Abstract
Background:
Acetylcholinesterase inhibitors (AChEis) including donepezil, galantamine and rivastigmine are used to treat Alzheimer’s disease (AD). This study aimed to evaluate evidence from the case report literature for an association between these agents and risk of QT interval prolongation and Torsades de Pointes (TdP) arrhythmia.
Methods:
Published literature was mined with predetermined MeSH terms for each of donepezil, galantamine and rivastigmine, to identify cases of QT interval prolongation and TdP. Case reports were analysed using causality scales and a QT interval nomogram.
Results:
A total of 13 case reports were found (10 for donepezil, 2 for galantamine and 1 for rivastigmine) with rate corrected QT interval (QTc) prolongation. Five cases with donepezil exhibited TdP. TdP was not reported in the cases with galantamine and rivastigmine. The use of a QT heart rate nomogram highlighted risk with donepezil compared with the other two drugs and the application of the Naranjo causality scale suggested probable or possible causation for all donepezil cases. All patients had at least two other risk factors for TdP, including modifiable risk factors such as electrolyte disturbances, bradycardia, co-administration of QT prolonging drugs. A number of recent cases involved recent changes in medication.
Conclusion:
Our evaluation of the case report literature suggests that there is evidence for a causal association between donepezil and QTc/TdP risk. Attention to risk factors for QTc prolongation/TdP should be exercised when prescribing donepezil and modifiable risk factors corrected. Owing to the low number of cases with galantamine and rivastigmine, further work is needed to establish whether these drugs may be more suitable than donepezil for patients with other risk factors for TdP.
Keywords: acetylcholinesterase inhibitor, Alzheimer’s disease, donepezil, galantamine, hERG, long QT, rivastigmine, Torsades de Pointes
Plain language summary
Evaluation of the link between Alzheimer’s drugs and altered electrical activity of the heart
Alzheimer’s disease (AD) is responsible for most cases of dementia. A loss of nerve cells in the brain leads to memory loss and impaired cognition. Current AD treatments aim to optimise the communication between the remaining nerve cells in key parts of the brain. They do this by helping increase levels of chemicals called neurotransmitters that are responsible for nerve cell communication. One group of such drugs, called acetylcholinesterase inhibitors, increases brain levels of a neurotransmitter called acetylcholine (ACh). The three main drugs in this class are donepezil, galantamine and rivastigmine. This study investigated evidence in the literature associating these drugs with unwanted effects on the heart that may predispose to dangerous disturbances (‘arrhythmias’) to the normal cardiac rhythm, by slowing the speed with which heart tissue recovers from electrical excitation. Our analysis suggests that data from medical case reports are consistent with some ability of donepezil to delay electrical recovery from electrical excitation and produce arrhythmia, particularly in patients with other risk factors that may increase arrhythmia susceptibility. Information from preclinical studies indicates that this may arise from an off-target interaction of donepezil with a particular protein that is involved in generating cardiac electrical activity. A low number of reports with galantamine and rivastigmine precluded firm conclusions in respect of these drugs; further experimental work is warranted to determine whether, in some settings, either of these drugs may offer a safer treatment alternative to donepezil.
Introduction
Approximately 24 million people globally have dementia, with Alzheimer’s disease (AD) likely accounting for the majority of cases.1 The prevalence of AD in Europe has been estimated to be 5.05%, affecting 3.31% of men and 7.13% of women.2 The condition is a major cause of morbidity and mortality and constitutes a significant burden on public health systems.1,3 The underlying aetiology of AD has not been fully elucidated; however, the ‘cholinergic hypothesis’ is considered to account, at least in part, for the progression of the condition.1,4 This proposes that a loss of cholinergic neurons leads to decreased transmission within areas in the brain, leading to memory loss and cognitive decline.4 Therefore, by increasing central levels of acetylcholine (ACh), the amount of transmission between the remaining cholinergic neurons in the brain of an AD patient is maximised and progression of the disease slowed.5 ACh is normally rapidly hydrolysed by acetylcholinesterase (AChE), however inhibition of this process can be expected to increase ACh levels.6,7
Drugs in the AChEi class include donepezil, galantamine and rivastigmine, which increase levels and action of ACh.8 Together these drugs form the mainstay of treatment for people suffering with AD, acting to slow the progression of cognitive decline. The NDMA receptor antagonist memantine is used only in very severe AD.8 The majority of drugs that enter the drug development pipeline for AD have failed, with memantine being the only notable success in terms of approval since 2004.9 Most specific phase II and phase III disease modification trials10 target amyloid; however, AChEis remain critically important for cognitive enhancement in current clinical practice. Donepezil is a non-competitive, reversible inhibitor of AChE,8 metabolised by cytochrome p450 enzymes CYP3A4 and CYP2D6.11 It is licensed for treatment of AD at therapeutic doses of 5 and 10 mg a day.12 Galantamine is a competitive, reversible inhibitor of AChE13 that also boosts ACh action by binding to nicotinic acetylcholine receptors (nAChRs), increasing the receptor response to ACh.14,15 Similar to donepezil, it is also metabolised by cytochrome p450 enzymes CYP3A4 and CYP2D6.16 Galantamine is licensed using doses of 4–24 mg a day.17,18 Rivastigmine is a pseudo-irreversible competitive inhibitor of AChE and acts by binding to AChE and undergoing hydrolysis, leaving the binding site inactivated for several hours.19,20 It does not undergo hepatic enzyme metabolism, and so of the three drugs is the least likely to have metabolic interactions with co-administered drugs.21,22
The most common side effects reported by patients taking AChEi drugs are gastrointestinal problems.23 However, increasing evidence suggests that AChEis can also be associated with cardiac side effects in patients: bradycardia, syncope, QT prolongation and Torsades de Pointes (TdP).23–27 Bradycardia with AChEis is unsurprising given that increased levels of ACh in the heart increase vagal tone leading to a decrease in heart rate.26 This increase in parasympathetic activity has also been linked to syncope in AD patients.24 Bradycardia has long been linked to AChEi therapy, with phase I and II clinical trials of donepezil showing a mean fall in heart rate of 1.2 bpm compared with control groups, despite patients with a history of bradycardia and syncope being excluded from the trials.28–30 A potential association between AChEis and TdP arrhythmia has been suggested previously.25,26,31 Examination of publicly available information on the Eudravigilance database32 indicates that, as of October 2019, donepezil has been associated with 46 reports of TdP, with one fatality, the majority of incidents occurring in elderly females (65 years or older). The same database has 14 reports of TdP with galantamine (all in individuals 65 years of age or older, with a majority of cases in females) and 4 reports of TdP with rivastigmine (all cases 65 or older and three-quarters in females). The aim of the present study was to evaluate the evidence in the peer-reviewed literature, with a particular focus on case reports that link TdP and rate corrected QT (QTc) interval with these three treatments for AD.
