Abstract
Background:
Immune checkpoint inhibitors (ICIs) are effective for advanced renal-cell carcinoma (aRCC) but can increase costs. This study compares the efficacy, safety and cost-effectiveness of ICIs for newly diagnosed aRCC patients in the first-line setting.
Methods:
Trials evaluating ICI regimens as first-line treatment for newly diagnosed aRCC were searched and included. A network meta-analysis (NMA) was conducted, and a cost-effectiveness analysis was performed from the US payer’s perspective. The key outcomes were overall survival (OS) and progression-free survival (PFS) in the NMA, and quality-adjusted life years (QALYs), costs and the incremental cost-effectiveness ratio (ICER) in the cost-effectiveness analysis.
Results:
Four randomized controlled trials (RCTs) involving 3758 patients receiving first-line ICIs treatment were analyzed. The NMA showed that pembrolizumab plus axitinib was ranked higher than the other three ICI regimens and sunitinib in the overall population. Nivolumab plus ipilimumab and pembrolizumab plus axitinib achieved more health benefits than the other ICI regimens and sunitinib in programmed death ligand 1 (PD-L1)-positive and negative tumors, respectively. Among the four ICI regimens, only the ICERs of nivolumab plus ipilimumab over sunitinib were lower than the willingness-to-pay threshold ($150,000/QALY) in the overall and PD-L1-positive populations, and none of four ICI regimens were lower than $150,000/QALY in PD-L1-negative populations.
Conclusions:
The NMA and cost-effectiveness analysis revealed that nivolumab plus ipilimumab is the most favorable first-line treatment for PD-L1-positive aRCC compared with other ICI regimens and sunitinib. Pembrolizumab plus axitinib is likely to be an alternative for PD-L1-negative aRCC due to its more favorable health advantages.
Keywords: cost-effectiveness analysis, immune checkpoint inhibitors, network meta-analysis, renal-cell carcinoma, systematic review
Introduction
The Global Burden of Disease Study 2017 showed that kidney cancer accounted for 1.40% of the disease burden from all neoplasms.1 As the most lethal of the common types of kidney cancer, advanced renal-cell carcinoma (aRCC) is diagnosed in approximately 30% of patients due to the generally asymptomatic nature of the disease at onset.2,3 Over the past decade, targeted agents, such as cabozantinib and sunitinib, have been widely prescribed for treating aRCC. Although notably prolonged survival has been achieved by these targeted agents, aRCC is still incurable, with a median overall survival (OS) of approximately 2 years.4–6 Therefore, the development of novel agents to treat this advanced disease is necessary.
Immune checkpoint inhibitor (ICI)-based regimens, which could block the programmed cell death 1 (PD-1) pathway or cytotoxic T lymphocyte-associated protein 4 (CTLA-4),7,8 are emerging as a new therapeutic modality for aRCC because these regimens can improve survival and quality of life. The recent CheckMate 214, KEYNOTE-426, IMmotion 151 and JAVELIN Renal 101 trials reported that nivolumab plus ipilimumab, pembrolizumab plus axitinib, atezolizumab plus bevacizumab and avelumab plus axitinib could be considered potential alternative first-line treatments for patients with previously untreated aRCC because these drugs are well tolerated and significantly reduce the risk of death in comparison with sunitinib.9–12 However, no studies have directly compared different ICI regimens with each other, so it is unclear which regimen should be advocated as the first option for most patients. The question of whether the expensive ICIs are balanced by improved health benefits, reduced health resource consumption of later-line treatment, or both, remains unresolved. In addition, the effects of ICI regimens may be mixed in the whole cohort because the efficacy for some patients was not fully realized. We performed a systematic review, network meta-analysis (NMA), and cost-effectiveness analysis to investigate regimens containing ICIs with each other and with sunitinib for the first-line management of aRCC, and constructed a rank order based on efficacy, safety and cost in the US.
Methods
The results were reported following the PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions and the consolidated health economic evaluation reporting standards statement (CHEERS) (Supplemental Appendix Tables 1 and 2, respectively).
Study eligibility and selection
The electronic databases including PubMed, EMBASE and the Cochrane Central Register of Controlled Trials were searched to identify eligible randomized controlled trials (RCTs) that compared regimens containing PD-1/programmed death ligand 1 (PD-L1) inhibitors with tyrosine kinase inhibitor (TKI) treatment in the first-line setting. The entry terms for the search included pembrolizumab, atezolizumab, avelumab, durvalumab, nivolumab, PD-1, PD-L1, renal-cell carcinoma, and randomized controlled trial (Supplemental Appendix File 1: supplemental methods of meta-analysis); we searched for studies that were reported in English until 19 March 2019. We also reviewed abstracts presented at the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO) and the American Association for Cancer Research (AACR). When duplicate trials were found, only the most complete and latest data of the trial were considered. Meeting abstracts without full-text original reports as well as case reports/letters/commentaries and reports not written in the English language were ineligible for this analysis. The inclusion and exclusion criteria are shown in Supplemental Appendix Table 3 of the supplemental methods of the meta-analysis.
