To the editor,
Several evidence have suggested the role of Interleukin‐6 (IL‐6) in the cytokine storm induced by severe acute respiratory syndrome Coronavirus 2 (SARS‐CoV2) infection and its correlation with the severity of acute lung injury. 1 , 2 Azis et al 3 have admirably analyzed the association between elevated IL‐6 and severe pneumonia. They have also clarified the necessity to define a cutoff of this cytokine in patients with high mortality risk.
We report a case of 47‐year‐old female Covid‐19 patient who had developed severe pneumonia complicated by Guillain‐Barré syndrome (GBS). The patient was self‐isolated at home after contact with a positive individual, referring fever and dry cough as onset symptoms. Respiratory worsening with severe hypoxemia, elevated lactates, and d‐dimer required sudden hospitalization in Intensive Care Unit (ICU) and subsequent mechanical ventilation. Lopinavir/Ritonavir, Hydroxychloroquine, Enoxaparin, Ceftriaxone, and Azithromycin therapy was administered with a poor clinical response. Blood test revealed high levels of IL‐6 (serum IL‐6: 402 pg/mL; reference value <3.5 pg/mL) and of its soluble receptor (soluble IL‐6 receptor >1900 pg/mL; reference value <46 pg/mL). Therefore, she was treated with two infusions of Tocilizumab, which resulted in clinical improvement and interruption of mechanical ventilation. In this patient, the evidence of right adrenal adenoma (Figure 1A), resistant hypertension, severe hypokalemia, and high serum levels of aldosterone (1194 pg/mL), with aldosterone/renin ratio of 373 ng/dL/(ng/mL/h), were also consistent with diagnosis of primary aldosteronism (PA). Thus, Spironolactone therapy was administered with improvements in clinical condition and mostly in respiratory symptoms. Finally, she was diagnosed with acute motor sensitive neuropathy (AMSAN) with prolonged distal motor/sensory latencies in the lower limbs at electromyography. Neurological symptoms were underestimated during the invasive ventilation period and late diagnosis did not allow for intravenous immune globulin therapy. Therefore, the patient began a rehabilitation phase and she was discharged with residual pulmonary fibrosis (Figure 1B).
Figure 1.
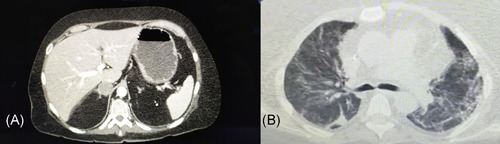
(A) Abdominal CT scan showing a right adrenal adenoma; (B) Thoracic CT scan with diffuse fibrosis and ground‐glass alterations in both the lungs. CT, computed tomography
In this case, serum IL‐6 is higher compared to the average values reported in the meta‐analysis. 3 Therefore, we suppose that an imbalance of renin angiotensin system (RAS) with high levels of aldosterone may directly stimulate IL‐6 production. The involvement of RAS dysfunction with increased angiotensin II levels in lung injury of Covid‐19 patients has previously been postulated. 4 , 5 , 6 In turn, higher levels of angiotensin II can increase aldosterone production by adrenal cortex cells. Thus, SARS‐CoV2 infection can directly enhance serum aldosterone levels by promoting its detrimental activity systemically, in the lungs and endothelial cells.
