Abstract
A 35-year-old male presented to our university hospital with night sweats, fevers, ulcerated skin lesions to the lower mouth and posterior neck, shortness of breath, and an enlarging cervical lymph node. The patient was evaluated 2 months prior for respiratory symptoms, cervical lymphadenopathy, and skin lesions resulting in a diagnosis of primary pulmonary coccidioidomycosis and was treated with a 4-week course of fluconazole. On presentation to our hospital, initial laboratory test results revealed leukocytosis, increased liver enzymes, elevated inflammatory markers, and hypercalcemia. Computed tomography scan of the chest revealed lung nodules in a miliary pattern and prominent mediastinal lymphadenopathy. Magnetic resonance imaging revealed multiple vertebral and iliac bone lesions, as well as bilateral psoas muscle lesions. Serum ELISA (enzyme linked immunosorbent assay) detected elevated serological markers against coccidioides, and sputum culture revealed coccidioides arthroconidia, confirming the presence of an acute coccidioides infection. Biopsy of the right iliac crest and cervical lymph node revealed spherules resembling coccidioides, escalating the diagnosis to disseminated coccidioidomycosis. The patient’s hospital course was complicated by septic shock, acute respiratory distress syndrome requiring several days of mechanical ventilation, and acute kidney injury. He was ultimately treated with several weeks of voriconazole and liposomal amphotericin-B. He made a full recovery and was discharged on an extended course of oral voriconazole. Our case highlights the importance of recognition and appropriate treatment duration of disseminated coccidioidomycosis at initial presentation. Failure to do so may lead to increased morbidity and mortality.
Keywords: disseminated coccidioidomycosis, valley fever, insufficient treatment, pulmonary coccidioidomycosis
Introduction
Coccidioides immitis is a soil-dwelling, dimorphic fungus endemic to the southwest region of the United States.1 It is usually inhaled into lungs as small barrel-shaped structures called arthroconidia. These arthroconidia develop into spherules in the lungs and are ingested by macrophages. Many cases only involve the lungs and are known as primary pulmonary coccidioidomycosis, or “Valley Fever,” due to the pathogen’s original discovery in the San Joaquin Valley in California.2 However, in some cases, macrophages that ingest the arthroconidia enter the lymphatic system and lead to disseminated disease.3 The severity of disease can vary from mild to life-threatening disease.4 The organs involved in disseminated disease include the skin, bones, joints, and central nervous system.5 Risk factors for the development of disseminated coccidioides (DC) include HIV/AIDS, immunosuppressive medication, race/ethnicity, old age, diabetes, and late-stage pregnancy.5-8
We are reporting a rare case of DC in a young, relatively healthy individual with no major risk factors for dissemination of disease. This patient was diagnosed with coccidioides 2 months prior to presentation to our hospital. However, his disease was only treated as a primary pulmonary infection, despite having features of disseminated disease (skin lesions, lymphadenopathy). Our case report highlights the unusual presentation of DC in a healthy young male. It also highlights the importance of recognizing and treating DC appropriately to prevent life-threatening progression of the disease.
Case Presentation
A 35-year-old Samoan American male from California with congenital deafness, hypertension, and asthma presented to a university hospital in San Antonio, Texas, with worsening night sweats, rash, productive cough, shortness of breath, fevers, intermittent back pain, and enlarging neck nodule. The patient had been diagnosed with Valley Fever 2 months prior while living in California. His initial symptoms included night sweats, facial rash, fever, worsening hearing loss, cough, skin lesions, cervical lymphadenopathy, and shortness of breath. At that time, he was given a 1-month course of fluconazole 400 mg orally daily, and no further treatment was recommended. While on fluconazole, he had noted some improvement in his symptoms. Physical examination at the time of hospital admission revealed scaling plaques to the right forehead, small ulcerated lesions to the right lower mouth and posterior neck, coarse breath sounds in the left upper lung fields, and a 3.5 cm × 3.5 cm nontender lymph node to the left anterior neck. He had no neurological deficits on examination. He was afebrile and tachycardic (120 bpm) with SpO2 of 91% on room air.