Collection of data and assessment of the risk of bias
Two reviewers (JF and YL) independently screened the studies. The extracted data included the study characteristics (author, publication time, design of the trial, number of patients enrolled), patient clinicopathological characteristics (age, histology, PD-L1 level), interventions including PD-1/PD-L1 therapy and the comparator regimen, and the outcome measures [hazard ratios (HRs) for OS and progression-free survival (PFS), and the odds ratio (OR) for adverse drug reactions (ADRs)]. The quality of the trials was evaluated with the Cochrane Collaboration’s tool.
Data synthesis and statistical analysis
We produced network plots of comparisons to annotate which regimens had been compared within randomized trials (head-to-head comparisons). Fixed-effects and random-effects models were explored. However, as typically only one trial informed each pair-wise comparison, and hence there were few data to inform the evaluation of heterogeneity between trials, a pragmatic decision was made to use the fixed-effects model; this model was applied to compare the relative efficacy and safety between different regimens by using frequentist methods.13 Ranking the different treatments in terms of their likelihood of showing the best results was performed using the p-value for each outcome, in which higher values represent better success. Statistical analysis was performed using R software (version 3.5.2) with the package ‘netmeta’.14
Cost-effectiveness analysis
We assessed the most cost-effective first-line regimens containing ICIs in patients with newly diagnosed aRCC from the perspective of the third-party payer in the US. A Markov multistate model with a cycle length of 1 week was adopted (Supplemental Appendix File 2: supplemental methods of the cost-effectiveness analysis). The baseline characteristics of the simulated patients were assumed to be similar to the patients in CheckMate 214, KEYNOTE-426, IMmotion 151 and JAVELIN Renal 101 trials.9–12 The expected total costs, quality-adjusted life years (QALYs) and incremental cost-effectiveness ratios (ICERs) were estimated over a lifetime horizon (10 years), as in previous economic studies.15,16 The model inputs of clinical data, cost and utility estimates were collected from the network meta-analysis and published literature (Table 1). To examine the potential drivers of economic outcomes, we performed both one-way and probabilistic sensitivity analyses (PSAs). Subgroup analysis was performed in patients categorized by PD-L1 status. Detailed information about the model development and clinical, cost and health preference inputs are shown in the Supplemental Appendix.
Table 1.
Key model inputs.
| Parameters | Values | Reference |
|---|---|---|
| Clinical data | ||
| Survival model of sunitinib | Motzer et al.9,10; Rini et al.12,17 | |
| Log-logistic model for PFS | Shape: 1.3705 (se: 0.0357), scale: 37.5566 (se: 1.2562); AIC: 10134.28 | |
| Log-logistic model for OS | Shape: 1.2800 (se: 0.05), scale: 135.14 (se: 7.02); AIC: 6398.76 | |
| HRs of ICI regimens against sunitinib | See the results of network meta-analysis | |
| Proportion (%) of receiving active second-line treatment | ||
| Sunitinib | 0.57 [Range: 0.52–0.63, distribution: beta (155.2, 117.1)] | Motzer et al.9,10; Rini et al.12,17 |
| Avelumab+axitinib | 0.43 [Range: 0.33–0.54, distribution: beta (8.7, 11.5)] | Motzer et al.10 |
| Pembrolizumab+axitinib | 0.5 [Range: 0.38–0.63, distribution: beta (7.4, 7.4)] | Rini et al.17 |
| Nivolumab+ipilimumab | 0.39 [Range: 0.29–0.49, distribution: beta (9.3, 14.5)] | Motzer et al.9 |
| Atezolizumab+bevacizumab# | 0.43 [Range: 0.32–0.54, distribution: beta (8.7, 11.5)] | Rini et al.12 |
| Cost data (US, $) | ||
| Price of sunitinib per 50 mg | 602 (Range: 301–602, distribution: fixed) | Red book online |