Methods
Literature evaluation
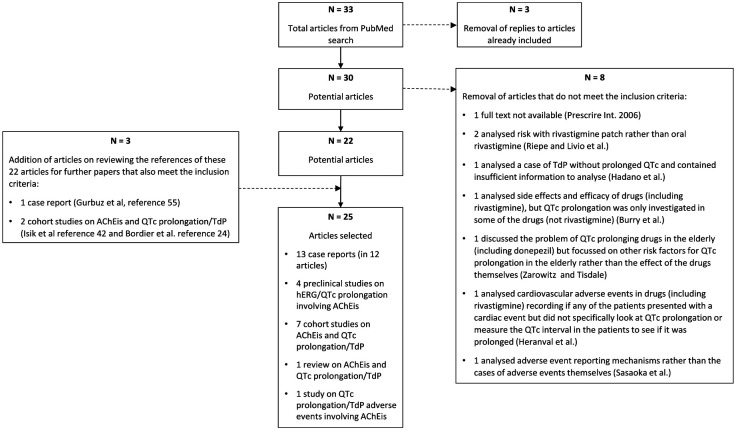
An analysis of the preclinical literature and of cohort and published case studies was conducted using PubMed to probe involvement of QT interval prolongation with AChEis. A combined search term was used that yielded 33 hits up to the time of submission of this article: ((hERG) OR (QT) OR (QTc) OR (torsad*)) AND ((donepezil) OR (aricept) OR (galantamine) OR (reminyl) OR (rivastigmine) OR (exelon)). Literature searches were initially conducted between 22 November 2017 and 7 January 2018 and repeated during the month of October 2019. Figure 1 summarises how hits from this literature search were filtered to arrive at the reports considered here. For inclusion: the full text of identified articles had to be available; case reports had mentioned QTc interval prolongation (with or without TdP occurrence), with sufficient information for analysis/evaluation; studies using adverse event databases for cases of QTc prolongation/TdP with any of the three drugs were acceptable; for cohort studies drug effects on QT/QTc interval must have been investigated for at least one of the three drugs; for consistency in the case of rivastigmine, the drug must have been administered orally and not by dermal patch; preclinical studies involving any of the three drugs on hERG channel inhibition or ECG repolarisation parameters were acceptable. Initial filtering was performed by KM as shown in the flowchart in Figure 1. As shown in this diagram, 3 studies included for analysis were identified from scrutiny of the reference lists of 22 articles selected through the literature search and filtering. Both authors reviewed abstracts of potential case and cohort studies and the reports finally selected for evaluation. This process yielded 13 case reports (in 12 articles) up to October 2019. All except one report was in English: the Shinozaki33 case involving donepezil was translated from Japanese.
Figure 1.
Flow diagram illustrating the steps adopted during literature evaluation that led to the selection of the literature evaluated in this report. This process applies to literature evaluated up to October 2019. PubMed IDs for the eight articles excluded are: Prescrire Int, 2006 (PMID: 16764099); Riepe, 2014 (PMID: 24717382); Livio et al., 2011 (PMID: 21309181); Hadano et al., 2013 (PMID: 30546746); Burry et al., 2019 (PMID: 31479532); Zarowitz and Tisdale, 2019 (PMID: 30747995); Heranval et al., 2016 (PMID: 27063094); Sasaoka et al., 2016 (PMID: 27723808).
Causality evaluation
Causality was evaluated using both the Naranjo scale34 and the World Health Organization (WHO) Uppsala Monitoring Centre (UMC) causality assessment.32 These evaluation tools are composed of questionnaires, applied here to each case in turn, to evaluate the likelihood of a causal link between the administration of each AChEi and the unwanted/adverse drug events of interest, namely QT/QTc interval prolongation and TdP. Causality assignments were reached by consensus. The Naranjo et al. method34 is based on empirically weighted answers to 10 questions, each of which has ‘yes’, ‘no’ or ‘don’t know’ answer options, with possible numerical scores of –1, 0, +1 or +2. The sum of the overall numerical scores (over a possible range from –4 to +13) indicates the strength of the causal relationship: ‘doubtful’, ‘possible’, ‘probable’ and ‘definite’.34 The WHO-UMC causality assessment is based on tabulated causality terms: Certain, Probable/Likely, Possible, Unlikely, Conditional/Unclassified, Unassessable/Unclassifiable. Assessment criteria matching each of these terms are defined.32 Causality assignments were reached by consensus. Potential limitations of these causality evaluation tools are considered in the discussion.
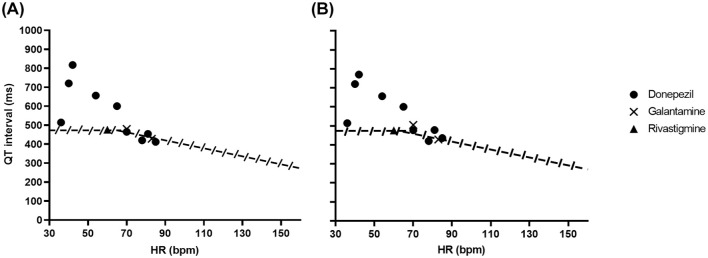
QT interval prolongation in the identified case reports was also interrogated using a QT nomogram.35,36 Chan et al. originally evaluated known cases of TdP and used the upper bound of a QT-RR cloud from human preclinical studies to develop the nomogram. With this, plotted QT-RR (or heart rate) values above the nomogram line are considered to represent an abnormally long QT interval.35 Waring et al. subsequently showed that this approach was more reliable than widely accepted QTc criteria in detecting QT interval prolongation with antidepressant drugs in overdose.36 The advantage in the use of the QT nomogram is that it takes into account the inherent rate dependence of the QT interval, without the potential for inaccurate rate correction at slow or fast heart rates.37 This is an important consideration for drugs that may produce bradycardia. For a number of the case reports examined here, QTc but not QT interval data were available and in those cases QT interval was derived using both Bazett’s and Fridericia’s correction formulae, with the two resulting nomogram plots given in Figure 2.
Figure 2.
QT interval nomogram. The figure contains plots of uncorrected QT intervals against corresponding heart rate for donepezil, galantamine and rivastigmine. With this nomogram, points plotted above the line indicate QT interval prolongation.35 For a number of the studies, uncorrected QTc interval values were not available, but heart rate and QTc interval values were. This allowed uncorrected QT interval values to be derived. QT intervals were required to be calculated from QTc and rate information in one galantamine case and five donepezil cases. As the correction formula used was unspecified in most reports, we derived uncorrected QT intervals where this was necessary using both Bazett’s and Fridericia’s rate correction formulae, plotted in panels A and B, respectively (Y axis labels in A also apply to B). For the sole rivastigmine case, both QT and QTc intervals were given in the case report, but heart rate was not. However, the very close proximity of QT and QTc intervals in that case yielded the same heart rate value with both correction formulae.
