistic regression identified associations between glucose measures and pregnancy outcomes (odds ratios and 95% CIs reported). Missing data were not imputed. A statistical significance level of 5% was used throughout.
2.1. Ethics
The OPHELIA study was approved by the London and Westminster research ethics committee (REC 18/LO/0477; research registry no.5528). The CUHFT data were collected as part of approved service evaluations. Further ethical approval was not required for this analysis.
3. RESULTS
3.1. Prediction of gestational diabetes diagnosis and association with pregnancy outcomes
All glucose measures were significantly associated with GDM diagnosis on ROC curves (Table 3 and Fig. 1). Among the alternative glucose measures, HbA1c at 28 weeks and random plasma glucose at booking (12±2 weeks) both performed reasonably well, with areas under the ROC (AUROCs) of 0.83 (95% CI 0.75–0.90, OPHELIA) and 0.81 (95% CI 0.79–0.83, older CUHFT cohort) to predict NICE‐defined GDM. 4 The AUROC for random plasma glucose at booking was comparable for GDM as defined ether by NICE or IADPSG criteria. FPG was slightly less predictive for GDM defined by the NICE criteria [AUROC 0.75 (95% CI 0.65–0.85), OPHELIA], but strongly predicted GDM defined according to the IADPSG criteria [AUROC 0.92 (95% CI 0.85–0.98) OPHELIA]. All glucose measures studied had associations with one or more outcomes (Table 3).
Table 3.
Associations between glucose measures, diagnosis of gestational diabetes and pregnancy outcomes
| Study | Population | Total, n | OGTT, n | Design | Outcome |
Random plasma glucose 12 weeks |
HbA1c 28 weeks |
FPG 28 weeks |
OGTT time 60 28 weeks |
OGTT time 120 28 weeks |
|---|---|---|---|---|---|---|---|---|---|---|
|
Diagnosis: receiver‐operating characteristic curves |
AUROC (95% CI) |
AUROC (95% CI) |
AUROC (95%CI) |
AUROC (95%CI) |
AUROC (95%CI) |
|||||
|
OPHELIA |
Antenatal population with ≥1 GDM risk factor |
361 | 361 | Prospective | NICE‐GDM | No data | 0.83 (0.75‐‐0.90)*** | 0.75 (0.65‐0.85)*** | no data | 0.93 (0.86‐1.00)*** |
| 361 | 361 | Prospective | IADPSG‐GDM | No data | 0.84 (0.77‐‐0.91)*** | 0.92 (0.85‐0.98)*** | no data | 0.83 (0.75‐0.92)*** | ||
|
Older CUHFT |
Antenatal population with positive glucose challenge test† | 17736 | 3764 | Retrospective | NICE‐GDM | 0.81 (0.79‐‐0.83)*** | No data | 0.69 (0.67‐0.71)*** | 0.83 (0.82‐0.85)*** | 0.99 (0.98‐0.99)*** |
| 17736 | 3764 | Retrospective | IADPSG‐GDM | 0.81 (0.79‐‐0.83)*** | No data | 0.79 (0.77‐0.80)*** | 0.93 (0.92‐0.94)*** | 0.85 (0.84‐0.86)*** | ||
|
Diagnosis: unadjusted logistic regression |
OR (95%CI) |
OR (95%CI) |
OR (95%CI) |
OR (95%CI) |
OR (95%CI) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Older CUHFT |