| Price of ipilimumab per 50 mg | 7324 (Range: 3662–7324, distribution: fixed) | Red book online |
| Price of nivolumab per 100 mg | 2670 (Range: 1335–2670, distribution: fixed) | Red book online |
| Price of nivolumab per 240 mg | 6427 (Range: 3213–6427, distribution: fixed) | Red book online |
| Price of avelumab per 200 mg | 1650 (Range: 825–1650, distribution: fixed) | Red book online |
| Price of pembrolizumab per 50 mg | 2295 (Range: 1148–2295, distribution: fixed) | Red book online |
| Price of axitinib per 10 mg | 525 (Range: 263–525, distribution: fixed) | Red book online |
| Price of atezolizumab per 1200 mg | 9280 (Range: 4640–9280, distribution: fixed) | Red book online |
| Price of bevacizumab per 100 mg | 841 (Range: 420–841, distribution: fixed) | Red book online |
| Cost of follow-up and monitoring per cycle | 422 [Range: 348–496, distribution: gamma (4731, 0.0892)] | Benedict et al.18 |
| Cost of second-line active treatment per patient | 27936 [Range: 26429–29443, distribution: gamma (1015855, 0.0275)] | Perrin et al.19 |
| Cost of BSC per cycle | 1213 [Range: 987–1438, distribution: gamma (4856, 0.2498)] | Henk et al.20 |
| Cost of terminal per patient | 10713 [Range: 8570–12856, distribution: gamma (105029, 0.102)] | Perrin et al.19; McCrea et al.21 |
| Cost of managing AEs (grade ⩾3) per event | ||
| Fatigue | 139 [Range: 1–2018, distribution: gamma (44, 3.1871)] | Perrin et al.19; Hansen et al.22; Liou et al.23 |
| Hypertension | 202 [Range: 1–6533, distribution: gamma (6, 32.3416)] | Perrin et al.19; Hansen et al.22; Liou et al.23 |
| Anemia | 4638 [Range: 3326–5949, distribution: gamma (32164, 0.1442)] | Perrin et al.19; Hansen et al.22; Liou et al.23 |
| Palmar–plantar erythrodysesthesia | 119 [Range: 3–1748, distribution: gamma (75, 1.5966)] | Perrin et al.19; Hansen et al.22; Liou et al.23 |
| Thrombocytopenia | 4014 [Range: 1716–9391, distribution: gamma (8229, 0.4878)] | Liou et al.23 |
| Cost of drug administration per unit | 292 [Range: 219–365, distribution: gamma (1168, 0.25)] | Sarfaty et al.16 |
| Health utility scores | ||
| Utility of PFS | 0.78 [Range: 0.71–0.85, distribution: beta (106.9, 30.2)] | McCrea et al.21; Amdahl et al.24; de Groot et al.25; Hoyle et al.26 |
| Utility of PS | 0.66 [Range: 0.45–0.82, distribution: beta (16.4, 8.4)] | McCrea et al.21; Amdahl et al.24; de Groot et al.25; Hoyle et al.26 |
| Disutility due to AEs (grade 1 and 2) | 0.01 [Range: 0.01–0.02, distribution: beta (9.4, 933.8)] | Amdahl et al.24 |
| Disutility due to AEs (grade ⩾3) | 0.16 [Range: 0.11–0.2, distribution: beta (37.2, 195.1)] | Amdahl et al.24 |
Due to no data reported by the IMmotion 151 at the present time point, an average proportion from the CheckMate 214, KEYNOTE-426, IMmotion 151 and JAVELIN Renal 101 trials was assumed.
AE, adverse event; BSC, best supportive care; HR, hazard ratio; OS, overall survival; PD-1, programmed cell death 1; PFS, progression-free survival; PS, progressed survival.
Results
Studies included and the risk of bias
After selecting abstracts and titles, four clinical trials involving 3758 patients were identified (see Supplemental Appendix Table 4 for a summary of the trial characteristics); these patients were treated with nivolumab plus ipilimumab (n = 550 patients), pembrolizumab plus axitinib (n = 432 patients), avelumab plus axitinib (n = 442 patients) and atezolizumab plus bevacizumab (n = 454 patients) or sunitinib (n = 1334 patients) as first-line treatment.9–12
The risk of bias judgments for the included studies are shown in Supplemental Appendix Figure 2. All four studies were judged to have an unclear risk of bias for sequence generation and allocation concealment and were judged to have a high risk of performance bias. Three studies were judged to have a low risk of bias for blinding of outcome assessment, and all four studies were judged to have a low risk of bias for incomplete outcome data and selective reporting.
