Thrombolysis with tissue plasminogen activator (tPA) is an established treatment strategy for patients with intermediate and high‐risk pulmonary embolism (PE) and signs of haemodynamic instability. The use of tPA in coronavirus disease 2019 (COVID‐19) patients with PE and acute respiratory distress syndrome (ARDS) may be of benefit due to the unusually high incidence of pulmonary embolism and pulmonary thrombosis, particularly microvascular thrombosis, which are thought to contribute significantly to hypoxaemia. 1 It may also ameliorate the effects of extravascular and intra‐alveolar fibrin deposition described in ARDS. 2 Inhaled delivery of tPA and two doses of tPA against placebo 3 are currently under trial. Small case series have reported transient improvements in oxygenation without significant bleeding from systemic fibrinolytic therapy in patients with ARDS and COVID‐19. 4 Here, we describe the largest cohort to date of patients with COVID‐19 treated with alteplase for severe hypoxia. This retrospective observational study was approved by the institutional review board as a service evaluation project and no further ethical approval was required.
All alteplase prescriptions from 17 April 2020 to 25 May 2020 were retrieved from pharmacy electronic records and those used for COVID‐19 were identified. Clinical and laboratory parameters were extracted from patient electronic records. Statistical analyses were performed using GraphPad Prism v8·4 (GraphPad Software, San Diego, CA, USA). Descriptive statistics were used to summarize the data; results are reported as medians and ranges or means and standard deviations, as appropriate. Categorical variables were summarized as counts and percentages. Pre‐ and post‐thrombolysis parameters were compared using a paired t‐test. A two‐sided P value < 0·05 was considered statistically significant.
During the study period, 12 patients received thrombolysis with alteplase for profound hypoxia on mechanical ventilation (except patient 5 on continuous positive airway pressure) and failed proning, with or without evidence of pulmonary thrombosis on computed tomography pulmonary angiography (CTPA). Baseline demographic features, clinical history, CTPA and echocardiographic findings prior to thrombolysis, dose of alteplase with infusion time and days since admission to thrombolysis are summarized in Table I. Only one patient 4 was on antithrombotic therapy (warfarin) and aspirin prior to admission due to a previous history of left apical mural thrombus. None of the patients had previous malignancy or autoimmune disease. Median (range) age of the group was 61·5 (51–75) years and 7/12 patients were male. Median duration from admission to thrombolysis was nine days (range 2–22). All patients received therapeutic heparin pre and post thrombolysis. Five of the 12 patients had multiorgan failure (defined as failure of two or more organ supports) and required renal replacement therapy. All except one (patient 5 on continuous positive airway pressure) were retained on mechanical ventilation following thrombolysis. The decision to use thrombolysis was made due to moderate to severe hypoxia with ratios of arterial pressure to inspired oxygen (PaO2/FiO2, PF ratio) <200 mm Hg on mechanical ventilation and failing all other interventions including proning and nitrates.
Table I.
Patients’ demographic features, clinical history and the time since hospital admission to thrombolysis.
| Patient |
Age (yrs) /sex |
BMI | Ethnicity |
Smoking |
Medical history | CTPA findings prior to thrombolysis | ECHO findings pre thrombolysis | Dose of alteplase | Days from admission to thrombolysis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 66/F | 32·2 | Black(B/A) | No | Type 2 DM, HT | B/L multiple PE | RV systolic function impaired pressure overload of RV. Possible large RA thrombus | 10 mg bolus + 90 mg over 2 h | 9 |
| 2 | 53/F | 31·1 | Asian | No | Fatty liver | Right‐sided PE, no evidence of right heart strain |
