Abstract
Background:
Immune checkpoint inhibitors (ICIs) have shown encouraging treatment efficacy for metastatic breast cancer in several clinical trials. However, response only occurred in a small population. Evidence predicting response and survival of patients with metastatic breast cancer following ICI treatment with existing biomarkers has not been well summarized. This review aimed to summarize the efficacy and predictive factors of immune checkpoint therapy in metastatic breast cancer, which is critical for clinical practice.
Methods:
PubMed, Embase, Cochrane Library, Web of Science, www.clinicaltrials.gov, and meeting abstracts were comprehensively searched to identify clinical trials. The outcomes were objective response rate (ORR), treatment-related adverse events (trAEs), immune-related adverse events (irAEs), progression-free survival (PFS), and overall survival (OS).
Results:
In this review, 27 studies with 1746 patients were included for quantitative synthesis. The pooled ORR was 19% [95% confidence interval (CI) = 12–27%]. Programmed death-ligand 1 (PD-L1)-positive patients had a higher response rate [odds ratio (OR) = 1.44, p = 0.01]. First-line immunotherapy had a better ORR than second-line immunotherapy (OR = 2.00, p = 0.02). Tumor-infiltrating lymphocytes (TILs) ⩾5% (OR = 2.53, p = 0.002) and high infiltrated CD8+ T-cell level (OR = 4.33, p = 0.006) were ideal predictors of immune checkpoint therapy response. Liver metastasis indicated poor response (OR = 0.19, p = 0.009). However, the difference was non-significant in ORR based on age, performance status score, lymph node metastasis, and lactate dehydrogenase (LDH) level. In addition, the PD-L1-positive subgroup had a better 1-year PFS (OR = 1.55, p = 0.04) and 2-year OS (OR = 2.28, p = 0.02) following ICI treatment. The pooled incidence during ICI therapy of grade 3–4 trAEs was 25% (95% CI = 16–34%), whereas for grade 3–4 irAEs it was 15% (95% CI = 11–19%).
Conclusions:
Metastatic breast cancer had modest response to ICI therapy. PD-L1-positive, first-line immunotherapy, non-liver metastasis, and high TIL and CD8+ T-cell infiltrating levels could predict better response to ICI treatment. Patients with PD-L1-positive tumor could gain more survival benefits from immune checkpoint therapy.
Keywords: biomarker, breast cancer, immune checkpoint inhibitor, immunotherapy, meta-analysis
Introduction
According to the estimates of global cancer statistics, breast cancer is known as the most common cancer and the second leading cause of cancer-related death among women.1 In the United States, approximately 268,600 new cases and 42,260 deaths due to female breast cancer are expected to occur in the year 2019.2 Despite the progress and advancements in the systematic treatment of breast cancer, approximately 20% of the patients will experience distant metastatic disease in the first 5 years.3 Patients with recurrence or metastatic breast cancer have a poor prognosis with a 5-year relative survival rate of 27%.2
In recent years, immune checkpoint therapy has been proved as an effective strategy in various advanced solid tumors and has rapidly become a hotspot in the research of antitumor drugs. Following the great success of immune checkpoint inhibitors (ICIs) in melanoma in 2010, multiple new monoclonal antibodies against cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), programmed cell death protein-1 (PD-1), and programmed cell death-ligand-1 (PD-L1) have been trialed and approved by the US Food and Drug Administration (FDA) in a diversity of solid tumors.4 Although breast cancer was once regarded as an immune-quiescent tumor, recent research has reported that some subtypes may respond favorably to immune checkpoint therapy.5 Patients with metastatic breast cancer have shown an objective response rate (ORR) of 3~45% following treatment with ICIs in different reported phase II clinical trials.6–10 In the newly reported phase III trial (IMpassion130), the PD-L1 inhibitor atezolizumab combined with nab-paclitaxel conferred a significant improvement of progression-free survival (PFS) compared with the nab-paclitaxel group in triple-negative breast cancer (7.2 months versus 5.5 months).11 In addition, patients with PD-L1-positive tumors had a better PFS compared with patients with PD-L1-negative tumors. In another study by Voorwerk et al., patients with a high level of tumor-infiltrating CD8+ T cells were also associated with a better ORR during the treatment with ICIs.12 Although immunotherapy demonstrated a promising efficiency in metastatic breast cancer, only a small proportion of patients would benefit from it in addition to a high rate of severe adverse events. To date, evidence predicting the response and survival of patients with metastatic breast cancer following ICI treatment with existing biomarkers has not been adequately summarized.
In this study, we performed a systematic review and meta-analysis of the reported clinical trials to evaluate the response and safety of immune checkpoint therapy in patients with metastatic breast cancer. In addition, the predictive role of several existing biomarkers for immunotherapy response was also investigated for the first time to identify the population who would potentially benefit.
Methods
Search strategy
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.13 Cochrane information (https://methods.cochrane.org/prognosis/tools) and prognostic meta-analysis guidelines were used as a guidance for biomarker analysis.14
The PICOTS system was used to describe the key items for framing this review and its objective and methodology:
Population – patients with metastatic breast cancer.
Index prognostic factors – particular biomarker (PD-L1 expression, line of ICI therapy, tumor-infiltrating lymphocyte [TIL] level, CD8+ T-cell infiltration level, liver metastasis, age, Eastern Cooperative Oncology Group [ECOG] performance status score, lymph node metastasis and lactate dehydrogenase [LDH] level).
Comparator prognostic factors – not applicable for this review.
Outcomes – objective response rate (ORR), treatment-related adverse events (trAEs), immune-related adverse events (irAEs), progression-free survival (PFS) and overall survival (OS).
Timing – biomarker measurements were performed before ICI treatment and all follow-up information on the outcomes were extracted from the studies.
Setting – hospital/treatment center.