Results
Cohort studies on donepezil, galantamine and rivastigmine
Cohort data on donepezil are mixed. In 2006 Bordier et al. published results of a study of patients presenting with mild-to-moderate AD who were given 5 mg/day of donepezil for 1 month followed by 10 mg/day for 7 months. A total of 22 patients completed the full 8 months’ time course of the study. There were no significant changes to QTc interval observed in the study.24 One patient experienced syncope, likely due to orthostatic hypotension. A significant decrease in heart rate was observed in patients receiving donepezil who were not on concomitant negatively chronotropic or dromotropic drugs, whilst PR interval lengthening was observed in patients receiving negatively chronotropic or dromotropic drugs to whom donepezil was administered.24 Isik et al. studied 71 newly diagnosed AD patients who were given 5 or 10 mg/day of donepezil; in 52 patients who completed their study no significant changes to baseline ECG parameters were found.38 Igeta et al. found significant changes to PR and RR interval in a group of 18 patients diagnosed with either dementia or cognitive disorder, who had been treated with donepezil. However, no significant changes to QT interval were reported.39 Wang et al. found decreased heart rate and prolonged PR interval but no significant changes to QT/QTc interval in a group of 60 elderly patients with ischaemic heart disease, receiving 5 mg/day of donepezil and followed for a month.40 Patients with bradycardia or using antiarrhythmic agents prior to the study were excluded from this study.40 By contrast, Poluzzi et al. have performed a datamining search of the public version of the FDA Adverse Event Reporting System (AERS) to find cases of reports of TdP across all drug classes.41 There were 1665 reports of TdP found over a 4-year period from 2004 to 2007. A total of 35 drugs with >10 reports of TdP were identified, including donepezil.41 Donepezil was considered to show a disproportionately high number of cases of TdP, despite the relatively low amount of evidence at the time of a link to TdP and it was noted that this was an area that needed further research.41
In 2010 Isik et al. reported results of a cohort study of 64 newly diagnosed AD patients with varying doses of galantamine (8–24 mg/day) and reported no significant changes in ECG parameters including the QT interval over the 4-month period of the study.42 In 2002, Morganroth and colleagues reported the results of a double-blind, multi-centre, placebo-controlled phase III trial of 2791 subjects with doses of rivastigmine over a 26-week period; 77% of the initial cohort completed treatment.43 They found no significant differences in ECG parameters between rivastigmine and placebo groups.43
Case report analysis: rivastigmine
Rivastigmine was the first of this group of AChEis to be associated with a case of QTc interval prolongation. In a 2002 report, a 78-year-old man displayed a prolonged QT interval a week after commencement of treatment with rivastigmine for worsening cognitive decline and behavioural difficulties.44 His ECG prior to rivastigmine had a QTc of 397 ms, despite evidence of an earlier MI and treatment with multiple medications and borderline hypokalaemia [3.4 mM (K+]e]. He had a QTc interval of 477 ms 7 days after rivastigmine treatment. Of the concurrent medications, citalopram is notable as this has been associated with QTc interval prolongation and TdP (e.g.45), but the patient’s QT interval was not prolonged prior to commencement of rivastigmine. Once the QT prolongation was identified the rivastigmine was withdrawn, as this was the only recent change to the patient’s medications. Two weeks later the patient’s QTc was 399 ms and remained normal in follow up.44 Application of Naranjo criteria34 and WHO-UMC scale for causality to the case here gave scores of ‘possible’ and ‘probable/likely’, respectively. Details of the case are summarised in Table 1.
Table 1.
Summary of case reports with galantamine and rivastigmine.
| Case | Age | Sex | AChEi | HR | QTc (ms) | TdP | Medical history | Drug history | Intervention | Naranjo | UMC-WHO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fisher and Davis46 | 85 | M | Galantamine (8 mg) | 83 | 503 | N | CAD, hypertension, osteoarthritis, hiatus hernia, BPH surgery Normal electrolytes |
Irbesartan, clopidogrel, simvastatin, pantoprazole, ergocalciferol, calcium carbonate, acetaminophen | Galantamine and irbesartan removed | Probable | Probable/likely |
| Nelson and Buchanon47 | 47 | M | Galantamine (12 mg) | 70 | 518 | N | Schizophrenia, diabetes, hypertension and hyperlipidaemia Normal electrolytes |
Aripiprazole, quetiapine, lithium, benztropine, trazadone, docusate, enalapril, insulin, metoprolol, ranitidine, simvastatin | Galantamine stopped immediately | Probable | Probable/likely |
| Walsh and Dourish44 | 78 | M | Rivastigmine | 60 | 477 | N | AD, MI, borderline hypokalaemia Normal electrolytes except for [K+]=3.4 mM |
Diltiazem, citalopram, furosemide, aspirin and ranitidine | Rivastigmine stopped | Possible | Probable/likely |
Summary information on each case is given under each of the subheading categories shown. Note that only the Fisher and Davis report stated the correction formula used (Bazett’s) to obtain QTc interval value shown.
AChEi, acetylcholinesterase inhibitor; AD, Alzheimer’s disease; BPH, benign prostatic hyperplasia; CAD, coronary artery disease; HR, heart rate; MI, myocardial infarction; TdP, Torsades de Pointes.
Case report analysis: galantamine
Two cases of QTc interval prolongation with galantamine were found.46,47 One patient was taking the drug as treatment for AD (8 mg/day)46 whereas the other patient was taking it for schizophrenia (12 mg/day).47 In both cases the patients were male, aged 85 and 47, respectively. The QTc was 503 and 518 ms, respectively, and neither patient had TdP recorded. In both cases there was history of cardiac pathology, but neither had congenital LQTS nor electrolyte disturbance. In each case the patient received medications in addition to galantamine. In particular, the schizophrenia patient received aripiprazole, quetiapine and trazadone.47 Hepatic and renal impairment was not noted in either case. The AD patient presented with bradycardia and the schizophrenia patient was taking a beta blocker to decrease the heart rate. The AD patient also exhibited other known AChEi side effects of syncope and gastrointestinal disturbance.46 There was a recent change in medication in both cases; the schizophrenia patient had undergone an increase in galantamine dose increased from 8 mg to 12 mg a day47 whereas the AD patient had recently been re-prescribed galantamine after previously having it withdrawn due to syncope and bradycardia.46 Once the QT prolongation was identified both patients had galantamine stopped immediately.46,47 Both cases saw a return to normal QTc and remained normal on follow up. This was despite the schizophrenia case still taking psychotropic drug associated with QTC/TdP risk.46,48 The Naranjo scale34 and WHO-UMC criteria were applied to the two cases to probe causality. Both cases scored ‘probable’ on the Naranjo scale and ‘probable/likely’ using WHO-UMC criteria. Details of these cases are summarised in Table 1.