Mildly dilated RV with good systolic function. No gross right heart strain |
10 mg bolus + 90 mg over 2 h |
8 |
| 3 | 75F | 32·6 | Asian | No | HT | B/L segmental PE |
Dilated RV and right heart strain |
50 mg over 90 mins | 9 |
| 4 | 60/M | 29·2 | Asian | No | Type 2DM, HI, IHD | B/L multiple small PE |
Right heart strain with raised RV pressures — TR max PG 17 mm Hg TAPSE 19 |
10 mg bolus + 90 mg over 2 h | 9 |
| 5 | 67/M | 18·8 | White | Yes | IHD | Massive B/L & evidence of right‐sided heart strain |
Mildly dilated left ventricle by volume. LV systolic function severely impaired (LVEF ~ 20%) Large LV echogenic structure measuring 8·33 cm × 5·6 cm consistent with thrombus Dilated RV with moderately impaired systolic function. Mild AR and MR. Mild to moderate TR. Estimated PASp = 49 mm Hg |
10 mg bolus + 90 mg over 2 h | 2 |
| 6 | 52/M | 34·0 | Asian | No | Type 2 DM, HT, hypercholesterolaemia | 'Presumed PE' no scan |
Dilated RV severely impaired systolic function with volume and pressure overload. Estimated PASP of 50 mm Hg |
10 mg bolus + 90 mg over 2 h | 19 |
| 7 | 69/F | 36·0 | Asian | No | Not significant | PE within the distal right main pulmonary artery extending to the right upper and middle lobe pulmonary arteries. There is some straightening of the interventricular septum and the RV:LV is high at 1·2 |
Mild LVH, good LV function RV mildly dilated: Mild TR |
50 mg over 90 min | 8 |
| 8 | 63/F | 31·0 | Black(B/A) | No |
Impaired glucose tolerance Asthma; bronchiectasis; pulmonary HTN |
Enlarged main pulmonary artery peripheral embolus in upper lobe on the left increased ground glass opacification more dense consolidation in dependent areas |
Dilated RV with evidence of RV strain Moderate TR. Estimated PASP 64–69 mm Hg. Dilated IVC size (2.2 cm) |
50 mg over 90 min | 24 |
| 9 | 59/M | 39·1 | Black(B/A) | Not recorded | Eczema; obesity | Left lower lobe segmental PE. Smaller subsegmental Pes obscured by the grossly abnormal lungs. Evidence of right heart strain | Dilated RV and right heart strain | 50 mg over 90 min | 21 |
| 10 | 57/M | 35·4 | Black(B/A) | Not recorded | Type 2 DM, HT, hypercholesterolaemia | Presumed PE based on ECHO | RV massively dilated, moderate‐severe RV pressure and volume overload, signs of LV intracavitary compromise. Unbalanced circulation. presumed PE |
50 mg over 90 min |
13 |
| 11 | 64/M | 32·2 | Black(B/A) | No | Type 2 DM, | Bilateral, multiple pulmonary embolism | Dilated RV and right heart strain | 50 mg over 90 min | 9 |
| 12 | 51/M | 31·1 | Asian | No | Not significant | Left‐sided PE, no evidence of right heart strain | Volume and some pressure overload of RV and Possible large RA thrombus | 10 mg bolus + 90 mg over 2 h | 11 |
B/A, British or African; M, Male; F, Female; BMI, body mass index (weight/height2); DM, diabetes mellitus; HT, hypertension; IHD, ischaemic heart disease; B/L, bilateral; PE, pulmonary embolism; LV, left ventricle; RV, right ventricle; RWMA, reginal wall motion abnormality, TAPSE, tricuspid annular plane systolic excursion; RA, right atrial; TR, tricuspid regurgitation; PG, pressure gradient; AR, aortic regurgitation; MR, mitral regurgitation; PASp, pulmonary artery systolic pressure; LVH, left ventricular hypertrophy.
PF ratios pre and 24 h post thrombolysis are shown in Fig 1, which showed a significant improvement in all patients (P = 0·002). Only three patients had a follow‐up CTPA and echocardiogram. These showed marked improvement in thrombotic occlusions and right ventricular strain (patients 5, 11 and 12). Seven patients survived to hospital discharge whilst others died from 2 to 11 days following thrombolysis due to multiorgan failure (patient 2, 3 4, 6 and 9). Overall mortality was 41·67%.
Fig 1.
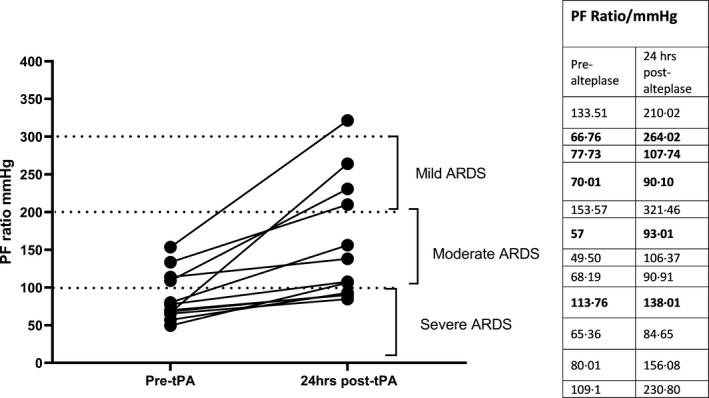
PF ratio pre and 24 h after thrombolysis with alteplase. PF ratios of five patients who died are presented in bold numbers.