Antenatal population with positive glucose challenge at 24 weeks | 17736 | 3764 | Retrospective | NICE‐GDM | 2.35 (2.24‐‐2.46)*** | No data | 4.84 (4.13‐5.67)*** | 2.29 (2.16‐2.44)*** | 348.82 (200.76‐606.07)*** |
| 17736 | 3764 | Retrospective | IADPSG‐GDM | 2.45 (2.34‐‐2.57)*** | No data | 16.00 (12.98‐19.71)*** | 5.76 (5.11‐6.49)*** | 3.66 (3.35‐4.01)*** | ||
|
Recent CUHFT |
Women with ≥1 GDM risk factor according to the IADPSG criteria |
826 | 826 | Prospective | NICE‐GDM | No data | n/a | n/a | no data | n/a |
| 826 | 826 | Prospective | IADPSG‐GDM | No data |
n/a |
n/a |
no data |
n/a |
||
|
Pregnancy outcomes: unadjusted logistic regression |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Older CUHFT |
Antenatal population with positive glucose challenge at 24 weeks | 17736 | 3764 | Retrospective | LGA infant | 1.10 (1.06‐‐1.13)*** | No data | 1.87 (1.63‐2.14)*** | 1.11 (1.06‐1.15)*** | 1.04 (0.99‐1.10) |
| 17736 | 3764 | Retrospective | CS | 1.15 (1.21‐1.17)*** | No data | 1.48 (1.32‐1.67)*** | 1.10 (1.06‐1.14)*** | 1.11 (1.06‐1.15)*** | ||
| 17736 | 3764 | Retrospective | NH | No data | No data | No data | No data | No data | ||
| 17736 | 3764 | Retrospective | NICU | 1.06 (1.02‐1.11)** | No data | 1.03 (0.82‐1.28) | 1.02 (0.95‐1.10) | 1.05 (0.97‐‐1.14) | ||
|
Recent CUHFT |
Women with ≥1 GDM risk factor according to the IADPSG criteria |
826 | 826 | Prospective | LGA infant | no data | 1.04 (1.00‐‐1.08)* | 1.28 (1.01‐‐1.62)* | No data | 1.02 (0.92‐‐1.14) |
| 826 | 826 | Prospective | CS | no data | 1.04 (1.01‐‐1.08)** | 1.06 (0.88‐‐1.28) | No data | 1.04 (0.95‐‐1.13) | ||
| 826 | 826 | Prospective | NH | no data | 1.05 (1.02‐‐1.08)** | 0.95 (0.79‐‐1.15) | No data | 1.00 (0.92‐‐1.08) | ||
| 826 | 826 | Prospective | NICU | no data | 1.02 (0.98‐‐1.06) | 1.21 (0.94‐‐1.54) | No data | 0.93 (0.83‐‐1.04) | ||
AUROC, area under the receiver‐operating characteristic curve; CS, Caesarean section; CUHFT, Cambridge University Hospital NHS Foundation Trust; FPG, fasting plasma glucose; GDM, gestational diabetes; IADPSG, International Association of Diabetes and Pregnancy Study Groups; LGA, large‐for‐gestational‐age; NICE, National Institute for Health and Care Excellence; NICU, neonatal intensive care unit; NH, neonatal hypoglycaemia; NICU. neonatal intensive care unit; OR, odds ratio.
NH defined as neonatal glucose <2.6 mmol/l at least 4 h after birth. NICU admission defined as >24 h. OPHELIA included 361 women (8.3% with NICE‐defined GDM) all offered standard clinical management. Older CUHFT data included 4.3% of women (4.5% using current NICE GDM criteria) offered standard clinical management. The recent CUHFT data includes women with IADPSG‐defined GDM, all of whom were offered standard clinical management. ORs were calculated using continuous measures. *P < 0.05; **P<0.01; ***P<0.001. †Glucose >7.8 mmol/l, 1 h after a universal 50‐g glucose challenge at 24 weeks' gestation.
FIGURE 1.