Case report analysis: donepezil
The first case of QTc interval prolongation and TdP with donepezil was reported in 2007.49 A 76-year-old woman with AD experienced two syncopal episodes. On admission to hospital her ECG showed bradycardia, marked QTc interval prolongation (590–777 ms) and TdP.49 Her serum electrolytes were normal, though her serum troponin T was borderline. In addition to donepezil, her medications included omeprazole, propranolol and escitalopram. Following withdrawal of donepezil, propranolol and escitalopram her QTc interval normalised (QTc interval of 436 ms). The authors considered donepezil to be the most likely cause of QT prolongation in this case, arguing that escitalopram could impair donepezil metabolism and omeprazole inhibit escitalopram metabolism. Escitalopram itself has now been linked with some cases of QTc interval prolongation/TdP45,50 and so it is possible that QTc prolongation and TdP in this case resulted from drug combination. However, mirtazapine was substituted for escitalopram, without adverse consequence; this is significant as mirtazapine can itself prolong the QT interval.51 Thus, donepezil administration is likely to have been important in this case. Including this initial report, ten cases of QTc prolongation with donepezil were identified, of which five had clearly identified episodes of TdP.33,49,52–58 Salient features of these cases are summarised in Table 2. Eight of the ten cases were in women and two in men. The mean age of affected individuals was 77.3 ± 5.8 years (mean ± SEM). However, one case was an outlier58 in the respect that it involved a young woman (26 years) treated with donepezil for cognitive rehabilitation rather than for AD. The mean age of the nine AD patients receiving donepezil was 83 ± 1.4 years. All cases but one involved therapeutic doses of donepezil, with the remaining case involving an accidental overdose.56 This case occurred in an 84-year-old male with previous cardiac pathology. He presented with a QTc interval of 502 ms, but no episode of TdP was recorded after he took seven times his usual 5 mg dose. As clearly shown in Table 2, all but two patients received additional medications and a number had multiple concurrent medical conditions. A pre-existing cardiovascular condition was noted in seven cases, whilst evidence of congenital LQTS was absent in all. An electrolyte imbalance (hypokalaemia) was recorded in only one case.53 No renal or hepatic insufficiencies were recorded. Bradycardia was present in five of ten cases. Several of the cases involved a recent change in the patient’s medication. Table 2 summarises clinical interventions given in the donepezil cases. Of note, one patient, an 80-year-old woman, became unresponsive with polymorphic ventricular tachycardia following admission, necessitating electrical cardioversion, which was successful.54 Another, an 87- year-old woman, experienced deterioration of TdP into ventricular fibrillation; but this recovered without intervention.52 None of these patients died; in all cases once QT prolongation had been identified, cessation of donepezil was associated with return of QTc intervals to the normal range.
Table 2.
Summary of information on case reports with donepezil.
| Case | Age | Sex | AChEi | HR | QTc (ms) | TdP | Medical history | Drug history | Intervention | Naranjo | WHO-UMC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leitch et al.49 | 76 | F | Donepezil (10 mg) | 42 | 590–777 | Y | Depression, AD Normal electrolytes |
Omeprazole, escitalopram, propranolol |
IV Mg and isoprenaline, temp cardiac pacing | Possible | Possible |
| Tanaka et al.52 | 90 | M | Donepezil (10 mg) | 36 | 398 | N | AD Normal (K+) (other electrolytes not mentioned) |
donepezil stopped, orciprenaline started | Probable | Probable/likely | |
| Tanaka et al.52 | 87 | F | Donepezil (5 mg) | 40 | 594 | Y | Hypertension, AF, AD and bradycardia Normal (K+) (other electrolytes not mentioned) |
Cilostazol, amlodipine, spironolactone, warfarin | donepezil stopped, orciprenaline started | Possible | Possible |
| Takaya et al.53 | 83 | F | Donepezil (5 mg) | 54 | 645 | Y | Hypertension, AD, AF, MI and diabetes Hypokalaemia [(K+)=3.3 mM]. |
Bisoprolol | Donepezil stopped, IV Mg, K and lidocaine, isoprenaline injection | Possible | Possible |
| Kitt et al.54 | 80 | F | Donepezil (10 mg) | 85 | 490 | Y | Cerebrovascular disease, mixed vascular and AD, AF (permanent pacemaker) and hypertension Normal electrolytes |
Bumetanide, perindopril, lansoprazole, atorvastatin, diltiazem M/R, fluoxetine | IV Mg/fluids, donepezil stopped, fluoxetine decreased, defibrillator | Probable | Probable/likely |
| Gurbuz et al.55 | 84 | F | Donepezil (10 mg) | 65 | 624 | Y | CAD, AD and hypertension Normal electrolytes |
Ramipril, aspirin | IV K and Mg and stopped donepezil | Probable | Certain |
| Pourmand et al.56 | 84 | M | Donepezil (35 mg) | 70 | 502 | N | AD, hypertension, stroke, BPH Normal electrolytes |
Donepezil overdose | dose of atropine and stopped donepezil | Probable | Certain |
| Shinozaki33 | 80 | F | Donepezil (5 mg) | 78 | 480 | N | AD, hypertension, hypercholesterolaemia, osteoporosis Normal electrolytes |
Benidipine, atorvastatin, vitamin D, risedronate | Changed benidipine to amlodipine | Possible | Possible |
| Jackson and Stowe57 | 83 | F | Donepezil (10 mg) | 638 | N | AD, unwitnessed syncope, history of falls, hip fracture, hypertension. Normal electrolytes |
Bendroflumethiazide, simvastatin |
Donepezil stopped | Probable | Probable/Likely | |
| Vogel et al.58 | 26 | F | Donepezil (20 mg) | 70–81 | 463–528 | N | Major depressive disorder, dysarthria, TBI, seizures, hemiplegia, GERD, tachycardia. Normal [K+] No [Mg2+] data |
Quetiapine, metoprolol, PEG-3350, cephalexin, diphenhydramine | Donepezil stopped | Probable | Probable/Likely |
Summary information on each case is given under each of the subheading categories shown. Note that only the Gurbuz et al. report stated the correction formula used (Bazett’s) to obtain QTc interval value shown.
AChEi, acetylcholinesterase inhibitor; AD, Alzheimer’s disease; AF, atrial fibrillation; BPH, benign prostatic hyperplasia; CAD, coronary artery disease; HR, heart rate; MI, myocardial infarction; TdP, Torsades de Pointes.
To assess causality the Naranjo scale34 and WHO-UMC scale were applied. This resulted in six probable and four possible results according to the Naranjo scale and two certain, four probable/likely and four possible according to the WHO-UMC criteria.