Efficacy and safety results
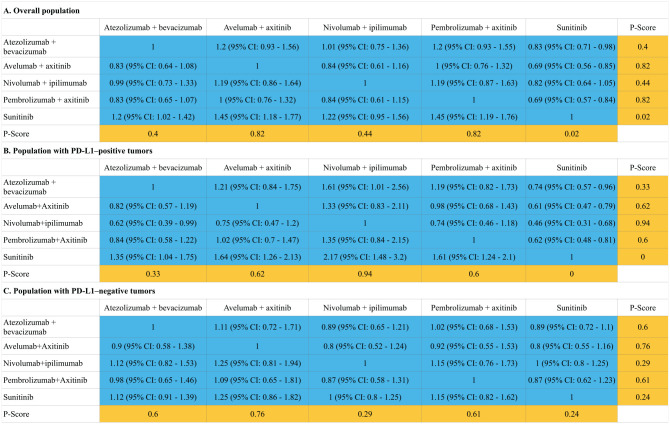
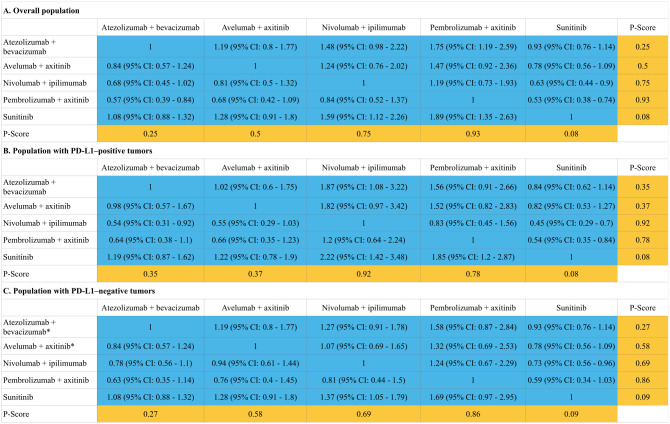
A total of four ICI regimens (atezolizumab plus bevacizumab, avelumab plus axitinib, nivolumab plus ipilimumab and pembrolizumab plus axitinib) and one control regimen (sunitinib) were included in the network (Supplemental Appendix Figure 3). Indirect comparisons demonstrated that both nivolumab plus ipilimumab [HR: 0.63, 95% confidence interval (CI): 0.44–0.9 and HR: 0.45, 95% CI: 0.29–0.7] and pembrolizumab plus axitinib (HR: 0.53, 95% CI: 0.38–0.74 and HR: 0.73, 95% CI: 0.56–0.96) could lead to statistically significant improvements in OS compared with sunitinib treatment in the overall population and PD-L1-positive patients (the definition of positivity is shown in Supplemental Appendix Table 5). The regimens of atezolizumab plus bevacizumab, avelumab plus axitinib and pembrolizumab plus axitinib for the overall population and all four ICI regimens for the population with PD-L1-positive tumors also achieved statistically significant improvements in PFS. The regimens with the highest p-value for OS in the overall and PD-L1-positive populations were pembrolizumab plus axitinib (HR: 0.53, 95% CI: 0.38–0.74; p = 0.93) and nivolumab plus ipilimumab (p = 0.92), respectively. Among the PD-L1-negative patients, the regimen with the highest p-value for OS was pembrolizumab plus axitinib (HR: 0.59, 95% CI: 0.34–1.03; p = 0.86) followed by nivolumab plus ipilimumab (HR: 0.73, 95% CI: 0.56–0.96; p = 0.69). No statistically significant differences in PFS and OS were found between the four ICI regimens. The results of indirect comparisons and the p-values of the PFS and OS of each regimen are shown in Figures 1 and 2, respectively.
Figure 1.
Hazard ratios (blue cell) and p-values (orange cell) of the network meta-analysis of the progression-free survival in the overall population (A), population with PD-L1-positive tumors (B) and population with PD-L1-negative tumors (C).
Figure 2.
Hazard ratios (blue cell) and p-values (orange cell) of the network meta-analysis of the overall survival in the overall population (A), population with PD-L1-positive tumors (B) and population with PD-L1-negative tumors (C).
*Due to the lack of reported data, we conservatively assumed that the HRs for OS in PD-L1-negative patients were equal to those of the overall population and applied these data in the network meta-analysis.
The forest plot revealed that the four ICI regimens had comparable safety profiles concerning any grade ADRs, and nivolumab plus ipilimumab had a lower likelihood of grade ⩾3 ADRs (OR: 0.73, 95% CI: 0.62–0.86) than sunitinib (Supplemental Appendix Figure 4). The regimen with the highest p-value for any grade (p = 0.64) and grade ⩾3 ADRs (p = 0.89) was nivolumab plus ipilimumab.