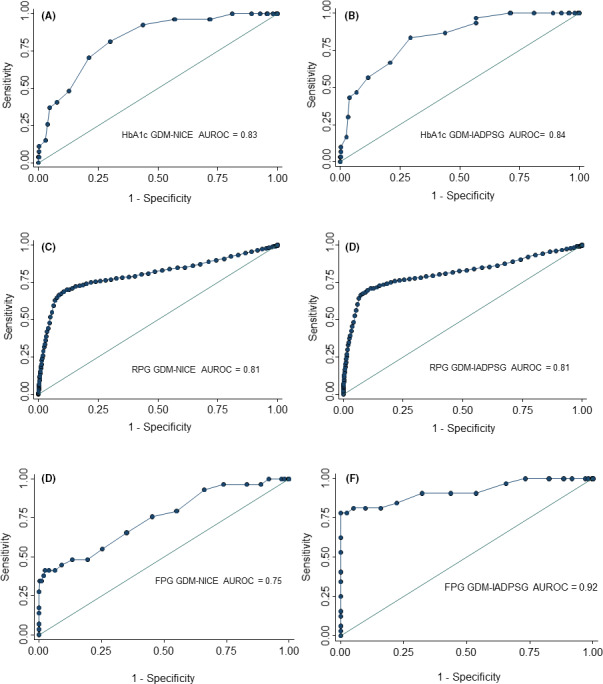
Receiver‐operating characteristic curves for (A & B) HbA1c at 28 weeks, (C & D) random plasma glucose at booking and fasting plasma glucose (FPG) at 28 weeks for predicting gestational diabetes diagnosis according to NICE and IADPSG criteria.
3.2. Assessment of potential thresholds
The sensitivity and specificity of thresholds for random plasma glucose, HbA1c and FPG to predict GDM using NICE or IADPSG diagnostic criteria are given in Table 4. For example, to identify a similar proportion of women as detected by the NICE criteria (~5%) would require a 12‐week random plasma glucose of ≥8.5 mmol/l (42% sensitivity, 96% specificity; older CUHFT cohort), a 28‐week HbA1c of ≥39 mmol/mol (26% sensitivity, 96% specificity; OPHELIA); or an FPG of ≥5.2–5.4 mmol/l (sensitivity 18–41%, specificity 97–98%; OPHELIA or older CUHFT).
3.3. Predictors for diabetes in pregnancy
Fasting plasma glucose at 28 weeks was the best predictor of diabetes in pregnancy [AUROC 0.9 (95% CI 0.86–0.95); older CUHFT), with a specificity of >90% and sensitivity of 50–70% at thresholds of ≥5.2 mmol/l (Table S1). Unfortunately, the number of women with HbA1c >48 mmol/mol was not available and the number with IADPSG‐defined ‘overt diabetes’ was too small (n = 19) to draw any conclusions.
4. DISCUSSION
There are limited data available to support a change in diagnostic criteria for GDM in a real‐world clinical setting. Despite this, our results provide some evidence regarding the use of routine antenatal blood tests, random plasma glucose at 12 weeks' gestation and HbA1c or FPG at 28 weeks' gestation for diagnosing hyperglycaemia in pregnancy during the COVID‐19 pandemic. 5 These established, affordable, widely available laboratory tests have reasonable ability to predict diagnosis of GDM and to identify women at highest risk of suboptimal glycaemic, obstetric and neonatal outcomes. Although the sensitivity of these measures is substantially lower than that of the OGTT, the specificity is sufficient to allow targeted assessment of women at highest risk.
Despite differences between the universal and selective risk factor screening procedures and patient characteristics, the results show consistent associations between routine antenatal glucose measures, GDM diagnosis and clinically relevant obstetric and neonatal outcomes (Caesarean delivery, LGA infant, neonatal hypoglycaemia, neonatal intensive care unit admission). However, the women included in the present study were not blinded to their diagnosis, had GDM diagnosed using different screening pathways and diagnostic criteria, and had no measures of glycaemic control later in pregnancy. The treatment of patients within these clinical datasets will not affect diagnostic predictions but may have reduced associations with maternal or neonatal outcomes. The use of unadjusted regression reflects clinical decision‐making; adjusted odds ratios may show different associations. In addition, these datasets do not have sufficient ethnic diversity to fully represent national or international populations, with more women belonging to higher‐risk ethnic groups. The older CUHFT dataset is large but not all women underwent an OGTT to exclude GDM and none of the datasets used universal screening for GDM with an OGTT at 28 weeks. These results reflect test performance in high‐risk cohorts. The OPHELIA study reflects a high‐risk cohort, chosen according to risk factors 3 (UK approach), while the older CUH cohort reflects a high‐risk cohort chosen from a two‐step strategy (used widely in USA). 5 Although these results are not directly comparable to assessments of test performance in an unselected population, they are comparable to current clinical practice internationally. Although preventing stillbirth is a priority during the pandemic, as this outcome is so rare, the datasets provide insufficient data to allow this outcome to be assessed accurately.
