Abstract
Background
Balloon pulmonary angioplasty is an evolving, interventional treatment option for inoperable patients with chronic thromboembolic pulmonary hypertension (CTEPH). Pulmonary hypertension at rest as well as exercise capacity is considered to be relevant outcome parameters. The aim of the present study was to determine whether measurement of pulmonary hemodynamics during exercise before and six months after balloon pulmonary angioplasty have an added value.
Methods
From March 2014 to July 2018, 172 consecutive patients underwent balloon pulmonary angioplasty. Of these, 64 consecutive patients with inoperable CTEPH underwent a comprehensive diagnostic workup that included right heart catheterization at rest and during exercise before balloon pulmonary angioplasty treatments and six months after the last intervention.
Results
Improvements in pulmonary hemodynamics at rest and during exercise, in quality of life, and in exercise capacity were observed six months after balloon pulmonary angioplasty: WHO functional class improved in 78% of patients. The mean pulmonary arterial pressure (mPAP) at rest was reduced from 41 ± 9 to 31 ± 9 mmHg (p < 0.0001). The mPAP/cardiac output slope decreased after balloon pulmonary angioplasty (11.2 ± 25.6 WU to 7.7 ± 4.1 WU; p < 0.0001), and correlated with N-terminal fragment of pro-brain natriuretic peptide (p = 0.035) and 6-minute walking distance (p = 0.01).
Conclusions
Exercise right heart catheterization provides valuable information on the changes of pulmonary hemodynamics after balloon pulmonary angioplasty in inoperable CTEPH patients that are not obtainable by measuring resting hemodynamics.
Keywords: Chronic thromboembolic pulmonary hypertension, balloon pulmonary angioplasty, right heart catheter, exercise
Introduction
An increasing number of inoperable patients with chronic thromboembolic pulmonary hypertension (CTEPH) are being treated with balloon pulmonary angioplasty (BPA) with or without targeted medication.1–7 As the level of evidence for BPA remains limited, with a lack of controlled clinical trials and very few long-term studies, this interventional therapy is not strongly recommended by current guidelines.8,9
Improvements in pulmonary hemodynamics are often used as the main outcome variable of BPA; the actual concept of the intervention, which is meanwhile used worldwide, has been reinvented and developed mainly in Japanese centers with reported normalization or near-normalization of pulmonary hemodynamics at rest in up to 50% of the patients.2–4,10 In European centers, outcomes of BPA have also been promising, with auspicious changes in physical capacity,5,7,11 although the improvements in pulmonary hemodynamics at rest were less pronounced than in the Japanese cohorts. These differences have been discussed,7,12,13 but it seems obvious that there is a need for a more thorough evaluation of hemodynamics. The assessment of pulmonary hemodynamics during exercise may provide additional information14 and has not yet been demonstrated in inoperable CTEPH patients after BPA.
The aim of the present study was to evaluate the changes in pulmonary hemodynamics at rest and during exercise due to BPA in patients with inoperable CTEPH.
Methods
Patient selection for BPA
Our hospital serves as an international reference center for CTEPH, with more than 150 pulmonary endarterectomies (PEA) and more than 200 BPA interventions per year. Patients are routinely evaluated in a multidisciplinary CTEPH conference. Between March 2014 and July 2018, technically inoperable patients were included in the present prospective, observational cohort study. CTEPH was diagnosed in symptomatic patients after three months of anticoagulation who presented in at least WHO functional class (WHO FC) II, with a mean pulmonary arterial pressure (mPAP) of at least 25 mmHg at rest and with persistent pulmonary vascular lesions on computed tomography and/or conventional biplanar pulmonary artery angiography. Patients were deemed technically inoperable by experienced PEA surgeons based on a comprehensive assessment of imaging findings; they were included in the study if target lesions for BPA (i.e. subsegmentally located short-term stenosis or a long-segment network forming organized thrombi) were found in pulmonary angiography. Patients were usually treated with targeted medication, and BPA was planned to be performed after a period of at least three months of drug therapy if WHO FC was still ≥II. Targeted medication was left unchanged until the six-month follow-up.
All patients were informed in detail about the investigational nature of the study, including potential risks and benefits, and gave written informed consent to participate. The local ethics committee approved this prospective observational study (AZ 43/14, Giessen University Ethics Committee). All CTEPH patients included were also enrolled in the New International CTEPH Database of the International CTEPH Association (NCT02656238).
Clinical assessment
All patients underwent standardized assessment contemporary prior to the first BPA, and six months after the last intervention. Assessment included WHO FC, Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR),15 6-minute walking distance (6MWD), serum levels of the N-terminal fragment of pro-brain natriuretic peptide (NT-proBNP), and right heart catheterization (RHC) at rest and during exercise.