Cost-effectiveness results
The base-case predicted mean costs, life years (LYs) and QALYs gained for each strategy are summarized in Table 2. For the overall population, the mean marginal costs and QALYs of avelumab plus axitinib, pembrolizumab plus axitinib, nivolumab plus ipilimumab and atezolizumab plus bevacizumab versus sunitinib were $197,793 and 0.53, $238,651 and 1.19, $121,948 and 0.86, and $81,010 and 0.18, which yielded ICERs of $371,360, $201,027, $141,120 and $448,952 per QALY gained, respectively. For the population with PD-L1-positive and negative tumors, the ICERs of avelumab plus axitinib, pembrolizumab plus axitinib, nivolumab plus ipilimumab and atezolizumab plus bevacizumab versus sunitinib were $406,644 and $389,229, $199,084 and $226,595, $92,262 and $180,251 and $245,355 and $500,910 per QALY, respectively. In the fixed dosing of nivolumab, the ICERs of nivolumab plus ipilimumab over sunitinib were $110,838, $71,641 and $138,285 per QALY gained in the overall, PD-L1-positive and PD-L1-negative population, respectively.
Table 2.
Summary of the cost ($) and outcome results in base-case analysis.
| Strategy | Cost | Overall LYs | QALYs | ICER* |
|---|---|---|---|---|
| Overall population | ||||
| Sunitinib | 291,572 | 4.03 | 2.59 | NA |
| Avelumab plus axitinib | 489,364 | 4.80 | 3.12 | 371,360 |
| Pembrolizumab plus axitinib | 530,223 | 5.96 | 3.77 | 201,027 |
| Nivolumab plus ipilimumab | 413,520 | 5.46 | 3.45 | 141,120 |
| Nivolumab plus ipilimumab with a fixed dose# | 387,352 | 5.4566 | 3.4513 | 110,838 |
| Atezolizumab plus bevacizumab | 372,582 | 4.26 | 2.77 | 448,952 |
| Population with PD-L1-positive tumors | ||||
| Sunitinib | 262,152 | 3.48 | 2.27 | NA |
| Avelumab plus axitinib | 455,503 | 4.08 | 2.74 | 406,644 |
| Pembrolizumab plus axitinib | 501,747 | 5.37 | 3.47 | 199,084 |
| Nivolumab plus ipilimumab | 411,000 | 5.90 | 3.88 | 92,262 |
| Nivolumab plus ipilimumab with a fixed dose# | 377,732 | 5.9043 | 3.8810 | 71,641 |
| Atezolizumab plus bevacizumab | 356,859 | 4.00 | 2.65 | 245,355 |
| Population with PD-L1-negative tumors | ||||
| Sunitinib | 309,256 | 4.37 | 2.78 | NA |
| Avelumab plus axitinib | 499,790 | 5.14 | 3.27 | 389,229 |
| Pembrolizumab plus axitinib | 521,157 | 5.96 | 3.71 | 226,595 |
| Nivolumab plus ipilimumab | 410,084 | 5.34 | 3.34 | 180,251 |
| Nivolumab plus ipilimumab with a fixed dose# | 386,609 | 5.3389 | 3.3382 | 138,285 |
| Atezolizumab plus bevacizumab | 391,217 | 4.59 | 2.94 | 500,910 |
Incremental cost per QALY (versus sunitinib).
Nivolumab dosing schedule, a single 480 mg iv dose every 4 weeks.
ICER, incremental cost-effectiveness ratio; LY, life year; PD-L1-, programmed death ligand 1; QALY, quality-adjusted life year.
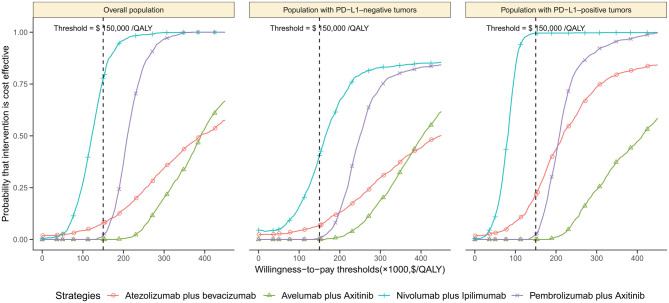
The one-way sensitivity analyses found that these results were substantially sensitive to the HRs of OS of ICI regimens against sunitinib (Supplemental Appendix Figure 12). The expected age, median duration for nivolumab treatment in the 2n-line setting and price of the ICI regimen also had considerable impacts on the ICERs. The longer expected survival after immunotherapy led to the smaller ICERs (Supplemental Appendix Figure 13). The other parameters included in the sensitivity analyses, such as the cost and utilities related to ADRs, had minimal impact on the ICER. At a willingness-to-pay threshold of $150,000/QALY, the cost-effectiveness acceptability curves showed that the probabilities of the avelumab plus axitinib, pembrolizumab plus axitinib and atezolizumab plus bevacizumab strategies being cost-effective were <25% in the overall, PD-L1-positive and PD-L1-negative population except for the nivolumab plus ipilimumab strategy, which were 77.8%, 99.5% and 40.5% compared with sunitinib (Figure 3), respectively.