Compared to an OGTT, random plasma glucose, HbA1c and FPG assessments have less evidence to support their use in the diagnosis of GDM. The ability of a first‐trimester OGTT to predict pregnancy outcomes is also unclear. However, random plasma glucose performs surprisingly well as a first‐trimester predictive tool for later GDM diagnosis. 15 An early‐pregnancy random plasma glucose also has consistent associations with Caesarean section, LGA infant, and neonatal intensive care unit admission, but the small odds ratios suggest that random plasma glucose alone lacks precision as a prognostic tool. The performance of and optimal thresholds for random plasma glucose in late pregnancy are unknown. Random plasma glucose was included pragmatically, alongside routine bloods, to minimize the logistical challenges associated with obtaining multiple early‐morning fasted samples within a short timeframe.
Fasting plasma glucose assessment, when performed as part of an OGTT at 24–28 weeks' gestation, has strong associations with the pregnancy outcomes attributed to fetal hyperinsulinism including primary Caesarean delivery, LGA infant and neonatal hypoglycaemia. 18 The necessity of having a test in the fasting state, and therefore in the morning, is associated with non‐attendance. 6 This was pertinent during the pandemic peak, when having all women attend during a small, timeframe was logistically difficult, and challenging for women without private transport. More data are required to better understand whether the requirement for an overnight fast and early‐morning attendance influences uptake of the OGTT, especially among socially disadvantaged women in real‐world clinical settings 6
Unfortunately, although included in the UK, Canadian, Australian and New Zealand recommendations 5 , 11 , 12 , 13 to detect overt and/or pre‐gestational diabetes, we did not have data for HbA1c in early pregnancy. Hughes et al. 19 previously demonstrated that an early‐pregnancy HbA1c ≥ 41 mmol/mol (5.9%) was predictive for diabetes and for identifying mothers and offspring at risk of complications. 19 In the present study, we found that HbA1c ≥39 mmol/mol (5.7%) at 28 weeks' gestation had good specificity (96%) and identified approximately 5% of women who were screened, a comparable number to those identified using the NICE or IADPSG criteria. 3 , 4 Outside pregnancy, HbA1c is widely used both for diabetes diagnosis and glycaemic monitoring, and is highly predictive of diabetes complications. 20 , 21 However, accuracy is reliant on stable red cell turnover and the absence of haematological disease, iron deficiency or inherited haemoglobin variants. 21 , 22 In early pregnancy, red cell turnover increases, contributing to the well‐recognized non‐glycaemic reduction in HbA1c in the late first/early second trimesters. 21 HbA1c is therefore a poor marker for individual glycaemia, but remains important for predicting obstetric and neonatal outcomes including preterm delivery, LGA infant and neonatal intensive care unit admission. 23 The proposed screening strategy includes an early HbA1c measurement to exclude overt diabetes and a 28‐week measurement for pragmatic reasons, with interpretation in conjunction with another glucose measure, either FPG or random plasma glucose.