The correlation between the serum levels of IL‐6 and the plasma aldosterone has already been demonstrated in patients affected by PA. 7
Therefore, we assume that the highest levels of aldosterone may induce IL‐6 and cytokine storm in patients with PA and Covid‐19 pneumonia, mostly by directing macrophages toward M1 proinflammatory phenotype and by activating dendritic cells. 8 , 9 Furthermore, aldosterone has direct effects on IL‐6 production in endothelial cells through the activation of mineralocorticoid receptor/nuclear factor kB (NF‐KB) pathway. 7 , 8
Intriguingly, the association between PA and Covid‐19 has not yet been described and proinflammatory role of aldosterone in SARS‐CoV2 infection remains unclear. 10 , 11
In addition, GBS has been reported as a neurological complication associated with SARS‐CoV2 infection, nevertheless AMSAN represents an uncommon phenotype. 9 To date, the axonal forms as AMSAN has been described during Covid‐19 acute phase as parainfectious disease. 9 Hyperaldosteronism can promote neurological complications as GBS/AMSAN by stimulating infiltration of macrophages/T‐cells and by enhancing axonal injury through secretion of IL‐22 and IL‐6. 12
Two major considerations emerge from this reported case. First, a correlation between IL‐6 and aldosterone levels needs to be confirmed in patients with Covid‐19 pneumonia and PA. Indeed, the association of IL‐6/hyperaldosteronism could have detrimental and synergic effect in the severe forms of pneumonia. Second, in Covid‐19 the parainfectious manifestation of GBS increases the risk of underestimating diagnoses in patients who require early treatment with mechanical ventilation. The increase in aldosterone activity/levels may also be involved in the severity of patients with SARS‐CoV2 infection and secondary aldosteronism as in patients with cardiovascular diseases, which have reported the highest rate of ICU hospitalization for Covid‐19.
In conclusion, the increased levels of aldosterone may be associated with severe forms of Covid‐19 by stimulating mostly IL‐6 production. Accordingly, the inhibition of IL‐6 effects induced by Tocilizumab could represent the main therapy in these patients. Serum aldosterone levels should be dosed in all patients with diagnosis of Covid‐19, especially in cases with increased levels of IL‐6 and/or secondary aldosteronism.
ETHICAL APPROVAL AND INFORMED CONSENT
All procedures performed in the study were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and conformed to the Declaration of Helsinki on human research. Informed consent was obtained from all individual participants included in the study.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117(20):10970‐10975. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐ 19: a single center experience. J Med Virol. 2020;92:814‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aziz M, Fatima R, Assaly R. Elevated interleukin‐6 and severe COVID‐19: A meta‐analysis. J Med Virol. 2020. 10.1002/jmv.25948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bavishi C, Maddox TM, Messerli FH. Coronavirus disease 2019 (COVID‐19) infection and renin angiotensin system blockers. JAMA Cardiol. 10.1001/jamacardio.2020.1282 [DOI] [PubMed] [Google Scholar]
- 5. Greco A, Buccheri S, D'Arrigo P, et al. Outcomes of renin‐angiotensin‐aldosterone system blockers in patients with COVID‐19: a systematic review and meta‐analysis. Eur Heart J Cardiovasc Pharmacother. 2020:pvaa074. 10.1093/ehjcvp/pvaa074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID‐19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:cvaa106‐cvaa1687. 10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chou CH, Hung CS, Liao CW, et al. IL‐6 trans‐signalling contributes to aldosterone‐induced cardiac fibrosis. Cardiovasc Res. 2018;114(5):690‐702. 10.1093/cvr/cvy013 [DOI] [PubMed] [Google Scholar]
- 8. Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrology. 2013;9(8):459‐469. 10.1038/nrneph.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toscano G, Palmerini F, Ravaglia S, et al. Guillain‐Barré syndrome associated with SARS‐CoV‐2. N Engl J Med. 2020;20:e00771. 10.1056/NEJMc2009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen D, Li X, Song Q, et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open. 2020;3(6):e2011122. 10.1001/jamanetworkopen.2020.11122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liaudet L, Szabo C. Blocking mineralocorticoid receptor with spironolactone may have a wide range of therapeutic actions in severe COVID‐19 disease. Crit Care. 2020;24(1):318. 10.1186/s13054-020-03055-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jasti AK, Selmi C, Sarmiento‐Monroy JC, Vega DA, Anaya JM, Gershwin ME. Guillain‐Barré syndrome: causes, immunopathogenic mechanisms and treatment. Expert Rev Clin Immunol. 2016;12(11):1175‐1189. 10.1080/1744666X.2016.1193006 [DOI] [PubMed] [Google Scholar]