Figure 3.
The cost-effectiveness acceptability curves for the avelumab plus axitinib, pembrolizumab plus axitinib, nivolumab plus ipilimumab and atezolizumab plus bevacizumab strategies compared to the sunitinib strategy in the overall population (A), population with PD-L1-negative tumors (B) and population with PD-L1-positive tumors (C).
Discussion
To the best of our knowledge, this is the first evaluation of all currently licensed ICI regimens for patients with untreated aRCC that has explored a p-value order for therapy options in terms of efficacy, safety outcomes and overall economic outcomes. Four ICI regimens appear to be superior to the standard sunitinib strategy for improving survival, especially in patients with PD-L1-positive tumors. The findings of the NMAs suggest that both the nivolumab plus ipilimumab and pembrolizumab plus axitinib regimens yielded more survival benefits than the other ICI regimens and sunitinib in patients with PD-L1-positive and PD-L1-negative tumors, respectively. All four ICI regimens are associated with a comparable risk for ADRs of all grades, and both nivolumab plus ipilimumab and atezolizumab plus bevacizumab were associated with a reduced risk of grade ⩾3 ADRs compared with sunitinib. The overall survival advantage of nivolumab plus ipilimumab and pembrolizumab plus axitinib translated into the highest QALYs in PD-L1-positive and PD-L1-negative patients, respectively. However, nivolumab plus ipilimumab is considered to be the most cost-effective alternative only because this regimen has the lowest ICER among all four ICI regimes in the overall, PD-L1-positive and negative patients. The findings in the scenario analyses are comparable with the US setting, which also found that nivolumab plus ipilimumab is the ICI regimen with the lowest ICER in the context of the UK and China (Supplemental Appendix Tables 7 and 8 and Supplemental Appendix Figure 14). Thus, the strengths of this assessment include universal profiles of the present research findings, a critical appraisal of the study quality, a focus on health outcomes, transferability of the findings across different regions and universal assessments that provide comparisons between ICI regimens as well as comparisons of ICI regimens with sunitinib.
Our economic analyses are sensitive to the relative efficacy of ICI regimens; the analysis found that the economic outcomes of the ICI regimens would become more favorable in patients with lower HRs of OS of ICI regimens against sunitinib and would become worse in patients with higher HRs. This finding is consistent with our subgroup analysis that showed that the economic outcomes of ICI regimens in PD-L1-positive patients were better than those in PD-L1-negative patients because the former has a better relative efficacy of ICI treatment. Several studies have found that PD-L1 expression is a poor prognostic factor and is predictive of better treatment responses from both PD-1 and PD-L1 inhibitors in a variety of tumor types, including aRCC.27,28 Due to the high cost of ICI agents, an early test for the validated biomarkers that predict the prognosis of immunotherapy is vital in the context of cost-efficiency and optimal logistics.
The current evaluation has several potential implications. Our study suggests that, of the presently available ICI regimens, nivolumab plus ipilimumab ranks as the best option for the population with PD-L1-positive tumors after accounting for efficacy, safety and cost. The CheckMate 214 trial showed nivolumab plus ipilimumab had better objective response rates (58% versus 37%) and overall survival in (HR 0.45 versus 0.73) patients with ⩾1% tumor PD-L1 than those with <1%, which is more distinguishable than the other three trials. Therefore, identifying the eligible patients receiving nivolumab plus ipilimumab might be a valuable and rational choice, although the current practice does not recommend checking PD-L1 status in patients with aRCC.29 It implied that PD-L1-positive tumors might receive nivolumab plus ipilimumab treatment and the rest of the people could receive a regimen containing ICIs and axitinib after the PD-L1 assay associated with nivolumab plus ipilimumab. These findings suggest that meaningful guidance on the hierarchy of ICI regimens to prolong the survival of patients with aRCC will be necessary, specifically for a therapy hierarchy and contexts under which alternative regimens from within similar classes should be administered (e.g. reserve therapies for those who have a treatment response based on PD-L1 status, specific ADRs or contraindications to the primary regimen). This approach should augment the consumption of this drug class, benefit patient health, and result in efficient and sustained health resource allocation.