A QT nomogram36,37 has been used to evaluate the severity of QT interval prolongation in relation to heart rate. Any points above the line are considered to indicate risk of TdP.36,37 Figure 1 shows the data from the case reports examined here. Heart rate data were unavailable for one donepezil case, so nine donepezil cases were analysed. It was necessary for some reports to calculate QT intervals from heart rate and QTc interval data and in only one donepezil report55 was the rate correction formula that had been used stated. Therefore, where it was necessary to calculate QT intervals in the absence of correction formula information, we calculated these using both Bazett’s and Fridericia’s formulae (Figure 2A and B, respectively). Irrespective of the method used, for five donepezil cases the QT interval lay clearly above the nomogram line, consistent with a QT-prolonging effect of the drug.37 Data for galantamine and rivastigmine cases were also included. The points for the galantamine and rivastigmine cases lie very close to the nomogram line, but there are not enough cases to make definitive conclusions from those data.
Discussion
Inferences from case reports
The present case report analysis shows a strong association between donepezil use and cases of QTc prolongation and risk of TdP arrhythmia. Case report data on galantamine and rivastigmine are sparse, making it difficult to make definitive conclusions for those drugs. The nature of the clinical indications for AChEis means that for the most part patients are older adults, who tend to have additional conditions and medications. The number of medications that some of the patients received was notable. This is significant because drug-induced QT prolongation and its associated risk of TdP are known to be exacerbated by the presence of at least one risk factor,59–61 which is the case here. It is also notable that one QTc prolongation case with donepezil occurred in a young adult,58 indicating that a risk of repolarisation delay with the drug can occur in younger individuals possessing additional risk factors (in this case receipt of quetiapine, expected to be synergistic in predisposing to QTc prolongation62). For most scenarios in which AChEis are likely to be deployed, however, older age is an unmodifiable risk factor. Initial administration of AChEis is commonly carried out using a two-step dosing schedule in order to increase tolerance and minimise the number of side effects the patient experiences.63 Several of the cases summarised in Tables 1 and 2 involved a recent increase in dose or sudden re-administration of the drugs at therapeutic dose after a long break in taking the drug in one step, whereas one was an overdose. Gastrointestinal side effects are also often present in those taking AChEis and this can lead to electrolyte disturbances.23 However, hypokalaemia was not a common feature of the case reports we identified, with borderline hypokalaemia present in the sole rivastigmine case44 and in only 1 of 10 donepezil cases.53 The preponderance of female:male cases (8:2) for donepezil is consistent with female sex as a known risk factor for QTc prolongation.60 Both case reports with galantamine and the single report with rivastigmine were in males, but these sample sizes are too small to provide conclusive information regarding sex as a risk factor for these drugs. However, it is notable that most cases of TdP with these drugs in publicly accessible information in the Eudravigilance database are in women. Several of the cases of QT prolongation involved drug–drug interactions where the metabolism of the AChEi would be affected. Rivastigmine is metabolised by AChE itself however donepezil and galantamine are metabolised by cytochrome p450 enzymes CYP3A4 and CYP2D6 which are inhibited by a range of commonly prescribed classes of drugs.11,16,21,64
Some of the cohort studies that found there to be no change in QT interval when taking AChEis are notable in respect of their design.38,42,43 Thus, Isik et al. excluded patients who were taking any form of cardio-stimulatory drugs, thereby effectively ruling out any patients with history of bradycardia and some other cardiac pathologies.38,42 The rivastigmine study by Morganroth et al. excluded patients who exhibited any abnormalities in their baseline ECG or who received, with some exceptions, psychotropic medications.43 On the one hand, study designs that limit risk factors present in the patients admitted are not directly comparable to case report data. On the other hand, such studies may point towards comparatively good drug safety in patients who possess fewer risk factors and therefore that risk can be reduced by ensuring patients have risk factors considered when prescribing.
Preclinical information on AChEis and ventricular repolarisation
It is useful to consider the evidence of QTc prolongation and TdP with donepezil, galantamine and rivastigmine in humans alongside preclinical experimental data that may provide insight into the basis for observed patient effects. Virtually all drugs associated with TdP and QTc interval prolongation inhibit potassium channels that mediate the cardiac rapid delayed rectifier current, IKr.65–67 These channels are encoded by KCNH2 or hERG (human Ether-à-go-go Related Gene) and determination of any propensity to block recombinant hERG channels is an important component of preclinical safety evaluation of novel pharmaceuticals.67,68 The unusually high pharmacological promiscuity of the hERG channel has been attributed to unique structural and functional features of the channel.66,67
Donepezil has been found to inhibit hERG channel ionic current (IhERG) recorded from hERG channels in a mammalian expression system, with a half maximal inhibitory concentration (IC50) of 1.30 μM.69 This study showed that the acute inhibitory action of donepezil on IhERG shows gating dependence, with interactions between the drug and both activated and inactivated channels.69 Furthermore, the same study showed that the donepezil metabolites 6-O-desmethyl donepezil and 5-O-desmethyl donepezil inhibited IhERG with similar potency to the parent compound. Moreover, in addition to its acute inhibitory effect on IhERG donepezil was found to inhibit hERG channel trafficking and expression in the plasma membrane.69 Galantamine effects on IhERG have also been investigated, with patch-clamp experiments demonstrating that galantamine produces low-potency inhibition of IhERG, with an IC50 of 760 μM; the observed voltage dependence of inhibition suggested that the drug interacts primarily with the activated state of the hERG channel.70 An additional small effect of galantamine on KCNQ1+KCNE1 channels (which mediate the ‘slow’ delayed rectifier, IKs) was also found. In the same study, galantamine was also applied to guinea pig Langendorff-perfused hearts, resulting in cycle-length dependent prolongation of monophasic action potentials (MAPD90), with a ~12 ms prolongation by 1 μM galantamine at a cycle length of 250 ms.70
Rivastigmine has been reported to inhibit transient outward and delayed rectifier potassium currents in rat dissociated hippocampal neurones.71 However, these are distinct from cardiomyocyte potassium conductances and expressed hERG channel currents and there appear to be no published data regarding inhibition of the hERG channel by rivastigmine. A recent study has compared the effects of rivastigmine, with those of galantamine and donepezil on ventricular repolarisation and propensity to generate early after-depolarisations (EADs).72 Ventricular repolarisation of Langendorff-perfused rabbit hearts was prolonged by donepezil but not galantamine, whereas spatial dispersion of repolarisation was prolonged by both drugs. Susceptibility to EADs and TdP was increased by both drugs. By contrast, rivastigmine prolonged repolarisation without increasing dispersion of repolarisation and did not increase susceptibility to EADs or TdP.72 Thus, in this study spatial dispersion of repolarisation but not duration of repolarisation, per se, was associated with proarrhythmic risk with AChEis, leading the authors to suggest that QT interval measurement alone may be insufficient to evaluate proarrhythmic risk with these drugs.72 Whilst further work is required to elucidate the underlying basis of the differences between the drugs, at least in this comparative setting rivastigmine appears to be safer than either galantamine or donepezil.72
In 2003, Redfern et al. evaluated the relationship between IhERG blockade and effective therapeutic plasma concentrations for a range of drugs and arrived at a provisional ‘safety margin’ of 30 (i.e. a 30-fold separation between plasma Cmax and IhERG IC50).73 The mean plasma levels of donepezil in patients receiving 5 and 10 mg/day of the drug have been reported to be 25.7 ± 0.7 and 50.6 ± 1.9 ng/ml respectively,74 equivalent to 67.7 and 133.3 nM and a safety margin of 9.8–19.4. Recent data from patients receiving 8 mg/day of galantamine for at least 6 months showed a mean plasma concentration of 83.7 ± 70 ng/ml,75 equivalent to 29.1 nM and a safety margin of 261.2 (falling to 49.4 for a mean + SEM concentration). The comparatively low (<30-fold) safety margin for donepezil is concordant with results for high risk of TdP shown on the QT nomogram (Figure 1). Galantamine has a higher safety margin, which is perhaps consistent with the inconclusive results from the QT nomogram plot (though clearly further data are needed). A hERG safety margin could not be calculated for rivastigmine as no published data from a hERG channel assay are available. It is notable that the ‘CredibleMeds’ database (https://www.crediblemeds.org), which classifies drugs based on their association with QT prolongation and TdP,76 lists rivastigmine as not classified: there is insufficient evidence to attribute a classification. Galantamine is classified as having ‘conditional risk of TdP’ (an association between a drug and TdP exists, but under certain conditions of use such as overdose or presence of additional risk factors). Donepezil is classified as having a ‘known risk of TdP’ (such drugs ‘prolong the QT interval AND are clearly associated with a known risk of TdP, even when taken as recommended’). The inability to ascribe any classification to rivastigmine highlights the need for further investigation of the drug, in order to understand more fully whether or not it carries any risk of QT prolongation and TdP.