A comprehensive search of PubMed, Embase, the Cochrane Library, Web of Science online databases and www.clinicaltrials.gov was performed on 5 August 2019. The retrieval strategy contained the following keywords: Nivolumab, Pembrolizumab, Atezolizumab, Durvalumab, Avelumab, Ipilimumab, Tremelimumab, immune checkpoint inhibitor, PD-1 inhibitor, PD-L1 inhibitor, CTLA-4 inhibitor, and breast cancer. The detailed protocol and search strategy are presented in Supplemental Appendices 1 and 2. We also reviewed abstracts from American Society of Clinical Oncology conferences using the same criteria reported in the following. The reference lists from these studies were hand searched for eligible articles. All search strategies were conducted following the guidelines.
Inclusion and exclusion criteria
Only prospective clinical trials of patients with metastatic breast cancer treated with an ICI (including anti-PD-1, anti-PD-L1, and anti-CTLA-4 inhibitor) that reported ORR, trAEs, irAEs, PFS or OS outcomes were included. Articles published online ‘ahead of print’ were included. Meeting abstracts without published full-text original articles were eligible for this study. Exclusion criteria were insufficient data, not advanced or metastatic breast cancer, preclinical studies, case reports, letters, commentaries, and reviews. In addition, retrospective studies were excluded in this review. When duplicate studies from the same trial were identified, only those with the most complete and updated data were included.
Study selection
All search results were independently inspected by two authors (YZ and XZ) and discrepancies were consulted with a third reviewer (SZ). Reviewers applied selection criteria after screening the potentially included studies. Duplicates were removed using Endnote X9 software or manually.
Data extraction
Baseline characteristics of each study (authors, year of publication, or conference presentation, line of ICI treatment, type of ICI agents, breast cancer subtype, number of patients enrolled, combination therapy, and median OS) were recorded by two reviewers independently. The primary outcome was the ORR, while the secondary outcomes were trAEs, irAEs, PFS, and OS. Data were extracted from different subgroups in the same trial to analyze biomarkers that predict ORR, PFS and OS of ICI treatment. These results were described by odds ratios (ORs) and 95% confidence intervals (CIs).
Methodology quality assessment
The quality of each randomized controlled trial (RCT) and non-randomized trial was assessed using the Cochrane risk-of-bias tool and the methodological index for non-randomized studies (MINORS), respectively. The risk of bias using the Cochrane risk-of-bias tool was expressed as low, high, or unclear risk including the aspects of selection, performance, detection, attrition, reporting, and other biases. MINORS is recognized as the most appropriate guideline to evaluate the methodological quality of non-randomized trials and contains eight specific items.15 The Quality In Prognosis Studies (QUIPS) tool was used to assess the risk of bias in the studies of prognostic factors.14,16 Six important domains were considered for evaluation, including study participation, study attrition, prognostic factor measurement, confounding measurement and account, outcome measurement, and analysis and reporting. Two reviewers made the assessments, with disagreements consulted with a third reviewer. All RCTs and non-randomized trials were scored and recorded.
Quality of evidence assessment
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the quality of evidence of the major outcomes. The following elements were included for evaluation: study design, risk of bias, inconsistencies, imprecision of the results, indirectness, and publication bias. The quality of evidence for the major outcomes was graded as high, moderate, low, or very low.
Data synthesis and analysis
The rates of ORR, trAEs, and irAEs were extracted and pooled using the Meta package in R software (version 3.5.0). ORs and 95% CIs describing the predictive outcomes (ORR, PFS, and OS) of biomarkers were synthesized using Review Manager software (version 5.3, Cochrane Collaboration). The difference was considered significant when 95% CI does not include 1.0. We used Cochrane’s Q test (reported with a χ2 value and p value) and the I2 test to estimate study heterogeneity. Heterogeneity was indicated if p < 0.1 and I2 > 50%. ORs and 95% CIs for each of the comparisons in the subgroup were pooled using the fixed-effects model (if heterogeneity Cochrane’s Q test p > 0.1) and the random-effects model (if heterogeneity Cochrane’s Q test p < 0.1) in the Review Manager software.17 Subgroup analysis was performed to address the possible sources of heterogeneity and identify the potential subsets of patients. Meta-regression was also performed to explain heterogeneity using the Meta package in R software. In addition, Egger’s test was performed with STATA software 15.1 (Stata Corp, College Station, TX, USA) to assess potential publication bias.18
Results
Baseline characteristics of included studies
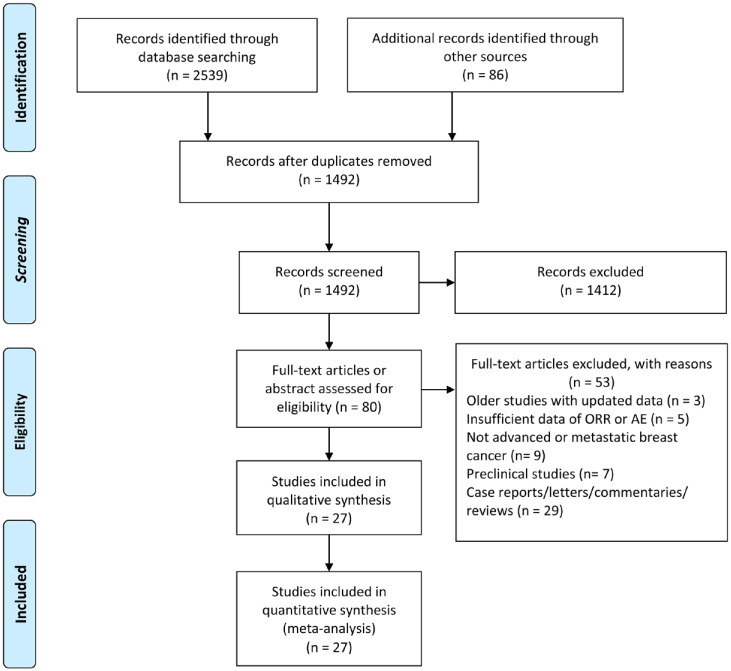
The details of our literature search are summarized in the PRISMA flow diagram (Figure 1). Overall, our electronic search strategy identified 1492 potential articles, of which 27 studies were included in the systematic review. Of the 27 studies, 16 were full-text articles, whereas 11 studies were abstracts. Sixteen studies used PD-1 antibody,7,8,12,19–31 eight studies used PD-L1 antibody,6,9–11,32–35 two studies used CTLA-4 antibody36,37 and one study used both PD-1 and CTLA-4 antibody.38 Patients with triple-negative breast cancer (TNBC), human epidermal growth factor receptor 2 (HER2) overexpression, and breast cancer with any subtypes were enrolled in 16, two, and nine studies, respectively. The main baseline characteristics of the included studies are reported in Table 1. Overall, 27 studies that enrolled 1746 patients were included in the final quantitative synthesis. The methodology quality of included studies was evaluated in both RCTs and non-randomized trials (Supplemental Tables 1 and 2). The risk of bias of included prognostic factors studies was assessed using the QUIPS tool (Supplemental Table 3).