There are several limitations. First, as evidenced in Supplemental Appendix Table 4, pharmaceutical companies have partnered with different PD-L1 assays. Due to the inability consistently to predict response across clinical trials and the limitations of PD-L1 immunohistochemistry staining, the limited role of PD-L1 as a predictive biomarker should be acknowledged in the comparisons across clinical trials. Therefore, the results in PD-L1-positive and PD-L1-negative tumors should be carefully interpreted. Second, a NMA approach was adopted for directly comparing between ICI regimens with the assumption that the included studies did not differ in patient characteristics, although no clear evidence of effect modification caused by the patient characteristics was observed (Supplemental Appendix Figure 10). Third, the short-term survival data of each trial were used to project the long-term outcomes in the cost-effectiveness analysis, which informed assumptions about treatment pathways and health state transitions. Third, although the guidelines have recommended cabozantinib to treat aRCC,30 we did not include it in this analysis because of its non-significant OS compared with sunitinib (HR 0.80, 95% CI 0.53–1.21) and other concerns.31,32 Fourth, the current analysis conservatively projected the outcomes over a 10-year time horizon. The cost-effectiveness of ICI regimes would become unfavorable when the shorter time horizon was adopted (Supplemental Appendix Figure 13). Nivolumab plus ipilimumab would cost more money than the other three ICI regimes in the first 3 months (Supplemental Appendix Table 9). Fifth, we did not investigate the economic outcomes in other subpopulations, such as the age and International Metastatic Renal Cell Carcinoma Database Consortium risk category. Finally, the analysis does not address the impact of different payment schemes, which is a much more tangible and relevant issue to most patients and providers, although it is not the focus of this analysis.
Regimens containing ICIs appear to be superior to standard sunitinib treatment in patients with untreated aRCC. Among the four ICI regimens, the combination regimen of nivolumab plus ipilimumab appears to maximize efficacy for those with PD-L1-positive tumors, with more favorable economic outcomes. Although pembrolizumab plus axitinib is likely to be a favorable option for PD-L1-negative patients due to its considerable trend of efficacy, the economic outcome is unlikely to be ideal. Further real-world data may bring new insights with respect to economic outcomes, and it is important to identify patients who may not benefit from ICIs treatment. New studies addressing the limitations of the present analysis may better inform physicians and decision-makers in this clinical context.
Supplemental Material
Supplemental material, CEA_appendix_4-13 for First-line treatments for advanced renal-cell carcinoma with immune checkpoint inhibitors: systematic review, network meta-analysis and cost-effectiveness analysis by Yingjie Su, Jie Fu, Jiangyang Du and Bin Wu in Therapeutic Advances in Medical Oncology
Footnotes
Contributors: BW was involved in the design of the study. YS and JF collected the data. YS and JD performed the meta-analysis. BW and YS performed the economic analysis. BW wrote the first draft of the manuscript, which was critically revised by YS, JF, JD and BW. All authors have approved this version for publication. BW is the study guarantor.
Conflict of interest: The authors declare that there is no conflict of interest.
Ethics approval: This study was based on a literature review and modelling techniques; this study did not require approval by an institutional research ethics board.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Disclaimer: The views expressed are those of the authors. The funding agencies played no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Bin Wu  https://orcid.org/0000-0002-6696-7471
https://orcid.org/0000-0002-6696-7471
Contributor Information
Yingjie Su, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China.
Jie Fu, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China.
Jiangyang Du, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China.
Bin Wu, Medical Decision and Economic Group, Department of Pharmacy, Ren Ji Hospital, School of Medicine, Shanghai Jiaotong University, South Campus, Jiangyue Road 1600, Shanghai 200127, China.