Twenty‐four hours after thrombolysis, median fibrinogen level fell from 7·0 (range 4·95–8·9) g/l to 3·40 (2·50–6·30) g/l (P = 0·03) and median D‐dimer level increased from 3502 (range 862–9929) ng/ml to 19450 (11495–>20000) (P = 0·002). There were no differences in haemoglobin, platelet count, C‐reactive protein, prothrombin time, activated partial thromboplastin time, renal or liver function tests pre and 24 h post thrombolysis.
There were no major or clinically significant minor bleeding complications of thrombolysis. However, one patient had intracranial bleeding 17 days after thrombolysis whilst on unfractionated heparin.
This report comprises the largest cohort to date of patients with severe COVID‐19 treated using alteplase. The indication in each case was progressive hypoxia with or without evident PE, despite mechanical ventilation (one on continuous positive airway pressure), nitrates, therapeutic anticoagulation and proning. The median PF ratio prior to thrombolysis was 73·87(range 49·5–153·57) mm Hg which improved to 122·86 (range 84·65‐321·46) mm Hg 24 h following thrombolysis (Fig 1). Previous studies of ARDS have shown a PF ratio of < 100 mm Hg is associated with a mortality rate of 53%, and 40% for a ratio of 100–300. 5 This group, therefore, had a very poor prognosis but current mortality rates for COVID‐19 after ICU admission are even higher, being 88% in a large series. 6 The mortality in this small series was 41·6% and assuming an expected mortality of 75% 3 in patients requiring advanced respiratory and renal support based on the intensive care national audit and research report (ICNARC) represents a significant improvement (P = 0·01, χ2). The rationale for use of tPA is straightforward. Patients with COVID‐19 have a systemic hypercoagulable state with an incidence of venous thrombosis (VT) and PE that exceeds that seen in other pneumonias. In addition, there appears to be gross inflammation of endothelium leading to inflammation‐driven local pulmonary thrombosis in medium and small vessels. Large and small thrombi cause vascular shunting and hypoxaemia which may be relieved by clot lysis. In addition, inflammation results in a vascular leak allowing extravascular fibrin formation in lung parenchyma tissue and in alveoli with associated hypofibrinolytic shutdown. 7 Therefore, improvement of the PF ratio following alteplase may reflect both improvement in alveolar perfusion and ventilation. In the absence of significant bleeding with preserved fibrinogen levels 24 h post thrombolysis and a significantly improved PF ratio this report suggests that fibrinolysis may be beneficial in a carefully selected group of patients with close monitoring. Therapeutic strategies targeting the pulmonary circulation in COVID‐19 require a multimodal approach. Thrombolysis in a carefully selected group of patients may be beneficial as shown in our cohort of patients. However, with the profound immune activation and intravascular thrombosis, and the short half‐life of thrombolytic agents, the lack of a sustained effect and absence of a control group are potential concerns. Randomized clinical trials with larger numbers may provide more solid evidence.
Funding information
No funding was received for this work.
Author contributions
DRJA was involved in the study concept and design, analysis and interpretation of data, review of the literature and prepared the first draft of the manuscript. FA and AS collected the data and reviewed the manuscript. MLinterpreted the data, reviewed the literature and revised the manuscript. MS interpreted the data and reviewed the manuscript. All authors approved the final version of the manuscript.
Conflicts of interest
The authors state that they have no conflict of interest.
Acknowledgements
Authors would like to thank all clinical staff at adult intensive care units, haemostasis and thrombosis consultants on call, the radiology department and the cardiology unit at St Mary’s Hospital, Imperial College Healthcare NHS Trust. Infrastructure support was provided by the National Institute for Health Research (NIHR) Imperial Biomedical Research Centre (BRC).
References
- 1. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383(2):120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buja LM, Wolf DA, Zhao B, Akkanti B, McDonald M, Lelenwa L, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID‐19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. clinicaltrials.gov NCT04357730 [.
- 4. Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue plasminogen activator (tPA) treatment for COVID‐19 associated acute respiratory distress syndrome (ARDS): A case series. J Thromb Haemost. 2020;18(7):1752–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Villar J, Blanco J, del Campo R, Andaluz‐Ojeda D, Díaz‐Domínguez FJ, Muriel A, et al. Assessment of PaO₂/FiO₂ for stratification of patients with moderate and severe acute respiratory distress syndrome. BMJ Open. 2015;5(3):e006812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized With COVID‐19 in the New York City Area. JAMA. 2020;323(20):2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel BV, Arachchillage DJ, Ridge CA, Bianchi P, Doyle JF, Garfield B, et al. Pulmonary angiopathy in severe COVID‐19: physiologic, imaging and hematologic observations. Am J Respir Crit Care Med. 2020. 10.1164/rccm.202004-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