Exercise right heart catheterization
RHC was performed using a Swan-Ganz catheter via brachial or jugular access under local anesthesia. The mid-thoracic level was used as zero reference in all supine measurements. Pressures (right atrial pressure (RAP); systolic pulmonary arterial pressure (PAPs); diastolic pulmonary arterial pressure (PAPd); mean pulmonary arterial pressure (mPAP); pulmonary arterial wedge pressure (PAWP)) were measured directly, and cardiac output (CO) was measured by the thermodilution technique, averaging three of five output determinations. Baseline parameters were assessed 30 min after insertion of the catheter.16 PAWP measurement at rest was performed by digitized mean at end-expiratory breathhold and during exercise without breathhold. Exercise was performed using a cycle ergometer in the supine position. Exercise was prolonged in a steady-state manner for at least 8 min, depending on the clinical condition of the patient, usually corresponding to a workload between 1 and 50 W.17,18 If the patient was able to continue cycling, the workload was increased to an adjusted higher level to reach at least submaximal exercise. SvO2 < 30% at the end of exercise was regarded as valid maximal effort. During a constant workload, hemodynamic parameters were measured for a duration of 5–7 min beginning 3 min after the initiation of exercise.19,20 Pulmonary pressures were averaged over several respiratory cycles.18,20 Mixed venous oxygen saturation (SvO2) was obtained via blood gas analysis at rest and at the end of exercise. The following hemodynamic parameters were calculated: pulmonary vascular resistance (PVR) = (mPAP – PAWP)/CO; cardiac index (CI) = CO/body surface area; total pulmonary resistance (TPR) = mPAP/CO; pulmonary arterial compliance (PAC) = (CO/heart rate)/(PAPs – PAPd); transpulmonary gradient (TPG) = mPAP – PAWP; diastolic pulmonary gradient (DPG) = PAPd – PAWP; slope of the mPAP/CO relationship; and the stroke volume = CO/heart rate.21
Balloon pulmonary angioplasty
BPA was performed as a staged procedure, with a limited number of pulmonary segments being treated during each session. All procedures were performed in conscious patients under local anesthesia. The standard procedure has been described previously in detail.7,22,23 Briefly, using femoral or jugular access, a sheath was placed in the pulmonary artery and a guiding catheter was inserted into the targeted segmental arteries. The guidewire was then advanced into the target subsegmental branches, which were subsequently dilated by multiple balloon inflations. Pulmonary angiography documented the final post-procedural morphological result. Antegrade flow with improved parenchymal perfusion and quick venous return was interpreted as successful treatment.
Statistical analysis
All data for continuous variables are expressed as mean ± SD or as median and interquartile range (IQR), as appropriate. Categorical variables are reported as number and percentage. Continuous variables were compared using the Wilcoxon signed-rank test. The cohort data were distributed parametrically, as determined by the Kolmogorov-Smirnov test. All statistical tests were performed with SPSS software, version 22.0. A two-tailed p value <0.05 was considered to indicate statistical significance. The reported p values are to be interpreted in the exploratory sense.
Results
Cohort selection and baseline characteristics
A total of 172 consecutive patients underwent BPA between March 2014 and July 2018. Of these, 64 patients with CTEPH had completed a full series of BPA interventions and underwent exercise RHC before the first intervention and six months after the last BPA procedure. Another 41 patients were still undergoing BPA treatments, 23 patients were waiting for the six-month follow-up, in four patients exercise RHC was contraindicated due to orthopedic disease or paraplegia, 15 patients were examined using very different exercise levels at baseline and at follow-up, 16 patients underwent their follow-up at another center (without exercise RHC), and 2 patients died in the early postinterventional phase. Seven patients were diagnosed with chronic thromboembolic disease (CTED; no PH at rest). Data from six of these CTED patients were already presented in a previous publication.11 The demographics and baseline characteristics of the present study cohort are given in Table 1.
Table 1.
Baseline characteristics and medication.
| Number of patients, n | 64 |
| Age, years | 61 ± 13 |
| Female, n (%) | 30 (47) |
| Body mass index, kg/m2 | 25 ± 3.7 |
| History of PEA, n (%) | 5 (7.8) |
| Anticoagulation with vitamin K antagonists, n (%) | 9 (14) |
| PDE 5 inhibitor, n (%) | 8 (12.5) |
| ERA, n (%) | 10 (15.6) |
| sGC, n (%) | 40 (62.5) |
| Inhaled analogue of prostacyclin, n (%) | 2 (3.1) |
| No medication, n (%) | 14 (21.9) |
| Monotherapy, n (%) | 41 (64.1) |
| Combination therapy, n (%) | 9 (14.1) |
Note: Values are given as mean ± SD unless otherwise indicated.
CTEPH: chronic thromboembolic pulmonary hypertensions; VTE: venous thromboembolism; PEA: pulmonary endarterectomy; PDE: phosphodiesterase; ERA: endothelin receptor antagonist; sGC: stimulator of soluble guanylate cyclase.