Figure 1.
PRISMA flow diagram of study retrieval and selection.
Table 1.
Main baseline characteristics and outcomes of the included studies.
| Study | Phase | Design and center | Breast cancer subtype | Line of therapy | Immune checkpoint inhibitor | Target | Sample size | ORR (%) | Median OS, month (range) |
|---|---|---|---|---|---|---|---|---|---|
| Schmid et al.11
(NCT02425891) |
III | RCT,1:1, Double-blinded; multicenter |
TNBC | First line | Atezolizumab | PD-L1 | E: 451 C: 451 |
E: 56 C: 47 |
E: 21.3 (17.3–23.4) |
| Emens et al.32
(NCT02924883) |
II | RCT,2:1, Double-blinded; multicenter |
HER2+ | ⩾Second line | Atezolizumab | PD-L1 | E: 132 C: 69 |
E: 45 C: 43 |
– |
| Sherene et al.29
(NCT02129556) |
I/II | Single-arm, open label; multicenter |
HER2+ | First/⩾second line | Pembrolizumab | PD-1 | 58 | 12 | – |
| Vinayak et al.28
(NCT02657889) |
I/II | Single-arm, open label; multicenter |
TNBC | First/⩾second line | Pembrolizumab | PD-1 | 47 | 22 | – |
| Voorwerk et al.12
(NCT02499367) |
II | Randomized, non-comparative, open-label; single center (Netherlands) |
TNBC | First/⩾second line | Nivolumab | PD-1 | 66 | 20 | – |
| Adams1 et al.31
(NCT02447003) |
II | Multicohort, open-label; multicenter |
TNBC | ⩾Second line | Pembrolizumab | PD-1 | 170 | 5.3 | 9.0 (7.6–11.2) |
| Adams2 et al.7
(NCT02447003) |
II | Multicohort, open-label; multicenter |
TNBC | First/⩾second line | Pembrolizumab | PD-1 | 84 | 21.4 | 18.0 (12.9–23.0) |
| Adams3 et al.9
(NCT01633970) |
Ib | Multicohort, open label; multicenter |
TNBC | First/⩾second line | Atezolizumab | PD-L1 | 33 | 39.4 | 14.7 (10.1–NE) |
| Dirix et al.6
(NCT01772004) |
Ib | Single-arm, Open-label; Multicenter |
Any subtype | ⩾Second line | Avelumab | PD-L1 | 168 | 3 | 8.1 (6.4–NE) |
| Emens et al.10 (NCT01375842) | I | Multicohort, open label; multicenter |
TNBC | First/⩾second line | Atezolizumab | PD-L1 | 115 | 10 | 8.9 (7.0–12.6) |
| Rugo et al.8
(NCT02054806) |
Ib | Non-randomized, multicohort, open-label; NA | ER+/HER2– with PD-L1+ | First/⩾second line | Pembrolizumab | PD-1 | 25 | 12 | 8.6 (7.3–11.6) |
| Santa-Maria et al.38
(NCT02536794) |
Pilot | Single arm, pilot study, open-label; single-center (USA) |
ER+ or TNBC | First/⩾second line | Durvalumab and tremelimumab | PD-L1 and CTLA-4 | 18 | 17 | – |
| Weiss et al.27
(NCT02331251) |
Ib/II | Non-randomized, parallel assignment, open label; NA |
Any subtype | First/⩾second line | Pembrolizumab | PD-1 | 12 | 8 | – |
| Nanda et al.30
(NCT01848834) |
Ib | Non-randomized, multicohort, open label; multicenter |
TNBC | First/⩾second line | Pembrolizumab | PD-1 | 27 | 18.5 | 11.2 (5.3–NE) |
| Anders et al.26
(NCT02768701) |
II | Single-arm, open label; multicenter |
TNBC | First/⩾second line | Pembrolizumab | PD-1 | 40 | 21 | 6.3 (2.8–8.4) |
| David et al.21
(NA) |
NA | NA; NA |
Any subtype | First/⩾second line | Pembrolizumab | PD-1 | 15 | 60 | – |
| Domchek et al.34
(NCT02734004) |
II | Single-arm, open label; multicenter |
HER2–, BRCA1/2 (–) | First/⩾second line | Durvalumab | PD-L1 | 32 | 56 | – |
| O’Day et al.23
(NCT02981303) |
II | Non-randomized, parallel assignment, open label; multicenter |
TNBC | ⩾Second line | Pembrolizumab | PD-1 | 12 | 25 | – |
| Page et al.19
(NCT02734290) |
I/II | Non-randomized, parallel assignment, open label; NA | TNBC | First/⩾second line | Pembrolizumab | PD-1 | 14 | 43 | – |
| Heather et al.20
(NCT02730130) |
II | Single-arm, two-stage, open label; multicenter |
TNBC | First/⩾second line | Pembrolizumab | PD-1 | 17 | 33 | – |