Limitations
The number of case reports for each of galantamine and rivastigmine was low. Further information is needed to make definitive conclusions regarding these drugs. In addition, both the Naranjo scale34 and the WHO-UMC scale, which suggested links between the AChEi and QTc prolongation/TdP, in each case have some limitations.77,78 For example, it can be difficult to determine what counted as an alternative cause for the adverse event as most cases had at least two other risk factors for QT prolongation. Importantly, re-administration of a drug, leading to a similar effect as seen initially, scores highly on both scales. However, the utility of this criterion is questionable when it would be unacceptable deliberately to re-administer a drug that may cause a life-threatening cardiac event. On the other hand, aside from the Naranjo and WHO-UMC evaluations, the use of the QT nomogram36 showed donepezil to have a clear risk of TdP. The nomogram was inconclusive in assessing risk of galantamine and rivastigmine due to the low number of cases.
Conclusion
In conclusion, the analysis conducted here is consistent with a link between the administration of donepezil and a risk of QTc prolongation and TdP. The donepezil case report data suggest that older women receiving agents that may interfere with donepezil metabolism or which may themselves predispose towards QT interval prolongation (including certain antidepressant or antipsychotic agents) may be particularly at risk. Case report analysis of galantamine is inconclusive, owing to the low number of case reports. However, comparison of safety-margins for donepezil and galantamine suggests that the latter may be safer than the former. Preclinical data show a clear repolarisation delay with donepezil, whereas conflicting data have been obtained in respect of galantamine.70,72 hERG data are lacking for rivastigmine, but repolarisation delay in rabbit hearts with rivastigmine was not associated with QT dispersion, EADs or TdP.72 This may suggest that rivastigmine has greater cardiac safety. Further comparative pre-clinical data on rivastigmine, galantamine and donepezil would be valuable, especially in respect of the current lack of information as to whether rivastigmine inhibits hERG channels, as well as comparative data on action potential prolongation by the three drugs under a standardised set of conditions. Owing to the seriousness of AD and lack of other viable treatment options, the use of AChEis is unavoidable. The present analysis suggests that modifiable risk factors should be screened for, regularly checked and corrected, whilst the combination of modifiable and unmodifiable risk factors may inform drug selection: it is possible that galantamine and rivastigmine may be preferable in the presence of several non-modifiable risk factors for QTc prolongation/TdP. However further investigation is needed to verify this and these drugs’ potential for and profile of non-cardiac side effects79 also need to be considered when making drug selection.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jules C. Hancox  https://orcid.org/0000-0002-2055-6482
https://orcid.org/0000-0002-2055-6482
Contributor Information
Katie Malone, School of Physiology, Pharmacology and Neuroscience, University of Bristol, University Walk, Bristol, UK.
Jules C. Hancox, School of Physiology, Pharmacology and Neuroscience, University of Bristol, University Walk, Biomedical Sciences Building, Bristol, BS8 1TD, UK.
References
- 1. Ballard C, Gauthier S, Corbett A, et al. Alzheimer’s disease. Lancet 2011; 377: 1019–1031. [DOI] [PubMed] [Google Scholar]
- 2. Niu H, varez-Alvarez I, Guillen-Grima F, et al. Prevalence and incidence of Alzheimer’s disease in Europe: a meta-analysis. Neurologia 2017; 32: 523–532. [DOI] [PubMed] [Google Scholar]
- 3. El-Hayek YH, Wiley RE, Khoury CP, et al. Tip of the Iceberg: assessing the global socioeconomic costs of Alzheimer’s disease and related dementias and strategic implications for stakeholders. J Alzheimers Dis 2019; 70: 323–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm (Vienna) 2006; 113: 1625–1644. [DOI] [PubMed] [Google Scholar]
- 5. Ladner CJ, Lee JM. Pharmacological drug treatment of Alzheimer disease: the cholinergic hypothesis revisited. J Neuropathol Exp Neurol 1998; 57: 719–731. [DOI] [PubMed] [Google Scholar]
- 6. Giacobini E. Do cholinesterase inhibitors have disease-modifying effects in Alzheimer’s disease? CNS Drugs 2001; 15: 85–91. [DOI] [PubMed] [Google Scholar]
- 7. Wilkinson DG, Francis PT, Schwam E, et al. Cholinesterase inhibitors used in the treatment of Alzheimer’s disease: the relationship between pharmacological effects and clinical efficacy. Drugs Aging 2004; 21: 453–478. [DOI] [PubMed] [Google Scholar]
- 8. Noetzli M, Eap CB. Pharmacodynamic, pharmacokinetic and pharmacogenetic aspects of drugs used in the treatment of Alzheimer’s disease. Clin Pharmacokinet 2013; 52: 225–241. [DOI] [PubMed] [Google Scholar]
- 9. Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther 2014; 6: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cummings J, Morstorf T, Lee G. Alzheimer’s drug-development pipeline: 2016. Alzheimers Dement (NY) 2016; 2: 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tiseo PJ, Perdomo CA, Friedhoff LT. Metabolism and elimination of 14C-donepezil in healthy volunteers: a single-dose study. Br J Clin Pharmacol 1998; 46(Suppl. 1): 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mihara M, Ohnishi A, Tomono Y, et al. Pharmacokinetics of E2020, a new compound for Alzheimer’s disease, in healthy male volunteers. Int J Clin Pharmacol Ther Toxicol 1993; 31: 223–229. [PubMed] [Google Scholar]
- 13. Farlow MR. Clinical pharmacokinetics of galantamine. Clin Pharmacokinet 2003; 42: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 14. Scott LJ, Goa KL. Galantamine: a review of its use in Alzheimer’s disease. Drugs 2000; 60: 1095–122. [DOI] [PubMed] [Google Scholar]