BPA procedures and effects on physical capacity and quality of life
The mean number of BPA sessions per patient was 5.6 ± 1.3. There were 11 ± 3 pulmonary segments targeted in all interventions. The mean time from diagnosis (=presentation in CTEPH conference) to first BPA was 3.5 (IQR 3.0–6.0) months. The effects of BPA on quality of life based on the CAMPHOR questionnaire (Table 2) showed improvement of scores for symptoms (10.1 ± 5.2 at baseline vs. 5.9 ± 3.8 after treatment, p < 0.0001), activity limitations (8.5 ± 4.6 vs. 5.3 ± 3.6, p < 0.0001), and quality of life (6.1 ± 4.5 vs. 3.4 ± 2.9, p < 0.0001). The WHO FC improved in 50 (78%) patients and was unchanged in 14 (22%). The 6MWD improved after BPA by an average of 47 m (10%).
Table 2.
Results of BPA.
| N | Prior to BPA | N | After BPA | p-value (exploratory) | ||
|---|---|---|---|---|---|---|
| Exercise capacity | ||||||
| WHO functional class, n (%) | 64 | 64 | ||||
| I | 0 (0) | 38 (59.4) | ||||
| II | 2 (3.2) | 23 (35.9) | ||||
| III | 49 (76.6) | 3 (4.7) | ||||
| IV | 13 (20.3) | 0 (0) | ||||
| 6MWD, m | 53 | 416 ± 94 | 60 | 463 ± 96 | <0.0001 | |
| Hemodynamics at rest | ||||||
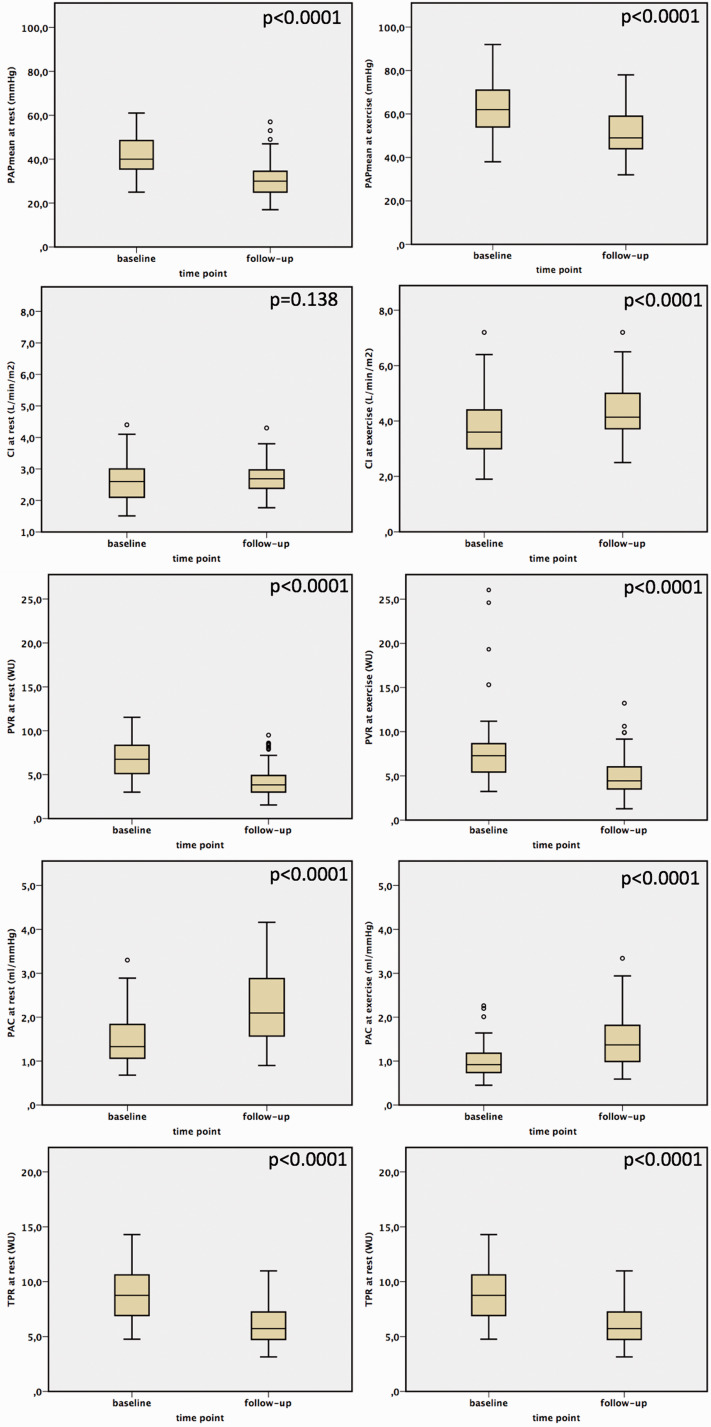
| Right atrial pressure, mmHg | 64 | 7 ± 4 | 64 | 5 ± 2 | <0.0001 | |
| mPAP, mmHg | 64 | 41 ± 9 | 64 | 31 ± 9 | <0.0001 | |
| PAPsyst, mmHg | 64 | 70 ± 16 | 64 | 52 ± 16 | <0.0001 | |
| PAPdiast, mmHg | 63 | 23 ± 6 | 64 | 16 ± 6 | <0.0001 | |
| PAWP, mmHg | 63 | 10 ± 2 | 64 | 9 ± 3 | 0.335 | |
| DPG, mmHg | 62 | 13.6 ± 6.2 | 57 | 6.6 ± 5.8 | <0.0001 | |
| TPG, mmHg | 63 | 31.8 ± 8.9 | 64 | 21.3 ± 8.4 | <0.0001 | |
| CO, L/min | 63 | 4.9 ± 1.2 | 64 | 5.1 ± 1.0 | 0.068 | |
| CI, L/min/m2 | 64 | 2.6 ± 0.6 | 64 | 2.7 ± 0.5 | 0.138 | |
| PVR, WU | 63 | 6.8 ± 2.3 | 64 | 4.3 ± 1.9 | <0.0001 | |
| TPR, WU | 63 | 8.9 ± 2.5 | 64 | 6.2 ± 2.1 | <0.0001 | |
| PAC, ml/mmHg | 63 | 1.5 ± 0.6 | 64 | 2.3 ± 0.9 | <0.0001 | |
| HR, bpm | 63 | 77 ± 13 | 64 | 71 ± 12 | <0.0001 | |
| stroke volume, ml | 63 | 66 ± 19 | 64 | 73 ± 15 | <0.0001 | |