Although the OGTT is the most widely used test for GDM diagnosis, this recommendation is largely based on research data with optimal pre‐analytical processing and analytical performance within specialist laboratory settings. 30 The OGTT has poor reproducibility in real‐world clinical settings. 24 Non‐pregnant individuals having two OGTTs within 1 week receive the same diagnosis of diabetes, prediabetes, or normal glucose tolerance on 27–80% of occasions. 25 FPG values can vary by 10–30% in adults with normal glucose tolerance. 25 The intra‐individual variation in OGTT glucose is predominantly determined by biological variation in normoglycaemic adults, with 95% of the test–retest variability of <15% for FPG and <46% for 2‐h post OGTT glucose. 26 The diagnostic performance of an OGTT is pertinent during pregnancy, where substantial variability was described by O’Sullivan et al. 27 more than 50 years ago. More recently, seasonal differences in OGTT performance have been highlighted: higher ambient temperatures may increase GDM diagnosis by ~30% in the UK summer. 7 Pre‐analytical processing is also critical: a recent Australian study reported that early centrifugation (<10 min) was associated with almost a doubling in GDM diagnoses. 24
A Canadian study found that almost 50% of women with GDM diagnosed using an OGTT were normoglycaemic using capillary glucose monitoring in daily life, emphasizing concerns about sensitivity. 28 Continuous glucose monitoring reflects fetal exposure to maternal glycaemia during the 24‐h day, providing substantially more detailed glucose measurements compared to an OGTT or capillary glucose monitoring. Preliminary data have identified the continuous glucose monitoring glucose profiles associated with LGA infants in women with GDM, although further research is needed to determine the feasibility and performance of continuous glucose monitoring for GDM diagnosis. 29
Despite its limitations, the OGTT has a strong evidence base in the diagnosis of GDM. 18 The Hyperglycaemia and Adverse Pregnancy Outcomes (HAPO) study demonstrated consistent linear associations between maternal glucose concentrations during an OGTT with pregnancy outcomes. 18 Furthermore, the OGTT identifies women who gain demonstrable benefit from standard clinical management, with reductions in adverse pregnancy outcomes in women with more severe hyperglycaemia and improvements in maternal infant metabolic outcomes in women with ‘milder’ GDM confirmed by high‐quality randomized controlled trial data. 1
The COVID‐19 pandemic has prompted a review of procedures for the screening and diagnosis of hyperglycaemia during pregnancy. Stacey et al. 9 have demonstrated no increase in stillbirth for women who are appropriately screened by OGTT and treated, but screening procedures are variably implemented and many high‐risk women are not screened. The HAPO follow‐up study highlights the longer‐term impact of antenatal hyperglycaemia on the risks of overweight, obesity and diabetes in both mother and child. 30 Women who are appropriately screened and managed for GDM can now have pregnancy outcomes comparable to the background maternity population, but we lack contemporary data on their progression to type 2 diabetes and/or the longer‐term cardio‐metabolic outcomes. After the pandemic, larger population‐based studies should seek to evaluate whether the compliance benefits of having opportunistic tests such as HbA1c and random plasma glucose performed in routine antenatal care settings can reduce the risks of perinatal death, especially among women in socially disadvantaged and higher‐risk ethnic groups. Various diagnostic thresholds at booking and at 28 weeks should be examined (separately and in combination) in relation to pregnancy outcomes alongside longer‐term cardio‐metabolic follow‐up programmes for women with treated GDM and their offspring.
In conclusion, the proposed changes to testing for hyperglycaemia during pregnancy facilitate identification of women at highest risk during the COVID‐19 pandemic peak, but should not be adopted long‐term. Future work should aim to identify pragmatic, evidence‐based alternatives to the OGTT, document the risks and benefits of opportunistic glycaemic testing in women from marginalized patient populations, and address longer‐term maternal and childhood cardio‐metabolic health outcomes.
COMPETING INTERESTS
C.L.M., E.M.S., C.E.A., J.M., R.M.R., D.S., J.M.Y. and D.M. have no relevant conflicts of interest to declare. R.S.L. has received advisory boards/speaker fees for Novo Nordisk, Eli Lilly and Servier. H.R.M. has received honoraria for speaking engagements from Medtronic, Roche, Novo Nordisk, Eli‐Lilly and is a member of the Medtronic European Advisory Board.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank the diabetes in pregnancy team, information management team and Rachel Fox, Benjamin Devoy and Preya Amin at the Cambridge University NHS Foundation Trust who contributed to data‐gathering and quality control for the studies.