- 15. Maelicke A, Schrattenholz A, Samochocki M, et al. Allosterically potentiating ligands of nicotinic receptors as a treatment strategy for Alzheimer’s disease. Behav Brain Res 2000; 13: 199–206. [DOI] [PubMed] [Google Scholar]
- 16. Mannens GS, Snel CA, Hendrickx J, et al. The metabolism and excretion of galantamine in rats, dogs, and humans. Drug Metab Dispos 2002; 30: 553–563. [DOI] [PubMed] [Google Scholar]
- 17. Bickel U, Thomsen T, Weber W, et al. Pharmacokinetics of galanthamine in humans and corresponding cholinesterase inhibition. Clin Pharmacol Ther 1991; 50: 420–428. [DOI] [PubMed] [Google Scholar]
- 18. Zhao Q, Janssens L, Verhaeghe T, et al. Pharmacokinetics of extended-release and immediate-release formulations of galantamine at steady state in healthy volunteers. Curr Med Res Opin 2005; 21: 1547–1554. [DOI] [PubMed] [Google Scholar]
- 19. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther 1998; 20: 634–647. [DOI] [PubMed] [Google Scholar]
- 20. Kennedy JS, Polinsky RJ, Johnson B, et al. Preferential cerebrospinal fluid acetylcholinesterase inhibition by rivastigmine in humans. J Clin Psychopharmacol 1999; 19: 513–521. [DOI] [PubMed] [Google Scholar]
- 21. Cutler NR, Polinsky RJ, Sramek JJ, et al. Dose-dependent CSF acetylcholinesterase inhibition by SDZ ENA 713 in Alzheimer’s disease. Acta Neurol Scand 1998; 97: 244–250. [DOI] [PubMed] [Google Scholar]
- 22. Lefevre G, Sedek G, Jhee SS, et al. Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice-daily capsules in Alzheimer’s disease patients. Clin Pharmacol Ther 2008; 83: 106–114. [DOI] [PubMed] [Google Scholar]
- 23. Pariente A, Pinet M, Moride Y, et al. Factors associated with persistence of cholinesterase inhibitor treatments in the elderly. Pharmacoepidemiol Drug Saf 2010; 19: 680–686. [DOI] [PubMed] [Google Scholar]
- 24. Bordier P, Garrigue S, Lanusse S, et al. Cardiovascular effects and risk of syncope related to donepezil in patients with Alzheimer’s disease. CNS Drugs 2006; 20: 411–417. [DOI] [PubMed] [Google Scholar]
- 25. Malone DM, Lindesay J. Cholinesterase inhibitors and cardiovascular disease: a survey of old age psychiatrists’ practice. Age Ageing 2007; 36: 331–333. [DOI] [PubMed] [Google Scholar]
- 26. Howes LG. Cardiovascular effects of drugs used to treat Alzheimer’s disease. Drug Saf 2014; 37: 391–395. [DOI] [PubMed] [Google Scholar]
- 27. Pu Z, Xu W, Lin Y, et al. Donepezil decreases heart rate in elderly patients with Alzheimer’s disease. Int J Clin Pharmacol Ther 2019; 57: 94–100. [DOI] [PubMed] [Google Scholar]
- 28. Pratt RD, Perdomo CA, Surick IW, et al. Donepezil: tolerability and safety in Alzheimer’s disease. Int J Clin Pract 2002; 56: 710–717. [PubMed] [Google Scholar]
- 29. Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 2006; 1: CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gill SS, Bronskill SE, Mamdani M, et al. Representation of patients with dementia in clinical trials of donepezil. Can J Clin Pharmacol 2004; 11: e274–e285. [PubMed] [Google Scholar]
- 31. Cubeddu LX. Drug-induced inhibition and trafficking disruption of ion channels: pathogenesis of QT abnormalities and drug-induced fatal arrhythmias. Curr Cardiol Rev 2016; 12: 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. The UPPSALA Monitoring Centre. The use of the WHO-UMC system for standardized causality assessment, https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf (accessed October 2019).
- 33. Shinozaki K. Shortening of donepezil-induced QTc prolongation with a change in the interacting drug, after electrocardiograph monitoring by community pharmacists: a case report. Yakugaku Zasshi 2012; 132: 237–241. [DOI] [PubMed] [Google Scholar]
- 34. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–245. [DOI] [PubMed] [Google Scholar]
- 35. Chan A, Isbister GK, Kirkpatrick CM, et al. Drug-induced QT prolongation and torsades de pointes: evaluation of a QT nomogram. QJM 2007; 100: 609–615. [DOI] [PubMed] [Google Scholar]
- 36. Waring WS, Graham A, Gray J, et al. Evaluation of a QT nomogram for risk assessment after antidepressant overdose. Br J Clin Pharmacol 2010; 70: 881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Isbister GK. Risk assessment of drug-induced QT prolongation. Aust Prescr 2015; 38: 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Isik AT, Yildiz GB, Bozoglu E, et al. Cardiac safety of donepezil in elderly patients with Alzheimer disease. Intern Med 2012; 51: 575–578. [DOI] [PubMed] [Google Scholar]
- 39. Igeta H, Suzuki Y, Tajiri M, et al. Cardiovascular pharmacodynamics of donepezil hydrochloride on the PR and QT intervals in patients with dementia. Hum Psychopharmacol 2014; 29: 292–294. [DOI] [PubMed] [Google Scholar]
- 40. Wang D, Wu Y, Wang A, et al. Electrocardiogram changes of donepezil administration in elderly patients with ischemic heart disease. Cardiol Res Pract 2018; 2018: 9141320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poluzzi E, Raschi E, Moretti U, et al. Drug-induced torsades de pointes: data mining of the public version of the FDA adverse event reporting system (AERS). Pharmacoepidemiol Drug Saf 2009; 18: 512–518. [DOI] [PubMed] [Google Scholar]
- 42. Isik AT, Bozoglu E, Naharci MI, et al. Evaluation of the effects of galantamine on cardiac function in elderly patients with Alzheimer’s disease. Am J Geriatr Pharmacother 2010; 8: 454–459. [DOI] [PubMed] [Google Scholar]
- 43. Morganroth J, Graham S, Hartman R, et al. Electrocardiographic effects of rivastigmine. J Clin Pharmacol 2002; 42: 558–568. [DOI] [PubMed] [Google Scholar]
- 44. Walsh E, Dourish J. Prolonged QT interval with rivastigmine. Br J Psychiatry 2002; 180: 466. [DOI] [PubMed] [Google Scholar]
- 45. Kogut C, Breden-Crouse E, Vieweg WV, et al. Selective serotonin reuptake inhibitors and torsades de pointes. New concepts and new directions derived from systematic review of case reports. Ther Adv Drug Saf 2013; 4: 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fisher AA, Davis MW. Prolonged QT interval, syncope, and delirium with galantamine. Ann Pharmacother 2008; 42: 278–283. [DOI] [PubMed] [Google Scholar]