| Quintela-Fandino et al.33
(NCT02802098) |
Ib | Single-arm, open label; NA |
HER2– | First/⩾second line | Durvalumab | PD-L1 | 24 | 0 | – |
| Quiroga et al.24
(NCT03025880) |
II | Single-arm, open label; NA |
HER2– | First/⩾second line | Pembrolizumab | PD-1 | 14 | 0 | – |
| Spira et al.25
(NCT02178722) |
I/II | Non-randomized, open-label; single-center (USA) |
TNBC | First/⩾second line | Pembrolizumab | PD-1 | 39 | 10 | – |
| Tolaney et al.22
(NCT02513472) |
Ib/II | Single-arm, open label; NA |
TNBC | First/⩾second line | Pembrolizumab | PD-1 | 82 | 26 | NE |
| Veitch et al.35
(NCT02644369) |
II | Single-arm, open label; NA |
TNBC | First/⩾second line | Pembrolizumab | PD-1 | 19 | 5 | 7.4 (6.2-10.7) |
| Jiang et al.36
(NA) |
I | Open label; NA |
Any subtype | First/⩾second line | Tremelimumab | CTLA-4 | 6 | 0 | 50.8 (–) |
| Vonderheide et al.37
(NA) |
I | Multicohort; open label; multicenter |
Any subtype | First/⩾second line | Tremelimumab | CTLA-4 | 26 | 0 | – |
BRCA1/2, BRCA1/2 DNA repair associated; C, group of control; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; E, group of experiment; ER+, estrogen receptor positive; HER2+, human epidermal growth factor receptor 2 overexpression; NA, not available; NCT, national clinical trial; ORR, overall response rate; OS, overall survival; PD-1, programmed death 1; PD-L1, programmed death ligand 1; RCT, randomized controlled trial; TNBC, triple negative breast cancer.
Objective response rates of ICI treatment
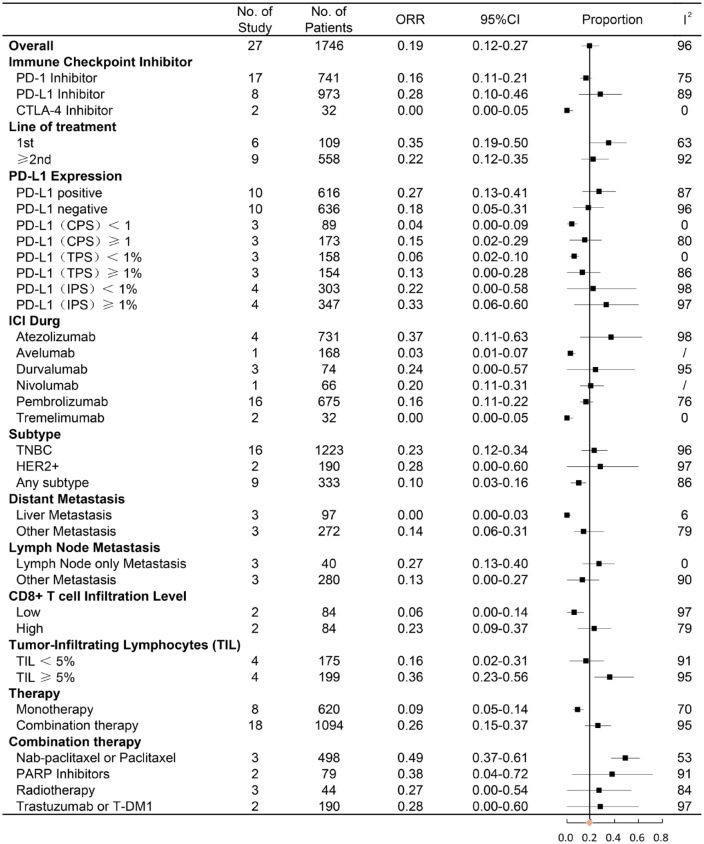
The data for ORR were available from 27 studies, including 1746 patients treated with immune checkpoint therapy. The pooled percentage for ORR was 19% (95% CI = 12–27%) (Supplemental Figure 1). In subgroup analysis, anti-PD-L1 immunotherapy had a higher rate of ORR compared with anti-PD-1 immunotherapy (28% versus 16%) (Figure 2). No response in patients was observed after receiving anti-CTLA-4 immunotherapy in both trials. An objective response was observed in 35% (95% CI = 19–50%) of patients who received the first-line immunotherapy and 22% (95% CI = 12–35%) of patients treated with second-line immunotherapy. With an ORR of 23% and 28%, TNBC and HER2 overexpression breast cancer had a relatively higher ORR than other breast cancer subtypes. The combination of immunotherapy with systematic therapy demonstrated a better ORR than monotherapy with ICI (26% versus 9%). In addition, 49% of patients achieved objective response after receiving a combination of ICI and nab-paclitaxel/paclitaxel chemotherapy, which had the highest ORR in all combined treatments. Other subgroup comparisons are shown in Figure 2. Subgroup analysis of ORR revealed that heterogeneity considerably decreased after division into specific subgroups in varying degrees (Figure 2). Given the heterogeneity, its contribution of median OS, proportion of PD-L1-positive patients, median age, sample size, year of publication, and quality score were analyzed by meta-regression analysis (Supplemental Figure 3A). ORR was significantly correlated with median OS, which was a contribution to overall heterogeneity (p = 0.004). Egger’s test (p = 0.196) indicated no publication bias existed in this meta-analysis for ORR.
Figure 2.
Forest plot of subgroup analysis of ORR in different immune checkpoint targets, line of ICI therapy, PD-L1 expression, ICI drug, subtype of breast cancer, metastatic site, CD8+ T-cell infiltration level, tumor-infiltrating lymphocytes, and combination therapy.