Meek CL, Lindsay RS, Scott EM, et al. Approaches to screening for hyperglycaemia in pregnant women during and after the COVID‐19 pandemic. Diabetic Medicine. 2021;38:e14380. 10.1111/dme.14380
Funding information
No specific funding was required for this study. The OPHELIA study was supported by a grant from the European Foundation for the Study of Diabetes ‐ Sanofi grant for innovative outcomes in diabetes (autoantibody pilot study) and subsequently by a National Institute of Health Research (NIHR) Clinical Research Network chief investigator award. The OPHELIA study is supported with staff time from the NIHR Clinical Research Network. H.R.M. conducts independent research supported by the NIHR (Career Development Fellowship, CDF‐2013‐06‐035), and is supported by Tommy’s charity. C.L.M. is supported by the Diabetes UK Harry Keen Intermediate Clinical Fellowship (DUK‐HKF 17/0005712) and the EFSD‐Novo Nordisk Foundation Future Leader’s Award (NNF19SA058974). R.R. acknowledges the support of Tommy's and the British Heart Foundation (RE/18/5/34216).
REFERENCES
- 1. Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. International Association of Diabetes and Pregnancy Study Groups Consensus Panel ; Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA et al. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Institute of Clinical Excellence (NICE) guideline NG3. Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. Available at https://www.nice.org.uk/guidance/ng3. Last accessed 12 June 2020.
- 4. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infect Dis. 2020; PMID: 32240634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thangaratinam S, Saravanan P, Huda MSB, Murphy HR, Williamson C. Guidance for maternal medicine in the evolving coronavirus Covid‐19 pandemic. Available at https://www.rcog.org.uk/globalassets/documents/guidelines/2020‐04‐09‐guidance‐for‐maternal‐medicine‐services‐in‐the‐evolving‐coronavirus‐covid‐19‐pandemic.pdf. Last accessed 12 June 2020.
- 6. Lachmann EH, Fox RA, Dennison RA, Usher‐Smith JA, Meek CL, Aiken CE. Barriers to completing oral glucose tolerance testing in women at risk of gestational diabetes. Diabet Med. 2020; 10.1111/dme.14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meek CL, Devoy B, Simmons D, et al. Seasonal variations in incidence and maternal‐fetal outcomes of gestational diabetes. Diabet Med. 2020;37:674‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sovio U, Murphy HR, Smith GC. Accelerated Fetal Growth Prior to Diagnosis of Gestational Diabetes Mellitus: A Prospective Cohort Study of Nulliparous Women. Diabetes Care. 2016;39:982‐987. [DOI] [PubMed] [Google Scholar]
- 9. Stacey T, Tennant P, McCowan L, et al. Gestational diabetes and the risk of late stillbirth: a case‐control study from England, UK. BJOG. 2019;126:973‐982. [DOI] [PubMed] [Google Scholar]
- 10. Draper ES, Kurinczuk JJ, Kenyon S. MBRRACE‐UK . 2015. Perinatal Confidential Enquiry: Term, singleton, normally‐formed, antepartum stillbirth. Available at https://www.npeu.ox.ac.uk/downloads/files/mbrrace‐uk/reports/MBRRACE‐UK%20Perinatal%20Report%202015.pdf. Last accessed 12 June 2020.
- 11. Diagnostic Testing for Gestational diabetes mellitus (GDM) during the COVID 19 pandemic: Antenatal and postnatal testing advice. A statement from the Australasian Diabetes in Pregnancy Society (ADIPS), the Australian Diabetes Society (ADS), the Australian Diabetes Educators Association (ADEA), and Diabetes Australia (DA). Available at https://www.adips.org/documents/COVID‐19GDMDiagnosis030420ADIPSADSADEADAforWebsite.pdf. Last accessed 12 June 2020.
- 12. Screening for GDM during COVID restrictions – Recommendations from New Zealand Society for the Study of Diabetes. Available at https://www.nzssd.org.nz/. Last accessed 12 June 2020.
- 13. Yamamoto JM, Donovan LE, Feig DS, Berger H. Urgent Update – Temporary Alternative Screening Strategy for Gestational Diabetes Screening During the COVID‐19 Pandemic: A Joint Consensus Statement from the Diabetes Canada Clinical Practice Guidelines Steering Committee* and the Society of Obstetricians and Gynecologists of Canada. Available at https://els‐jbs‐prod‐cdn.jbs.elsevierhealth.com/pb/assets/raw/Health%20Advance/journals/jcjd/JCJD_COVID_guidelines_020420‐1585856697530.pdf. Last accessed 12 June 2020.