References
- 1. GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018; 392: 1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhatt JR, Finelli A. Landmarks in the diagnosis and treatment of renal cell carcinoma. Nat Rev Urol 2014; 11: 517–525. [DOI] [PubMed] [Google Scholar]
- 3. Amzal B, Fu S, Meng J, et al. Cabozantinib versus everolimus, nivolumab, axitinib, sorafenib and best supportive care: a network meta-analysis of progression-free survival and overall survival in second line treatment of advanced renal cell carcinoma. PLoS One 2017; 12: e184423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sheng X, Chi Z, Cui C, et al. Efficacy and safety of sorafenib versus sunitinib as first-line treatment in patients with metastatic renal cell carcinoma: largest single-center retrospective analysis. Oncotarget 2016; 7: 27044–27054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandez-Pello S, Hofmann F, Tahbaz R, et al. A systematic review and meta-analysis comparing the effectiveness and adverse effects of different systemic treatments for non-clear cell renal cell carcinoma. Eur Urol 2017; 71: 426–436. [DOI] [PubMed] [Google Scholar]
- 6. Gu W, Zhu Y, Wang H, et al. Prognostic value of components of body composition in patients treated with targeted therapy for advanced renal cell carcinoma: a retrospective case series. PLoS One 2015; 10: e118022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018; 359: 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luke JJ, Ott PA. PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget 2015; 6: 3479–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018; 378: 1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380: 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Motzer RJ, Powles T, Atkins MB, et al. IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC). J Clin Oncol 2018; 36 (Suppl. 6): 578. [Google Scholar]
- 12. Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019; 393: 2404–2415. [DOI] [PubMed] [Google Scholar]
- 13. Rucker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol 2015; 15: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neupane B, Richer D, Bonner AJ, et al. Network meta-analysis using R: a review of currently available automated packages. PLoS One 2014; 9: e115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Remak E, Charbonneau C, Negrier S, et al. Economic evaluation of sunitinib malate for the first-line treatment of metastatic renal cell carcinoma. J Clin Oncol 2008; 26: 3995–4000. [DOI] [PubMed] [Google Scholar]
- 16. Sarfaty M, Leshno M, Gordon N, et al. Cost effectiveness of nivolumab in advanced renal cell carcinoma. Eur Urol 2018; 73: 628–634. [DOI] [PubMed] [Google Scholar]
- 17. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 18. Benedict A, Figlin RA, Sandstrom P, et al. Economic evaluation of new targeted therapies for the first-line treatment of patients with metastatic renal cell carcinoma. BJU Int 2011; 108: 665–672. [DOI] [PubMed] [Google Scholar]
- 19. Perrin A, Sherman S, Pal S, et al. Lifetime cost of everolimus vs axitinib in patients with advanced renal cell carcinoma who failed prior sunitinib therapy in the US. J Med Econ 2015; 18: 200–209. [DOI] [PubMed] [Google Scholar]
- 20. Henk HJ, Chen C, Benedict A, et al. Retrospective claims analysis of best supportive care costs and survival in a US metastatic renal cell population. Clinicoecon Outcomes Res 2013; 5: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCrea C, Johal S, Yang S, et al. Cost-effectiveness of nivolumab in patients with advanced renal cell carcinoma treated in the United States. Exp Hematol Oncol 2018; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hansen RN, Hackshaw MD, Nagar SP, et al. Health care costs among renal cancer patients using pazopanib and sunitinib. J Manag Care Spec Pharm 2015; 21: 37–44, 44a–d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liou SY, Stephens JM, Carpiuc KT, et al. Economic burden of haematological adverse effects in cancer patients: a systematic review. Clin Drug Invest 2007; 27: 381–396. [DOI] [PubMed] [Google Scholar]
- 24. Amdahl J, Diaz J, Park J, et al. Cost-effectiveness of pazopanib compared with sunitinib in metastatic renal cell carcinoma in Canada. Curr Oncol 2016; 23: e340–e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Groot S, Redekop WK, Versteegh MM, et al. Health-related quality of life and its determinants in patients with metastatic renal cell carcinoma. Qual Life Res 2018; 27: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoyle M, Green C, Thompson-Coon J, et al. Cost-effectiveness of temsirolimus for first line treatment of advanced renal cell carcinoma. Value Health 2010; 13: 61–68. [DOI] [PubMed] [Google Scholar]
- 27. Lopez-Beltran A, Henriques V, Cimadamore A, et al. The identification of immunological biomarkers in kidney cancers. Front Oncol 2018; 8: 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gnjatic S, Bronte V, Brunet LR, et al. Identifying baseline immune-related biomarkers to predict clinical outcome of immunotherapy. J Immunother Cancer 2017; 5: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hahn AW, Sirohi D, Agarwal N. The role of PD-L1 testing in advanced genitourinary malignancies. Eur Urol Focus 2019; 6: 11–13. [DOI] [PubMed] [Google Scholar]
- 30. Lyseng-Williamson KA. Cabozantinib as first-line treatment in advanced renal cell carcinoma: a profile of its use. Drugs Ther Perspect 2018; 34: 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Choueiri TK, Hessel C, Halabi S, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression-free survival by independent review and overall survival update. Eur J Cancer 2018; 94: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buti S, Bersanelli M. Is cabozantinib really better than sunitinib as first-line treatment of metastatic renal cell carcinoma? J Clin Oncol 2017; 35: 1858–1859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CEA_appendix_4-13 for First-line treatments for advanced renal-cell carcinoma with immune checkpoint inhibitors: systematic review, network meta-analysis and cost-effectiveness analysis by Yingjie Su, Jie Fu, Jiangyang Du and Bin Wu in Therapeutic Advances in Medical Oncology