Initial laboratory results were significant for leukocytosis with predominant neutrophilia, elevated erythrocyte sedimentation rate and C-reactive protein, elevated liver enzymes, total protein of 10.0 g/dL, albumin of 1.7 g/dL, corrected calcium of 11.0 mg/dL, elevated alkaline phosphatase of 167 U/L, elevated lactic acid of 2.7 mmol/L, elevated lactate dehydrogenase of 341 U/L, and ferritin of 3366 ng/mL. The patient also had anemia of chronic disease. A respiratory viral panel was positive for respiratory syncytial virus.
Our differential diagnosis included disseminated fungal diseases such as coccidioidomycosis, histoplasmosis, or blastomycosis, as well as tuberculosis, secondary hemophagocytic lymphohistiocytosis, HIV, and malignancy. Infectious Diseases was consulted given high likelihood of disseminated coccidioidomycosis.
Serum coccidioides IgG ELISA (enzyme linked immunosorbent assay) was 8.3 IV (reference range ≤0.9 IV) and IgM ELISA was 3.0 IV (reference range ≤0.9 IV). Direct microscopy of the sputum revealed spherules resembling coccidioides. Magnetic resonance imaging (MRI) was obtained to further workup the elevated alkaline phosphatase and hypercalcemia. MRI of the spine (Figures 1-3) revealed a diffusely abnormal signaling with numerous discrete variable sized hyperintense lesions throughout the spine, sacrum, and iliac bones. Indeterminate hyperintense lesions in the bilateral psoas muscles were also present. To confirm that these lesions represented infection and not a potential malignancy, a computed tomography–guided biopsy was performed of the right iliac crest and cervical lymph node. Both biopsies revealed spherules and cultures grew coccidioidomycosis, confirming the presence of a disseminated infection. Lumbar puncture revealed no evidence of cerebrospinal fluid involvement. Laboratory testing for blastomycosis, histoplasmosis, Epstein-Barr virus, cytomegalovirus, HIV, syphilis, tuberculosis, hemophagocytic lymphohistiocytosis, multiple myeloma, and other malignancies were all negative.
Figure 1.

Magnetic resonance imaging of the spine, sagittal view (without contrast): numerous discrete, variable-sized lesions throughout the spine, most notably seen in T12, L1, and L3-L5 vertebrae.
Figure 2.
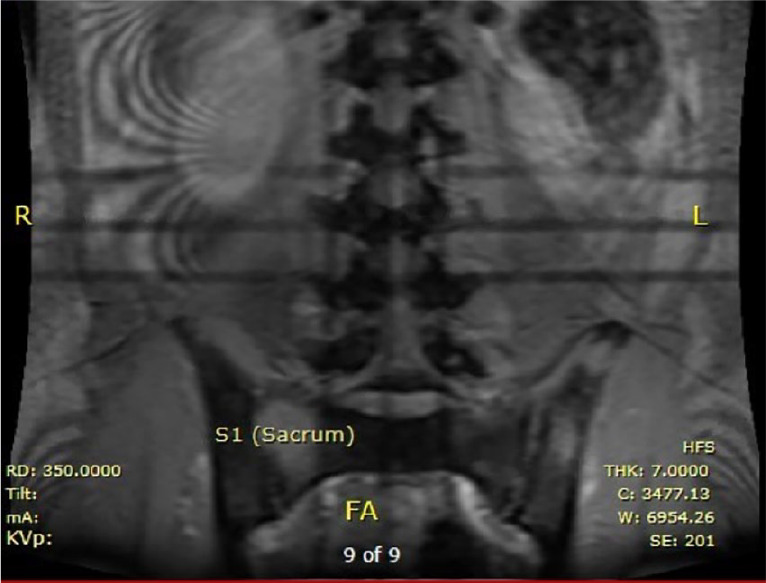
Magnetic resonance imaging of the spine, coronal view (without contrast): lesions in the sacrum.