- 14. Aiken CEM, Hone L, Murphy HR, Meek CL. Improving outcomes in gestational diabetes: does gestational weight gain matter? Diabet Med 2019; 36: 167–176. [DOI] [PubMed] [Google Scholar]
- 15. Meek CL, Murphy HR, Simmons D. Random plasma glucose in early pregnancy is a better predictor of gestational diabetes diagnosis than maternal obesity. Diabetologia. 2016;59:445‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Institute for Health and Care Excellence (NICE) . Clinical Guideline 63. Diabetes in pregnancy: Management of diabetes and its complications from pre‐conception to the postnatal period. London: NICE, 2008. [Google Scholar]
- 17. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477‐2486. [DOI] [PubMed] [Google Scholar]
- 18. HAPO Study Cooperative Research Group ; Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991‐2002. [DOI] [PubMed] [Google Scholar]
- 19. Hughes RC, Moore MP, Gullam JE, Mohamed K, Rowan J. An early pregnancy HbA1c ≥5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care. 2014;37:2953‐2959. [DOI] [PubMed] [Google Scholar]
- 20. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837‐853. [PubMed] [Google Scholar]
- 21. World Health Organisation . Use of glycated haemoglobin (HbA1C) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. Available at: http://www.who.int/cardiovascular_diseases/report‐hba1c_2011_edited.pdf. Last accessed 12 June 2020. [PubMed]
- 22. Panzer S, Kronik G, Lechner K, Bettelheim P, Neumann E, Dudczak R. Glycosylated hemoglobins (GHb): an index of red cell survival. Blood. 1982;59:1348‐1350. [PubMed] [Google Scholar]
- 23. Murphy HR. Intensive Glycemic Treatment During Type 1 Diabetes Pregnancy: A Story of (Mostly) Sweet Success! Diabetes Care. 2018;41:1563‐1571. [DOI] [PubMed] [Google Scholar]
- 24. Potter JM, Hickman PE, Oakman C, Woods C, Nolan CJ. Strict Preanalytical Oral Glucose Tolerance Test Blood Sample Handling Is Essential for Diagnosing Gestational Diabetes Mellitus. Diabetes Care. 2020;43:1438‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olefsky JM, Reaven GM. Insulin and Glucose Responses to Identical Oral Glucose Tolerance Tests Performed Forty‐eight Hours Apart. Diabetes. 1974;23:449. [DOI] [PubMed] [Google Scholar]
- 26. Mooy JM, Grootenhuis PA, de Vries H, et al. Intra‐individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia. 1996;39:298‐305. [DOI] [PubMed] [Google Scholar]
- 27. O'Sullivan JB, Mahan CM. Glucose tolerance test. Variability in pregnant and nonpregnant women. Am J Clin Nutr. 1966;19:345‐351. [DOI] [PubMed] [Google Scholar]
- 28. Ardilouze A, Bouchard P, Hivert MF, Simard C, Allard C, Garant M‐P et al. Self‐Monitoring of Blood Glucose: A Complementary Method Beyond the Oral Glucose Tolerance Test to Identify Hyperglycemia During Pregnancy. Can J Diabetes. 2019;43:627‐635. [DOI] [PubMed] [Google Scholar]
- 29. Law GR, Alnaji A, Alrefaii L, et al. Suboptimal Nocturnal Glucose Control Is Associated With Large for Gestational Age in Treated Gestational Diabetes Mellitus. Diabetes Care. 2019;42:810‐815. [DOI] [PubMed] [Google Scholar]
- 30. Lowe WL Jr, Scholtens DM, Lowe LP, et al. Association of Gestational Diabetes With Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA. 2018;320:1005‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Church D, Halsall D, Meek C, Parker RA, Murphy HR, Simmons D. Random blood glucose measurement at antenatal booking to screen for overt diabetes in pregnancy: a retrospective study. Diabetes Care. 2011;34:2217‐2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