| Hemodynamics during exercise | ||||||
| Work load, W | 64 | 27 ± 14 | 64 | 28 ± 15 | 0.187 | |
| Right atrial pressure, mmHg | 64 | 15 ± 7 | 64 | 11 ± 5 | <0.0001 | |
| mPAP, mmHg | 64 | 62 ± 11 | 64 | 52 ± 11 | <0.0001 | |
| PAPsyst, mmHg | 64 | 102 ± 22 | 64 | 86 ± 20 | <0.0001 | |
| PAPdiast, mmHg | 63 | 33 ± 8 | 64 | 26 ± 4 | <0.0001 | |
| PAWP, mmHg | 58 | 14 ± 7 | 63 | 15 ± 4 | 0.164 | |
| DPG, mmHg | 57 | 18.5 ± 10.7 | 63 | 11.5 ± 7.0 | <0.0001 | |
| TPG, mmHg | 58 | 48.1 ± 13.7 | 63 | 37.3 ± 11.3 | <0.0001 | |
| CO, L/min | 62 | 7.0 ± 2.0 | 63 | 8.3 ± 2.0 | <0.0001 | |
| CI, L/min/m2 | 62 | 3.8 ± 1.1 | 63 | 4.4 ± 1.1 | <0.0001 | |
| PVR, WU | 56 | 8.0 ± 4.4 | 63 | 5 ± 2.4 | <0.0001 | |
| TPR, WU | 62 | 9.6 ± 3.0 | 63 | 6.7 ± 2.5 | <0.0001 | |
| PAC, ml/mmHg | 62 | 0.9 ± 0.8 | 63 | 1.5 ± 0.6 | <0.0001 | |
| HR, bpm | 63 | 104 ± 15 | 64 | 101 ± 15 | 0.022 | |
| mPAP/CO slope, WU | 62 | 11.2 ± 25.6 | 63 | 7.7 ± 4.1 | <0.0001 | |
| stroke volume, ml | 62 | 69 ± 22 | 64 | 83 ± 21 | <0.0001 | |
| Laboratory findings | ||||||
| NT-proBNP, ng/L, median (IQR) | 63 | 741 (192–1425) | 64 | 139 (60–266) | <0.0001 | |
| CAMPHOR scores | ||||||
| Symptoms | 47 | 10.1 ± 5.2 | 34 | 5.9 ± 3.8 | <0.0001 | |
| Activity | 46 | 8.5 ± 4.6 | 38 | 5.3 ± 3.6 | <0.0001 | |
| Quality of life | 39 | 6.1 ± 4.5 | 34 | 3.4 ± 2.9 | <0.0001 | |
Note: Values are given as mean ± SD unless otherwise indicated.
WHO: World Health Organization; 6MWD: 6-min walking distance; mPAP: mean pulmonary arterial pressure; PAPsyst: systolic pulmonary arterial pressure; PAPdiast: diastolic pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure; DPG: diastolic pressure gradient; TPG: transpulmonary gradient; CO: cardiac output; CI: cardiac index; PVR: pulmonary vascular resistance; TPR: total pulmonary resistance; PAC: pulmonary arterial compliance; HR: heart rate; NT-proBNP: N-terminal fragment of pro-brain natriuretic peptide; CAMPHOR: Cambridge Pulmonary Hypertension Outcome Review; BPA: balloon pulmonary angioplasty.