CI, confidence interval; CPS, combined positive score; HER2+, human epidermal growth factor receptor 2; IPS, immune cell proportion score; ORR, objective response rate, PARP inhibitors, poly ADP-ribose polymerase inhibitors; TPS, tumor proportion score; TNBC, triple-negative breast cancer.
Biomarkers for ORR, PFS, and OS following ICI treatment
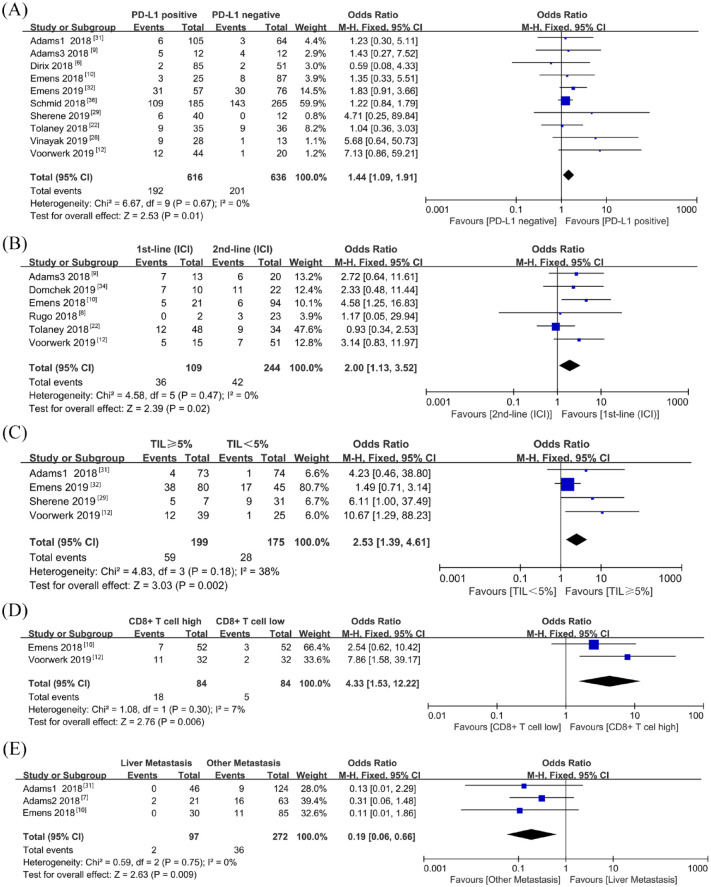
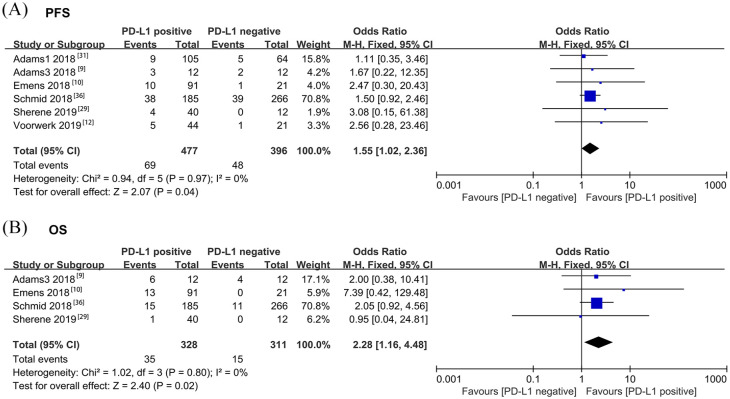
To determine the prognostic factors of ORR, PFS, and OS in patients with metastatic breast cancer receiving ICI treatment, 13 studies with subgroup data were included for further analysis. PD-L1-positive patients had a higher ORR than those with PD-L1-negative tumors (OR = 1.44, 95% CI = 1.09–1.91, p = 0.01, I2 = 0%) (Figure 3A). As PD-L1 expression on immune cells is more prevalent than that on tumor cells in breast cancer,39 we also evaluated the predicted value based on PD-L1 expression on immune cells. However, PD-L1 expression on infiltrating immune cells (OR = 1.33, 95% CI = 0.93–1.90, p = 0.12, I2 = 0%) was not able to predict ORR of immunotherapy (Supplemental Figure 2A). First-line immunotherapy showed a better ORR than second-line immunotherapy (OR = 2.00, 95% CI = 1.13–3.52, p = 0.02, I2 = 0%) (Figure 3B). There was an association between TIL and tumor-infiltrated CD8+ T-cell level and ORR in favor of TIL ⩾5% (OR = 2.53, 95% CI = 1.39–4.61, p = 0.002, I2 = 38%) and patients with high CD8+ T cells (OR = 4.33, 95% CI = 1.53–12.22, p = 0.006, I2 = 7%) (Figure 3C–D). Patients with liver metastasis had a poorer ORR compared with those with metastasis in other sites (OR = 0.19, 95% CI = 0.06–0.66, P = 0.009, I2 = 0%) (Figure 3E). In addition, the difference was non-significant in ORR based on age, performance status score, lymph node metastasis, and LDH level (Supplemental Figure 2B–E). Tumor mutation burden (TMB) was only reported in one study, which revealed a non-significant difference between the response and non-response group.12 Microsatellite instability (MSI) was also reported in one study to predict response and survival. One patient with MSI breast metastatic tumor had an ongoing remission for 102 weeks after receiving ICI for 1 year.12 Data of 1-year PFS, 1-year OS, and 2-year OS were collected from subgroups of the previously mentioned studies. Pooled analysis demonstrated that patients with PD-L1-positive tumor had a better 1-year PFS than those with PD-L1-negative tumor following ICI therapy (OR = 1.55, 95% CI = 1.02–2.36, P = 0.04, I2 = 0%) (Figure 4A). PD-L1-positive expression was not a predictive biomarker for 1-year OS following immunotherapy, possibly due to the short follow-up time (OR = 1.19, 95% CI = 0.91–1.56, p = 0.20, I2 = 15%) (Supplemental Figure 4). However, patients with PD-L1-positive tumor had better 2-year OS after receiving ICI therapy (OR = 2.28, 95% CI = 1.16–4.48, p = 0.02, I2 = 0%) (Figure 4B). With atezolizumab, patients with PD-L1-positive tumor in a phase III trial (IMpassion130) had an improved OS, which indicated that immune checkpoint therapy has better effect in certain populations (hazard ratio = 0.62; 95% CI = 0.45–0.86). Egger’s test was conducted to assess publication bias in the biomarker meta-analysis. No potential publication bias was observed except in the case of performance status score (Supplemental Figure 5). GRADE quality of evidence assessment and its clinical importance is summarized in Supplemental Table 4.