Figure 3.

Magnetic resonance imaging of pelvis, axial view (without contrast): variable-sized lesions in bilateral iliac crests.
Initially, the patient was admitted to the general medicine floor. He required high-flow nasal cannula and multiple boluses of intravenous fluids to maintain mean arterial pressure of 65 mm Hg. Despite treatment with itraconazole, liposomal amphotericin B, and supplemental oxygen, his respiratory rate continued to increase, and he developed worsening hypoxemia. He was transferred to the intensive care unit, intubated, and given vasopressors. A chest X-ray at that time demonstrated diffuse bilateral reticulonodular opacities consistent with acute respiratory distress syndrome (Figure 4).
Figure 4.

Chest X-ray: Diffuse bilateral fine nodular reticular pattern of interstitial prominence with some fullness to the mediastinum and hila concerning for adenopathy.
The patient required increased FiO2 and PEEP (positive end-expiratory pressure) while intubated; therefore, a computed tomography scan of the chest was done. The imaging found a miliary pattern of pulmonary nodules with focal consolidations posteriorly, along with prominent and confluent superior mediastinal lymphadenopathy obliterating the left upper bronchus resulting in postobstructive consolidation of the left upper lobe. In addition to antifungals, ceftriaxone and azithromycin were started for possible concomitant community-acquired pneumonia. Despite broad spectrum antifungals and antibiotics, his respiratory status continued to worsen. Liposomal amphotericin B treatment resulted in acute kidney injury with creatinine peaking at 4.2 mg/dL (patient’s baseline 0.7 mg/dL). His course was further complicated by paralytic ileus, right internal jugular deep vein thrombosis, and a gluteal hematoma. After weeks of treatment with liposomal amphotericin B, voriconazole, and mechanical ventilation, the patient made a full pulmonary and renal recovery. On the day of discharge, his creatinine was within normal limits, and he did not need supplemental oxygen. He was discharged on an extended course of oral voriconazole with plans to reevaluate treatment after outpatient infectious disease specialist follow-up. He was discharged to an intensive inpatient rehabilitation program for severe physical deconditioning after a prolonged hospitalization.
Discussion
The patient described in this report resided in an area endemic to coccidioidomycosis; however, he possessed no major risk factors for dissemination of this disease. Our workup for HIV/AIDS and other immunocompromising processes was negative, and he denied the use of outpatient immunosuppressant medications. There have been previously documented cases of low-risk individuals developing DC.3,9 Many cases reported that the progression of disease in these individuals was due to a delay in diagnosis at initial presentation.9 Although our patient’s treatment was not delayed, the duration of his treatment was inappropriate. His initial presentation indicated the presence of extrapulmonary disease, evidenced by the presence of ulcerating skin lesions and cervical lymphadenopathy. Therefore, his diagnosis 2 months prior to presenting to our hospital should have been disseminated coccidioidomycosis rather than primary pulmonary coccidioidomycosis, and the duration of treatment should have been much longer than 4 weeks.
The guidelines for the treatment of coccidioidomycosis vary depending on the extent of disease.10 For uncomplicated coccidioidal pneumonia with mild or nondebilitating symptoms, patients may be treated with education, close observation, and supportive care. For those with uncomplicated disease but significant debility or comorbidities, a 3- to 6-month course of an oral azole (such as fluconazole) is recommended. For patients with evidence of soft tissue coccidioidomycosis, such as skin lesions and subcutaneous abscesses, at least 6 to 12 months of oral azole therapy is recommended. For patients with evidence of bone or joint involvement, treatment with an oral azole (itraconazole or fluconazole) is given for a period of 3 years or up to a lifetime. Amphotericin B may also be used in severe bone involvement, especially when there is concern for cord compression with vertebral lesions.2
The appropriate treatment regimen for this patient would have been a minimum of 6 to 12 months of an oral azole, given the presence of extrapulmonary soft tissue infection at initial presentation.2 However, our patient was treated with fluconazole 400 mg daily for 1 month. We believe that this inadequate treatment led to further progression of his respiratory disease and the development of additional extrapulmonary manifestations. The progression of his infection resulted in a complicated and life-threatening disease course. The patient spent 6 weeks in the hospital, developed septic shock, acute respiratory distress syndrome, and significant physical deconditioning requiring an additional 2 weeks of inpatient physical rehabilitation.