Hemodynamic responses before and six months after BPA
In CTEPH patients, mPAP at rest was reduced from 41 ± 9 to 31 ± 9 mmHg (p < 0.0001) (Table 2 and Figure 1), with 14 (22%) patients having an mPAP < 25 mmHg six months after BPA. Of these patients, 10 had a PVR < 3.0 WU at rest. All patients with mPAP < 25 mmHg at rest six months after BPA had a substantial decrease of NT-proBNP values (baseline: median 298 ng/L (IQR 79–1048 ng/L) vs. six months after BPA: median 53 ng/L (IQR 31–189 ng/L); p < 0.001) and increase of 6MWD (baseline: mean 433.9 m ± SD 69.8 m vs. 6 months after BPA: mean 480.9 m ± SD 68.2 m; p = 0.003). At exercise, SvO2 was 44 ± 10 %, in seven patients SvO2 was below 30%.
Figure 1.
The effects of BPA on pulmonary hemodynamics.
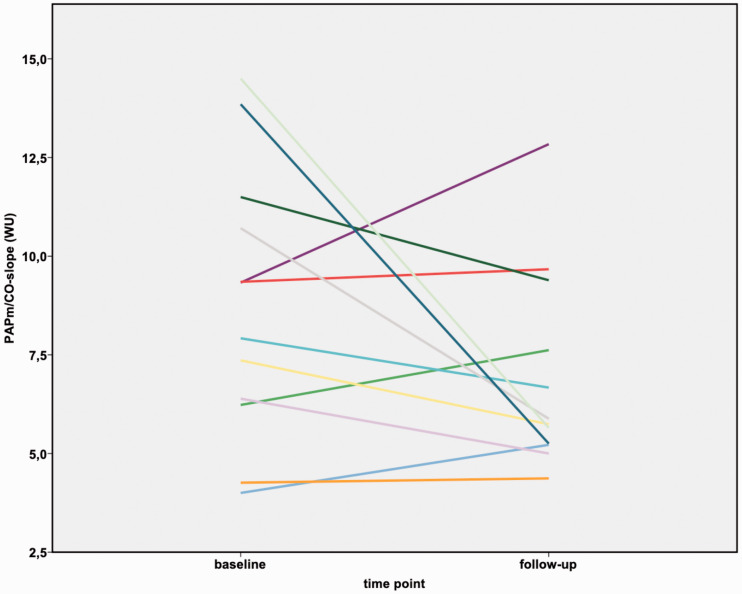
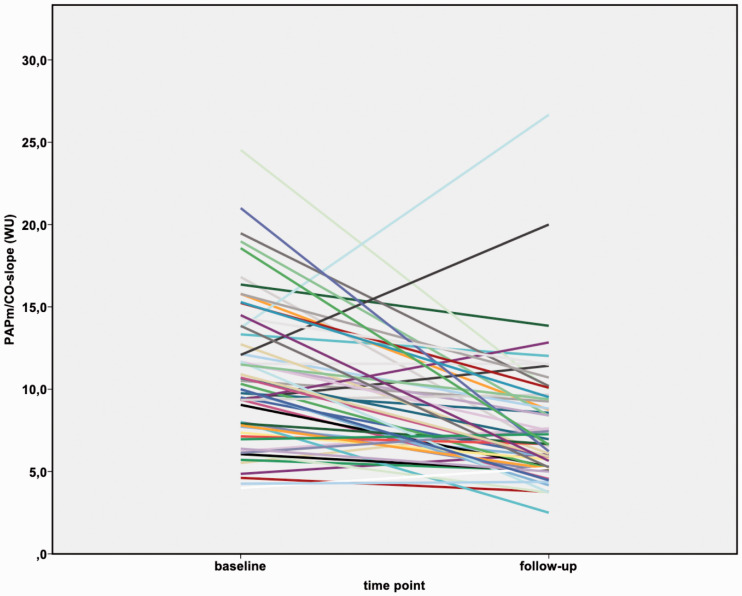
Before BPA, the slope of the mPAP/CO relationship was above 3 WU in all patients (range: 4.0–24.5 WU), and the TPR was also above 3 WU. After BPA, the mPAP/CO slope decreased in all patients (range: 2.5–26.7 WU), although it was still above 3 WU in all but one patient. In the subgroup of patients having an mPAP < 25 mmHg after BPA (n = 14), the mPAP/CO slope decreased from median 8.6 WU (IQR 6.3–11.3 WU) to median 5.7 WU (IQR 4.8–8.1 WU). With that, after BPA all patients still fulfilled the proposed criteria of exercise PH, with mPAP > 30 mmHg and TPR > 3 WU at the six-month follow-up.14 Both the TPR and the mPAP/CO slope decreased after BPA (p < 0.0001) (Table 2; Figures 1 to 3) and correlated significantly with NT-proBNP (TPR 0.462 (p < 0.0001); mPAP/CO slope 0.252 (p = 0.035)) and 6MWD (TPR −0,450 (p < 0.0001); mPAP/CO slope −0.313 (p = 0.01)). Pulmonary artery compliance at rest and during exercise increased after BPA (p < 0.0001) and also did the stroke volume at rest and at exercise (p < 0.0001).
Figure 3.
Changes in mPAP/CO slope before and after BPA in patients with mPAP < 25 mmHg at rest six months after BPA.
mPAP: mean pulmonary arterial pressure; CO: cardiac output.