Figure 3.
Forest plots of ORR comparisons based on (A) PD-L1 expression, (B) line of ICI therapy, (C) TIL level, (D) CD8+ T-cell infiltration level, and (E) liver metastasis
Odds ratio for each study is presented and horizontal lines indicate the 95% CI.
CI, confidence interval; ICI, immune checkpoint inhibitor; ORR, objective response rate; TIL, tumor-infiltrating lymphocytes.
Figure 4.
Forest plots of comparison of (A) PFS rate at the first year and (B) OS rate at the second year based on PD-L1 expression level after receiving ICI treatment.
Odds ratio for each study is presented, and horizontal lines indicate the 95% CI.
CI, confidence interval; ICI, immune checkpoint inhibitor; OS, overall survival; PFS, progression-free survival.
Incidence of adverse events
The pooled analysis of safety outcomes was conducted in 21 studies, including trAEs and irAEs. The ICI treatment had a relative high frequency of trAEs of any grade (70%, 95% CI = 58–82%) (Supplemental Figure 6A) and trAEs of grade 3 or more severity (25%, 95% CI = 16–34%) (Supplemental Figure 7A). Combination of ICI treatment with systematic therapy (91%, 95% CI = 85–97%) had a higher incidence of trAEs of any grade compared with monotherapy (64%, 95% CI = 64% to 68%) (Supplemental Figure 8A). Combination of ICI with nab-paclitaxel/paclitaxel chemotherapy had the highest rate of trAEs of any grade (98%, 95% CI = 94–100%) in all combinations. The incidence of irAEs of any grade and 3–4 grade was 34% (95% CI = 18–51%) and 15% (95% CI = 11–19%), respectively (Supplemental Figures 6B and 7B). All grade irAEs occurred in 28% (95% CI = 12–44%) of patients treated with PD-1 inhibitors and were found in 53% (95% CI = 11–94%) of patients treated with PD-L1 inhibitors (Supplemental Figure 8B). Pembrolizumab (18%, 95% CI = 12–25%) and avelumab (10%, 95% CI = 6–16%) had a significantly lower rate of irAEs compared with atezolizumab (74%, 95% CI = 41–100%) and nivolumab (81%, 95% CI = 70–89%) (Supplemental Figure 8B).
Ongoing randomized controlled phase III trials
We identified five ongoing randomized controlled phase III trials evaluating immune checkpoint therapy in combination with chemotherapy in metastatic breast cancer (Table 2). ICI was used as the first-line and second-line therapies in four and one study, respectively, for metastatic breast cancer. Pembrolizumab (PD-1 inhibitor) and atezolizumab (PD-L1 inhibitor) were investigated in three and two trials, respectively. Only one trial had enrolled patients with HER2+ breast cancer and evaluated the impact of atezolizumab on PFS in combination with pertuzumab, trastuzumab, and paclitaxel. The estimated completion dates of these trials range from 11 April 2019 to 1 January 2023.
Table 2.
Ongoing randomized controlled phase III trials with immune checkpoint therapy in advanced or metastatic breast cancer.
| Trial | Phase | Line of therapy | Experimental arm | Control arm | Primary endpoint | Subtype | Estimated completion date |
|---|---|---|---|---|---|---|---|
| NCT02555657 | III | ⩾2nd line | Pembrolizumab | Capecitabine, Eribulin, Gemcitabine, or Vinorelbine | OS | TNBC | 11 April 2019 |
| NCT02819518 | III | 1st line | Pembrolizumab + (Nab-paclitaxel or Paclitaxel or (Gemcitabine + Carboplatin)) | Placebo + (Nab-paclitaxel or Paclitaxel or (Gemcitabine + Carboplatin)) | OS, PFS | TNBC | 30 December 2019 |
| NCT03125902 | III | 1st line | Atezolizumab + Paclitaxel | Placebo + Paclitaxel | PFS | TNBC | 30 January 2020 |
| NCT03371017 | III | 1st line | Atezolizumab + Gemcitabine + Capecitabine + Carboplatin | Placebo + Gemcitabine + Capecitabine + Carboplatin |
OS | TNBC | 1 January 2023 |
| NCT03199885 | III | 1st line | Atezolizumab + + Trastuzumab +Paclitaxel | Placebo + Pertuzumab + Trastuzumab + Paclitaxel |
PFS | HER2+ | 31 December 2020 |
NCT, national clinical trial; OS, overall survival; PFS, progression-free survival; TNBC, triple negative breast cancer; HER2+, human epidermal growth factor receptor 2 overexpression.