The likely culprit behind this patient’s complicated infection was inadequate duration of treatment, but other factors may have contributed to his prolonged disease course. Inpatient evaluation for an immunodeficiency included HIV testing and measurement of T-lymphocytes. Although the patient had a normal CD4 count, it was on the lower side of normal (462 cells/mcL). It is possible the patient has an unknown form of immunodeficiency that may be evaluated in the future by immunogenetics. On admission, the patient also had respiratory syncytial virus and quickly developed hypoxic respiratory failure. The compounded effect of a viral and fungal pneumonia is unknown, but may have contributed to the patient’s rapid decline. Even though the role of dual antifungal therapy in coccidioidomycosis is unknown (unless the patient has additional dissemination to central nervous system), we added itraconazole and later switched it to voriconazole in addition to liposomal amphotericin B before clinical improvement was seen. The minimum inhibitory concentration for his Coccidioides immitis was 8 µg/mL for fluconazole and 0.25 µg/mL for voriconazole. No minimum inhibitory concentration was measured for itraconazole by our hospital’s microbiology laboratory.
Our report presents a rare case of disseminated coccidioidomycosis in a young, relatively healthy male. This case highlights the importance of adjusting treatment regimens based on the symptoms and physical examination findings identified at initial presentation in order to prevent further dissemination of the disease and reduce the risk of morbidity and mortality.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs: Ameesh Dev  https://orcid.org/0000-0003-4441-6705
https://orcid.org/0000-0003-4441-6705
James Gnecco IV  https://orcid.org/0000-0001-5054-0492
https://orcid.org/0000-0001-5054-0492
References
- 1. Akram SM, Koirala J. Coccidioidomycosis. StatPearls; 2020. Accessed May 10, 2009 https://www.ncbi.nlm.nih.gov/books/NBK448161/ [Google Scholar]
- 2. Galgiani JN, Ampel NM, Blair JE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis. 2016;63:e112-e146. [DOI] [PubMed] [Google Scholar]
- 3. Malik U, Cheema H, Kandikatla R, et al. Disseminated coccidioidomycosis presenting as carcinomatosis peritonei and intestinal coccidioidomycosis in a patient with HIV. Case Rep Gastroenterol. 2017;11:114-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown J, Benedict K, Park BJ, Thompson GR., 3rd Coccidioidomycosis: epidemiology. Clin Epidemiol. 2013;5:185-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garcia SCG, Alanis JCS, Flores MG, Gonzalez SEG, Cabrera LV, Candiani JO. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015;90:610-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Odio CD, Marciano BE, Galgiani JN, Holland SM. Risk factors for disseminated coccidioidomycosis, United States. Emerg Infect Dis. 2017;23:308-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santelli AC, Blair JE, Roust LR. Coccidioidomycosis in patients with diabetes mellitus. Am J Med. 2006;119:964-969. [DOI] [PubMed] [Google Scholar]
- 8. Bercovitch RS, Catanzaro A, Schwartz BS, Pappagianis D, Watts DH, Ampel NM. Coccidioidomycosis during pregnancy: a review and recommendations for management. Clin Infect Dis. 2011;53:363-368. [DOI] [PubMed] [Google Scholar]
- 9. Crum NF, Lederman ER, Hale BR, Lim ML, Wallace MR. A cluster of disseminated coccidioidomycosis cases at a US military hospital. Mil Med. 2003;168:460-464. [PubMed] [Google Scholar]
- 10. Ampel NM. The treatment of coccidioidomycosis. Rev Inst Med Trop Sao Paulo. 2015;57(suppl 19):51-56. [DOI] [PMC free article] [PubMed] [Google Scholar]