In the subgroup of patients, that did not receive targeted medication (n = 14), mPAP at rest was reduced from 38 ± 8 to 31 ± 8 mmHg (p = 0.007), mPAP at exercise improved from 60 ± 10 to 55 ± 10 mmHg (p = 0.007). CI at rest remained stable after BPA (mean 2.6 ± SD 0.5 l/min/m2 vs. mean 2.4 ± SD 0.4 l/min/m2; p = 0.27), while there was non-significant improvement at exercise (mean 3.9 ± SD 1.4 l/min/m2 vs. mean 4.2 ± SD 1.2 l/min/m2; p = 0.23). PVR at rest (mean 6.6 ± SD 2.2 WU vs. mean 5.2 ± SD 1.8 WU; p = 0.013) and at exercise (mean 8.6 ± SD 5.6 WU vs. mean 6.0 ± SD 2.2 WU; p = 0.15) improved non-significantly. These patients further had a substantial decrease of NT-proBNP values (baseline: median 264 ng/L (IQR 82–1121 ng/L) vs. six months after BPA: median 148 ng/L (IQR 48–371 ng/L); p < 0.0001) and non-significant increase of 6MWD (baseline: mean 398 m ± SD 84 m vs. six months after BPA: mean 412 m ± SD 101 m; p = 0.49).
Complications of BPA and exercise RHC
Twenty-five procedure-related complications occurred during the 363 interventions (6.9% of all interventions). These adverse events were mostly caused by wire perforation of the pulmonary vasculature, resulting in parenchymal bleeding with mild hemoptysis in some cases. Four patients developed reperfusion edema with clinical symptoms (coughing of frothy secretion, desaturation) and consolidations in chest X-rays during the post-procedural period of 6 to 24 h. Non-invasive ventilation was frequently performed after interventions for complications (mostly as a routinely performed procedure to avoid dystelectasis following parenchymal hemorrhage); invasive ventilation was not necessary. These complications are in line with our experience in the whole group of in our center interventionally treated inoperable CTEPH patients.7,11,13,22,23 There were no complications due to exercise RHC, and there were no deaths in the study cohort.
Discussion
In the present study, we examined the effects of BPA on pulmonary hemodynamics at rest and during exercise six months after completion of the interventions in patients with inoperable CTEPH. The main findings of this study are: (1) BPA with or without targeted medication leads to significant improvements in pulmonary hemodynamics at rest and during exercise in CTEPH patients; (2) even when patients no longer presented with PH at rest after BPA, they still had signs of PH during exercise; (3) BPA leads to significant improvements in exercise capacity (WHO FC, 6MWD) and quality of life. To the best of our knowledge, this is the first study to compare the results of exercise RHC before and after BPA in CTEPH patients.
There is some evidence from smaller and mid-sized case series that BPA exerts beneficial effects on pulmonary hemodynamics and physical capacity in inoperable CTEPH patients, but the results differ from center to center with reported normalization or near-normalization of pulmonary hemodynamics in up to 50% of the patients2–4,10 and less pronounced hemodynamic improvements in European centers.5,7 The main reason for variations in the data may be differing indications for PEA and for BPA in different centers. In our study, patients selected for BPA comprised only those with clearly inoperable disease. However, pulmonary hemodynamics have thus far only been examined at rest.2–5,7,10
Exercise pulmonary hemodynamics can reveal pathophysiological details in patients with PH that are not apparent from measurements made at rest.14 Our observations illustrate in particular the influence of BPA on exercise pulmonary hemodynamics in inoperable patients with CTEPH. Defining exercise PH is still a matter of debate, but an mPAP >30 mmHg with a TPR or mPAP/CO slope >3.0 WU is a reasonable definition among experts in the field.17,21,24,25 In our cohort, there were 14 patients (22% of all patients) with an mPAP <25 mmHg at rest after BPA, but all still fulfilled the criteria of exercise PH. The relevance of these findings for the previously published outcomes of BPA treatment may be important, as they suggest that BPA does not necessarily lead to a normalization of pulmonary hemodynamics. Aside from secondary vasculopathy, an explanation for residual PH during exercise might be that obstructing material is left behind after BPA, which is, in fact, one of the major differences between BPA and surgical treatment by PEA. These findings underline the clinical relevance of exercise RHC for CTEPH patients, as advanced targeted medical therapy may be evaluated as an additional option.
Recently, the results of the World Symposium on Pulmonary Hypertension from 2018 in Nice were published26 that suggest a change in the definition of PH: precapillary PH may be diagnosed in patients with a mPAP of >20 mmHg, a PAWP ≤15 mmHg, and a PVR of ≥3 WU. These novel ideas are currently still being critically discussed, however, and have not yet been transferred to a guideline recommendation. It is complex to interpret this shift in the threshold in mPAP, but it seems obvious that an mPAP in the range between 20 and 25 mmHg indicates a pathological change in pulmonary hemodynamics. In our CTEPH patients with a resting mPAP <25 mmHg after BPA, we were nevertheless able to demonstrate pathological pulmonary hemodynamics during exercise (Figure 3).