- 47. Nelson MW, Buchanan RW. Galantamine-induced QTc prolongation. J Clin Psychiatry 2006; 67: 166–167. [DOI] [PubMed] [Google Scholar]
- 48. Hasnain M, Vieweg WV. QTc interval prolongation and torsade de pointes associated with second-generation antipsychotics and antidepressants: a comprehensive review. CNS Drugs 2014; 28: 887–920. [DOI] [PubMed] [Google Scholar]
- 49. Leitch A, McGinness P, Wallbridge D. Calculate the QT interval in patients taking drugs for dementia. BMJ 2007; 335: 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baranchuk A, Simpson CS, Methot M, et al. Corrected QT interval prolongation after an overdose of escitalopram, morphine, oxycodone, zopiclone and benzodiazepines. Can J Cardiol 2008; 24: e38–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jasiak NM, Bostwick JR. Risk of QT/QTc prolongation among newer non-SSRI antidepressants. Ann Pharmacother 2014; 48: 1620–1628. [DOI] [PubMed] [Google Scholar]
- 52. Tanaka A, Koga S, Hiramatsu Y. Donepezil-induced adverse side effects of cardiac rhythm: 2 cases report of atrioventricular block and Torsade de Pointes. Intern Med 2009; 48: 1219–1223. [DOI] [PubMed] [Google Scholar]
- 53. Takaya T, Okamoto M, Yodoi K, et al. Torsades de Pointes with QT prolongation related to donepezil use. J Cardiol 2009; 54: 507–511. [DOI] [PubMed] [Google Scholar]
- 54. Kitt J, Irons R, Al-Obaidi M, et al. A case of donepezil-related torsades de pointes. BMJ Case Rep 2015; 2015: bcr2015211900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gurbuz AS, Ozturk S, Acar E, et al. Acquired long QT syndrome and Torsades de Pointes related to donepezil use in a patient with Alzheimer disease. Egypt Heart J 2016; 68: 197–199. [Google Scholar]
- 56. Pourmand A, Shay C, Redha W, et al. Cholinergic symptoms and QTc prolongation following donepezil overdose. Am J Emerg Med 2017; 35: 1386. [DOI] [PubMed] [Google Scholar]
- 57. Jackson EG, Stowe S. Lesson of the month 1: prolonged QT syndrome due to donepezil: a reversible cause of falls? Clin Med (Lond) 2019; 19: 80–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vogel SM, Mican LM, Smith TL. Donepezil-induced QTc prolongation: a case report. Ment Health Clin 2019; 9: 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Viskin S. Long QT syndromes and torsade de pointes. Lancet 1999; 354: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 60. Zeltser D, Justo D, Halkin A, et al. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore) 2003; 82: 282–290. [DOI] [PubMed] [Google Scholar]
- 61. Trinkley KE, Page RL, Lien H, et al. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin 2013; 29: 1719–1726. [DOI] [PubMed] [Google Scholar]
- 62. Hasnain M, Vieweg WV, Howland RH, et al. Quetiapine, QTc interval prolongation, and torsade de pointes: a review of case reports. Ther Adv Psychopharmacol 2014; 4: 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl 2002; 127: 45–63. [PubMed] [Google Scholar]
- 64. Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet 2000; 38: 41–57. [DOI] [PubMed] [Google Scholar]
- 65. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart 2003; 89: 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature 2006; 440: 463–469. [DOI] [PubMed] [Google Scholar]
- 67. Hancox JC, McPate MJ, El Harchi A, et al. The hERG potassium channel and hERG screening for drug-induced torsades de pointes. Pharmacol Ther 2008; 119: 118–132. [DOI] [PubMed] [Google Scholar]
- 68. Gintant GA. Preclinical Torsades-de-Pointes screens: advantages and limitations of surrogate and direct approaches in evaluating proarrhythmic risk. Pharmacol Ther 2008; 119: 199–209. [DOI] [PubMed] [Google Scholar]
- 69. Chae YJ, Lee HJ, Jeon JH, et al. Effects of donepezil on hERG potassium channels. Brain Res 2015; 1597: 77–85. [DOI] [PubMed] [Google Scholar]
- 70. Vigneault P, Bourgault S, Kaddar N, et al. Galantamine (Reminyl) delays cardiac ventricular repolarization and prolongs the QT interval by blocking the HERG current. Eur J Pharmacol 2012; 681: 68–74. [DOI] [PubMed] [Google Scholar]
- 71. Pan Y, Xu X, Wang X. Rivastigmine blocks voltage-activated K+ currents in dissociated rat hippocampal neurons. Br J Pharmacol 2003; 140: 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ellermann C, Coenen A, Niehues P, et al. Proarrhythmic effect of acetylcholine-esterase inhibitors used in the treatment of Alzheimer’s disease: benefit of rivastigmine in an experimental whole-heart model. Cardiovasc Toxicol 2020; 20: 168–175. [DOI] [PubMed] [Google Scholar]
- 73. Redfern WS, Carlsson L, Davis AS, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovas Res 2003; 58: 32–45. [DOI] [PubMed] [Google Scholar]
- 74. Rogers SL, Doody RS, Mohs RC, et al. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil study group. Arch Intern Med 1998;158: 1021–1031. [DOI] [PubMed] [Google Scholar]
- 75. Lin YT, Chou MC, Wu SJ, et al. Galantamine plasma concentration and cognitive response in Alzheimer’s disease. Peer J 2019; 7: e6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Woosley RL, Black K, Heise CW, et al. CredibleMeds.org: what does it offer? Trends Cardiovasc Med 2018; 28: 94–99. [DOI] [PubMed] [Google Scholar]
- 77. Avner M, Finkelstein Y, Hackam D, et al. Establishing causality in pediatric adverse drug reactions: use of the Naranjo probability scale. Paediatr Drugs 2007; 9: 267–270. [DOI] [PubMed] [Google Scholar]
- 78. Agbabiaka TB, Savovic J, Ernst E. Methods for causality assessment of adverse drug reactions: a systematic review. Drug Saf 2008; 31: 21–37. [DOI] [PubMed] [Google Scholar]
- 79. Khoury R, Rajamanickam J, Grossberg GT. An update on the safety of current therapies for Alzheimer’s disease: focus on rivastigmine. Ther Adv Drug Saf 2018; 9: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]