Short abstract
LINKED CONTENT
This article is linked to Taxonera et al papers. To view these articles, visit https://doi.org/10.1111/apt.15804 and https://doi.org/10.1111/apt.15955
1.
Editors,
With interest, we read the article of Taxonera et al on symptoms and the risk of COVID‐19 in inflammatory bowel disease (IBD). 1 We would like to provide data from our IBD centre during the outbreak of SARS‐CoV‐2 concerning patients receiving immunotherapies.
When SARS‐CoV‐2 spread pandemically, 2 , 3 the first German patient was described in January 28th in Munich, 4 which later became a hotspot 5 with an infection rate of 0.40% and a mortality of 2.9% in May 2020, particularly among old and sick patients. 2 Therefore, soon two questions raised great concern: are IBD patients who receive immunotherapies more susceptible to SARS‐CoV‐2 infections than the general population and are infected patients exposed to a more severe course?
Our tertiary outpatient clinic oversees more than 1200 IBD patients. According to the suggestions of the ECCO in March 2020, 6 we decided to continue our regular ambulatory service for all patients and to start an observational study. Only appointments of patients who felt un‐well, reported fever, cough or had contact with SARS‐CoV‐2‐positive patients, were postponed for at least 2 weeks.
Since the estimated number of silent SARS‐CoV‐2 infections is high 7 and most individuals show only moderate symptoms of respiratory tract infection, we called or invited our patients to fill‐in a questionnaire, 8 asking whether they had any of those symptoms since the start of the pandemic (January 1st until May 20th) (Figure 1), and inquiring about hospitalisations, contact with SARS‐CoV‐2 patients, results of SARS‐CoV‐2 swab tests and IBD‐related information.
FIGURE 1.
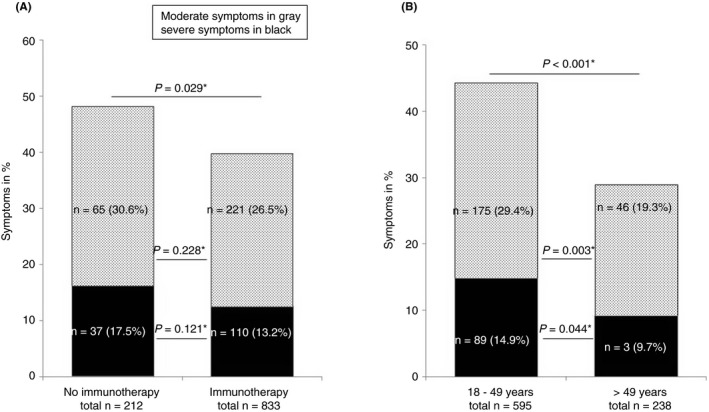
Symptoms of respiratory tract infections: moderate in grey (cough, rhinitis and sore throat) and severe in black (fever, chills, anosmia) in (A) patients with or without immunotherapy and (B) patients with immunotherapy according to their age. *two‐sided Fisher's exact test
Until May 20th, we had contacted 1172 patients, of whom 1045 had successfully completed the questionnaire. Of those, 51.5% had Crohn's disease, 48.5% had ulcerative or indeterminate colitis and 52.1% were females. The median age was 39 years (18‐89), and 81.8% of all patients were in remission as defined by CDAI and MAYO scores. Immunotherapies were given in 79.7%, consisting mainly of biologic therapies (anti‐TNF‐alpha 42.0%, ustekinumab 13.0%, vedolizumab 11.7%, biologic combinations 11.4% and immunosuppressants or systemic steroids 1.6%), 20.3% received no therapy, mesalazine or topical steroids. In 20 patients, immunotherapy was started for the first time between March and May 2020.
Symptoms of respiratory tract infections occurred less frequently in IBD patients with immunotherapies as compared with those without (Figure 1A), and occurred less frequently in older (>49 years) than in younger patients with immunotherapies (Figure 1B).
Of all patients, 55 had received a SARS‐CoV‐2 swab test and five were positive with severe symptoms (0.49% of all participating patients): Three received regular infliximab therapy (32, 45 and 53 years), a 45‐year old received ustekinumab (90 mg) and azathioprine (50 mg/d). A 49‐year old had no immunotherapy. All five were home quarantined, their course remained uneventful and immunotherapies were continued.
Our registry data show that even in an endemic area, IBD patients receiving immunotherapies have no increased risk for respiratory tract and SARS‐CoV‐2 infections. Immunotherapies do not expose older people to a risk of respiratory tract infections. The course of SARS‐CoV‐2 infections in IBD patients receiving immunotherapies was mild and uneventful.
Our data confirm the recommended practice that immunotherapies should not be stopped or delayed during the COVID‐19 crisis.
AUTHORSHIP
Guarantor of the article: Thomas Ochsenkühn.
Author contributions: Thomas Ochsenkühn, Cornelia Tillack‐Schreiber, Constanze Waggershauser, Daniel Szokodi and Christine Berchtold‐Benchieb performed the research, Thomas Ochsenkühn, Stefanie Howaldt and Constanze Waggershauser collected and analysed the data, Thomas Ochsenkühn and Constanze Waggershauser designed the research study and wrote the paper, and Stefanie Howaldt contributed to the design of the study. All authors approved the final version of the manuscript.
REFERENCES
- 1. Taxonera C, Sagastagoitia I, Alba C, Mañas N, Olivares D, Rey E. 2019 novel coronavirus disease (COVID‐19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2020;52:276‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morens DM, Daszak P, Taubenberger JK. Escaping Pandora's Box ‐ another novel coronavirus. N Engl J Med. 2020;382:1293‐1295. [DOI] [PubMed] [Google Scholar]
- 4. www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Fallzahlen.html. 2020. Accessed January 28, 2020
- 5. www.covid19.who.int. 2020. Accessed March 20, 2020
- 6. COVID‐19 information – eNews. 2020/023/13. 2020. www.ecco‐ibd.eu/publications/covid‐19.html. Accessed March 13, 2020
- 7. Raj K, Rohit, Ghosh A, Singh S. Coronavirus as silent killer: recent advancement to pathogenesis, therapeutic strategy and future perspectives. Virusdisease. 2020;1‐9. 10.1007/s13337-020-00580-4. Epub:2020/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. CEDUR ‐registry . Ethikkommission der Ärztekammer Hamburg. PV 5539. 2018.
ACKNOWLEDGEMENTS
Declaration of personal interests: Thomas Ochsenkühn and Stefanie Howaldt have served as speaker, consultants and advisory board member for Abbvie, Amgen, Biogen, Janssen, MSD, Pfizer, Sandoz and Takeda. Cornelia Tillack‐Schreiber has served as a speaker and a consultant for Pfizer and Takeda. Daniel Szokodi has served as a speaker for Pfizer. All other authors have no conflicts to declare. There was no funding for this study.