Discussion
To date, this study is the first meta-analysis investigating the efficacy and safety of ICI treatment in patients with metastatic breast cancer. In particular, this review is the first to summarize the several potential biomarkers for response and survival prediction which is essential to identify the patients who benefited from treatment with ICIs. The results showed that immune checkpoint therapy had an ORR of 19% (95% CI = 12–27%). Subgroup analysis demonstrated that PD-L1-positive tumor, first-line immunotherapy, high TIL level, non-liver metastasis, and high CD8+ T-cell infiltrating level predict high ORR in ICI treatment. PD-L1 expression on infiltrating immune cells was not an ideal biomarker for response prediction. With respect to survival prediction, PD-L1 expression was a potential prognostic factor for 1-year PFS and 2-year OS following immune checkpoint therapy. TNBC and HER2 overexpression breast cancer had a relatively higher ORR than other breast cancer subtypes. The combination of immunotherapy with systematic therapy showed a better ORR than ICI (26% versus 9%) monotherapy. Approximately half of the patients achieved objective response following combination of ICI with nab-paclitaxel/paclitaxel chemotherapy, which had the highest ORR in all combination treatment. The incidence of grade 3–4 trAEs was 25% (95% CI = 16–34%) while grade 3–4 irAEs was 15% (95% CI = 11–19%) during immunotherapy. All grade trAEs occurred in almost all patients treated with the combination of ICI and nab-paclitaxel/paclitaxel chemotherapy. PD-1 inhibitors showed fewer all grade irAEs than PD-L1 inhibitors.
Given the remarkable innovation and progress made in immunotherapeutic strategies to treat cancer, novel active agents have emerged as the saviors of patients with multiple advanced or metastatic cancers.40 Over the past decade, strategies such as monoclonal antibodies, immune enhancing adjuvants, vaccines against oncogenic viruses, and adoptive cell therapies have been well established.41 Targeting regulatory pathways in T cells, immune checkpoint therapy has demonstrated its efficacy and benefit in improving the survival in metastatic melanoma, non-small cell lung cancer, and renal cell carcinoma.42–44 The efficacy of immune checkpoint therapy in breast cancer has been examined in initial clinical trials, which revealed modest but interesting responses.45 In the phase III randomized clinical trial (IMpassion130), the PD-L1 inhibitor atezolizumab combined with nab-paclitaxel conferred a significant improvement of PFS compared with the nab-paclitaxel group in TNBC (hazard ratio = 0.80; 95% CI = 0.69–0.92; p = 0.002). As a result, atezolizumab became the first FDA-approved immune checkpoint agent for use in combination with nab-paclitaxel for patients with metastatic TNBC in March 2019. Although significant benefit for PFS was found in the atezolizumab treatment group, OS was non-significant between the two groups in all patients (hazard ratio = 0.84; 95% CI = 0.69–1.02; p = 0.08). However, atezolizumab revealed an improved OS in patients with PD-L1-positive tumors, indicating that immune checkpoint therapy has a better effect in certain populations (hazard ratio = 0.62; 95% CI = 0.45–0.86). In this study, positive PD-L1 expression was found to be associated with an improved 1-year PFS and 2-year OS in patients receiving immune checkpoint therapy. These results are critical for clinical practice in selecting patients who would potentially benefit.
Historically, breast cancer was considered immunologically quiescent compared with other solid tumors such as non-small cell lung cancer and melanoma. With a lower TMB, breast cancer may have fewer neoantigen generations to stimulate antitumor immune response.46 TNBC and HER2 overexpression breast cancers are known to have higher TMB and TIL rates compared with luminal breast cancer.47–49 The immune microenvironment can exert great influence on the progression of breast cancer, which results in the different clinical prognosis of patients.50–52 We found that patients with TNBC and HER2 overexpression breast cancer had a better response to immune checkpoint therapy, which could be attributed to their high TMB and TIL rate. In addition, a high TIL level was associated with a high response rate. The PD-L1 expression level is an acknowledged prognostic factor in different cancers.53–55 PD-L1 expression in tumor cells is a confirmative biomarker for predicting response to PD-1/PD-L1 checkpoint inhibition according to the analyses of more than 10 different solid tumors, which was the same as that for breast cancer in our results.56–58 PD-L1 mainly expresses on tumor-infiltrating immune cells rather than on tumor cells in patients with TNBC, thus it might be a prognostic factor for response.59 We performed a pooled analysis of the response rate based on the PD-L1 status on tumor-infiltrating immune cells and found a non-significant positive trend. Liver metastasis of breast cancer indicates a poor response to immune checkpoint therapy compared with other metastatic sites. The liver is an immune tolerogenic organ because of its exposure to various antigens (toxins, gut-derived microbial products, etc.) and chronic inflammatory state.60,61 Several mechanisms have been proved to explain liver-induced immune tolerance, including trapping and inactivation of CD8+ T cells,62,63 activation of Treg cells by Kupffer cells64 and poor activation of CD4+ T cells.65 In addition, chronic hepatitis B virus (HBV) infection can remarkably induce natural killer cell receptor imbalance and dysfunction which results in immune tolerance.66 Therefore, liver metastatic cancers have a lower response to ICI treatment by means of these mechanisms to evade the immune system and facilitate tumor progression.
Detection of PD-L1 is always an important research direction of cancer immunotherapy. Immunohistochemistry (IHC) is the most common method to determine PD-L1 expression and multiple monoclonal PD-L1 antibodies have been well developed, such as clone 22C3,67 clone 28-8,68 and clone SP142.69 However, the accuracy of these PD-L1 IHC detection methods has remained controversial. PD-L1 assessment by IHC can sometimes show as false negative, which means some patients with PD-L1 positive are misdiagnosed as PD-L1 negative. For instance, glycosylation of cell surface PD-L1 can render its polypeptide antigens inaccessible to PD-L1 antibodies, which can lead to false negative IHC judgement and inconsistent therapeutic outcomes of ICI treatment.70 As a result, a small proportion of patients with PD-L1-negative tumor can still gain benefit from ICI therapy according to the outcomes of clinical trials. Therefore, developing advanced methods or using multiple biomarkers to make joint prediction is the focus of future investigation. For example, removing the glycosylation and exposing the antigens before PD-L1 IHC staining to reduce the false-negative rate of detection.70
This analysis had several limitations. First, given the lack of randomized clinical trials in the initial stages of immunotherapy research in breast cancer, bias was inevitable to some extent. Second, several important prognostic values for response and survival were not included in the subgroup meta-analysis (TMB, MSI, etc.). Third, the small number of patients enrolled in each clinical trial contributed to the high heterogeneity in the analysis of response and adverse events. Fourth, as the clinical exploration of ICI treatment in breast cancer is still in the early stages, results of biomarker prediction from multivariate analysis have not been reported. Outcomes from multivariate analysis, by which the prognostic effect of this biomarker can be evaluated together with other prognostic factors after proper adjustment, are far more informative than those from univariate analysis. A new biomarker might not add to existing predictors if adjusted results are unavailable. Therefore, more multicenter RCTs with high quality, large sample size, multivariate analysis, and adequate follow-up are required for further validation.