Another aspect of the evaluation of a PH patient is the description of their physical capacity and quality of life. For BPA, several groups have already shown improvements of exercise capacity,27,28 but there are no data with regard to the CAMPHOR questionnaire. This offers much more discriminating information than WHO functional class.15 In inoperable CTEPH patients, we observed significant improvements in activity, symptoms, and quality of life after BPA. Furthermore, NT-proBNP is often used as a biomarker in the clinical assessment of CTEPH patients,23 which also showed significant improvement in the presented cohort and correlated strongly with the changes in exercise hemodynamics. According to these beneficial changes, also PAC improved significantly, which is believed to be an important marker of prognosis in PH patients29; these findings underline the beneficial effects of BPA for inoperable CTEPH, but a normalization of all parameters has not been observed in the study cohort. The meaning of these findings with regard to the long-term management of this particular group of patients cannot be answered by the presented data. But against this background, a continuation of targeted medical treatment may be reasonable. On the other hand, Cannon et al. presented data on residual PH after PEA, showing a worse prognosis of patients with an mPAP > 38 mmHg and a PVR of >5.3 WU at rest.30 But with actually only rare long-term data for inoperable CTEPH patients treated with BPA,10 it can only be speculated, whether these data can be extrapolated on this group.
Our study must be evaluated with caution due to several potential limitations. There was no control group of patients with no specific treatment or with only targeted medication. But it is well known, that CTEPH is a progressive disease with a poor prognosis if left untreated.31 Another important limitation is that the majority of patients did not undergo cardiopulmonary exercise testing as a standard examination.
In conclusion, patients with inoperable CTEPH benefit from BPA treatment as evidenced by measurement of pulmonary hemodynamics during exercise and assessment of functional capacity and quality of life. The complication rate of BPA was low and exercise RHC was without complications. Exercise RHC offers a differentiated insight into the hemodynamic changes after BPA. It is not easy to outline the clinical benefit of exercise hemodynamics, and the evaluation of a CTEPH patient is based on multiple findings. But our results indicate that improving exercise hemodynamics explain much better the improvement of physical capacity than mPAP at rest. On the other hand, our findings demonstrate that inoperable CTEPH is not cured by BPA. In particular, even in patients without PH at rest after BPA, signs of exercise PH were detectable.
Figure 2.
Changes in mPAP/CO slope before and after BPA in inoperable CTEPH patients.
mPAP: mean pulmonary arterial pressure; CO: cardiac output.
Acknowledgements
We are grateful to Elizabeth Martinson, PhD, of the KHFI Editorial Office for her editorial assistance.
Footnotes
These authors contributed equally as last authors.
Conflict of interest
CB Wiedenroth has received speaker fees and/or consultant honoraria from Actelion, Bayer AG, BTG, MSD, and Pfizer.
AJ Rieth received speaker fees and/or consultant honoraria from Servier, St. Jude Medical/Abbott, Novartis and Actelion, and traveling support by Orion Pharma.
S Kriechbaum has nothing to disclose.
HA Ghofrani has reported receiving fees for serving as a board member for Bellerophon Pulse Technologies, Medscape, OMT, UCB Celltech, and Web MD Global; receiving consultancy fees and fees for serving on a steering committee for Actelion Pharmaceuticals, Bayer, Gilead Sciences, GlaxoSmithKline, Merck, Novartis, and Pfizer; receiving lecture fees from Actelion Pharmaceuticals, Bayer, GlaxoSmithKline, Merck, Novartis, and Pfizer; and receiving grant support from Actelion Pharmaceuticals, Bayer, Novartis, and Pfizer.
A Breithecker has nothing to disclose.
M Haas has received speaker fees from Daiichi-Sankyo and Pfizer.
F Roller has nothing to disclose.
MJ Richter has nothing to disclose.
M Lankeit reports having received consultancy and lecture honoraria from Actelion, Bayer, Daiichi-Sankyo, MSD and Pfizer – Bristol-Myers Squibb; an institutional grant from the German Federal Ministry of Education and Research (BMBF 01EO1003 and 01EO1503) and research funding from BRAHMS – Thermo Fisher Scientific.
L Mielzarek has nothing to disclose.
A Rolf has nothing to disclose.
C Hamm has nothing to disclose.
E Mayer has received speaker fees and/or honoraria for consultations from Actelion, Bayer, GSK, MSD, and Pfizer.
S Guth has received speaker fees from Actelion, Bayer, GSK, MSD and Pfizer.
C Liebetrau has received speaker fees from Abbott, Astra Zeneca, Bayer, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Elixir Medical, and Pfizer.
Funding
This study is funded by the Deutsche Forschungsgemeinschaft (DFG) and the Sonderforschungsbereich (SFB) 1213, project CP01, and project B07.
References
- 1.Feinstein JA, Goldhaber SZ, Lock JE, et al. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001; 103: 10–13. [DOI] [PubMed] [Google Scholar]
- 2.Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 748–755. [DOI] [PubMed] [Google Scholar]
- 3.Kataoka M, Inami T, Hayashida K, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 756–762. [DOI] [PubMed] [Google Scholar]
- 4.Sugimura K, Fukumoto Y, Satoh K, et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 2012; 76: 485–488. [DOI] [PubMed] [Google Scholar]
- 5.Andreassen AK, Ragnarsson A, Gude E, et al. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 2013; 99: 1415–1420. [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi Y, Miyagawa K, Nakayama K, et al. Balloon pulmonary angioplasty: an additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. EuroIntervention 2014; 10: 518–525. [DOI] [PubMed] [Google Scholar]
- 7.Olsson KM, Wiedenroth CB, Kamp JC, et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J 2017, pp. 49. [DOI] [PubMed] [Google Scholar]
- 8.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 9.Wilkens H, Konstantinides S, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol 2018; 272S: 69–78. [DOI] [PubMed] [Google Scholar]
- 10.Aoki T, Sugimura K, Tatebe S, et al. Comprehensive evaluation of the effectiveness and safety of balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension: long-term effects and procedure-related complications. Eur Heart J 2017. [DOI] [PubMed] [Google Scholar]
- 11.Wiedenroth CB, Olsson KM, Guth S, et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic disease. Pulm Circ 2018; 8: 2045893217753122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsubara H, Ogawa A. A long way to go after the initial experience with balloon pulmonary angioplasty. Eur Respir J 2017, pp. 49. [DOI] [PubMed] [Google Scholar]
- 13.Wiedenroth CB, Ghofrani HA, Adameit MSD, et al. Sequential treatment with riociguat and balloon pulmonary angioplasty for patients with inoperable chronic thromboembolic pulmonary hypertension. Pulm Circ 2018; 8: 2045894018783996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovacs G, Herve P, Barbera JA, et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J 2017, pp. 50. [DOI] [PubMed] [Google Scholar]
- 15.McCabe C, Bennett M, Doughty N, et al. Patient-reported outcomes assessed by the CAMPHOR questionnaire predict clinical deterioration in idiopathic pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Chest 2013; 144: 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol (Engl Ed) 2016; 69: 177. [DOI] [PubMed] [Google Scholar]
- 17.Kovacs G, Avian A, Tscherner M, et al. Characterization of patients with borderline pulmonary arterial pressure. Chest 2014; 146: 1486–1493. [DOI] [PubMed] [Google Scholar]
- 18.Rieth A, Richter MJ, Gall H, et al. Hemodynamic phenotyping based on exercise catheterization predicts outcome in patients with heart failure and reduced ejection fraction. J Heart Lung Transplant 2017; 36: 880–889. [DOI] [PubMed] [Google Scholar]
- 19.Chaouat A, Sitbon O, Mercy M, et al. Prognostic value of exercise pulmonary haemodynamics in pulmonary arterial hypertension. Eur Respir J 2014; 44: 704–713. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs G, Avian A, Pienn M, et al. Reading pulmonary vascular pressure tracings. How to handle the problems of zero leveling and respiratory swings. Am J Respir Crit Care Med 2014; 190: 252–257. [DOI] [PubMed] [Google Scholar]
- 21.Lewis GD, Bossone E, Naeije R, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 2013; 128: 1470–1479. [DOI] [PubMed] [Google Scholar]
- 22.Kriechbaum SD, Wiedenroth CB, Keller T, et al. Dynamics of high-sensitivity cardiac troponin T during therapy with balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. PLoS One 2018; 13: e0204683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kriechbaum SD, Wiedenroth CB, Wolter JS, et al. N-terminal pro-B-type natriuretic peptide for monitoring after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2018; 37: 639–646. [DOI] [PubMed] [Google Scholar]
- 24.Naeije R, Saggar R, Badesch D, et al. Exercise-induced pulmonary hypertension: translating pathophysiological concepts into clinical practice. Chest 2018; 154: 10–15. [DOI] [PubMed] [Google Scholar]
- 25.Naeije R, Vanderpool R, Dhakal BP, et al. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med 2013; 187: 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2018. pii: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takei M, Kawakami T, Kataoka M, et al. Residual high intrapulmonary shunt fraction limits exercise capacity in patients treated with balloon pulmonary angioplasty. Heart Vessels 2019; 34: 868–874. [DOI] [PubMed] [Google Scholar]
- 28.Shinkura Y, Nakayama K, Yanaka K, et al. Extensive revascularisation by balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension beyond haemodynamic normalisation. EuroIntervention 2018; 13: 2060–2068. [DOI] [PubMed] [Google Scholar]
- 29.Mahapatra S, Nishimura RA, Sorajja P, et al. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 2006; 47: 799–803. [DOI] [PubMed] [Google Scholar]
- 30.Cannon JE, Su L, Kiely DG, et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the United Kingdom National Cohort. Circulation 2016; 133: 1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riedel M, Stanek V, Widimsky J, et al. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982; 81: 151158. [DOI] [PubMed] [Google Scholar]