In conclusion, although immune checkpoint therapy has demonstrated its promising efficacy in metastatic breast cancer, the majority of patients are not likely to benefit from it. Different populations appeared to have varying responses to ICI treatment. Our analysis found that PD-L1-positive tumor, first-line immunotherapy, non-liver metastasis, high TIL, and CD8+ T-cell infiltrating level could predict a better response to ICI therapy. The PD-L1-positive subgroup could gain more survival benefits from immune checkpoint therapy.
Supplemental Material
Supplemental material, Supplementary_Materials_3 for Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: a systematic review and meta-analysis by Yutian Zou, Xuxiazi Zou, Shaoquan Zheng, Hailin Tang, Lijuan Zhang, Peng Liu and Xiaoming Xie in Therapeutic Advances in Medical Oncology
Acknowledgments
Authors Yutian Zou, Xuxiazi Zou and Shaoquan Zheng contributed equally to this work.
Footnotes
Author contributions: Conception and design, XX and PL; development of methodology, YZ and XZ; acquisition of data, YZ, XZ, SZ and HT; formal analysis, YZ and LZ; writing, YZ, XZ and SZ; reviewing and editing, XX and PL. All authors read and approved the final manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Natural Science Foundation of China (81872152, Xiaoming Xie).
ORCID iD: Yutian Zou  https://orcid.org/0000-0002-5205-9923
https://orcid.org/0000-0002-5205-9923
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yutian Zou, Department of Breast Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China.
Xuxiazi Zou, Department of Breast Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China.
Shaoquan Zheng, Department of Breast Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China.
Hailin Tang, Department of Breast Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China.
Lijuan Zhang, Department of Breast Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China.
Peng Liu, Department of Breast Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, 651 East Dongfeng Road, Guangzhou 510060, People’s Republic of China.
Xiaoming Xie, Department of Breast Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, 651 East Dongfeng Road, Guangzhou 510060, People’s Republic of China.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 3. Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010; 28: 3271–3277. [DOI] [PubMed] [Google Scholar]
- 4. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller LD, Chou JA, Black MA, et al. Immunogenic subtypes of breast cancer delineated by gene classifiers of immune responsiveness. Cancer Immunol Res 2016; 4: 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat 2018; 167: 671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 2019; 30: 405–411. [DOI] [PubMed] [Google Scholar]
- 8. Rugo HS, Delord JP, Im SA, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res 2018; 24: 2804–2811. [DOI] [PubMed] [Google Scholar]
- 9. Adams S, Diamond JR, Hamilton E, et al. Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up: a phase 1b clinical trial. JAMA Oncol 2019; 5: 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol 2019; 5: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018; 379: 2108–2121. [DOI] [PubMed] [Google Scholar]
- 12. Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med 2019; 25: 920–928. [DOI] [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Riley RD, Moons KGM, Snell KIE, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ 2019; 364: k4597. [DOI] [PubMed] [Google Scholar]
- 15. Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003; 73: 712–716. [DOI] [PubMed] [Google Scholar]
- 16. Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158: 280–286. [DOI] [PubMed] [Google Scholar]
- 17. Debray TP, Damen JA, Snell KI, et al. A guide to systematic review and meta-analysis of prediction model performance. BMJ 2017; 356: i6460. [DOI] [PubMed] [Google Scholar]
- 18. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Page D, Pucilowska J, Bennetts L, et al. Updated efficacy of first or second-line pembrolizumab (pembro) plus capecitabine (cape) in metastatic triple negative breast cancer (mTNBC) and correlations with baseline lymphocyte and naïve CD4+ T-cell count. Cancer Res 2019; 79 (Suppl. 4): Abstract P2-09-03. [Google Scholar]
- 20. McArthur HL, Barker CA, Gucalp A, et al. A single-arm, phase II study assessing the efficacy of pembrolizumab (pembro) plus radiotherapy (RT) in metastatic triple negative breast cancer (mTNBC). Ann Oncol 2017; 28 (Suppl. 5): v74–v108. [Google Scholar]
- 21. David SP, Savas P, Neeson PJ, et al. Safety and efficacy of stereotactic body radiotherapy and pembrolizumab in advanced breast cancer patients with 1 to 5 metastases. Cancer Res 2019; 79 (Suppl. 4): Abstract P2-09-06. [Google Scholar]
- 22. Tolaney S, Kalinsky K, Kaklamani V, et al. Phase 1b/2 study to evaluate eribulin mesylate in combination with pembrolizumab in patients with metastatic triple-negative breast cancer. Cancer Res 2018; 78 (Suppl. 4): Abstract PD6-13. [Google Scholar]
- 23. O’Day S, Borges V, Chmielowski B, et al. Imprime PGG, a novel innate immune modulator, combined with pembrolizumab in a phase 2 multicenter, open label study in chemotherapy-resistant metastatic triple negative breast cancer (TNBC). Cancer Res 2019; 79 (Suppl. 4): Abstract P2-09-08. [Google Scholar]
- 24. Quiroga V, Holgado E, Alonso JL, et al. Run-in-phase results from a multicenter phase II trial to evaluate pembrolizumab (P) and gemcitabine (Gem) in patients (pts) with HER2-negative advanced breast cancer (ABC): GEICAM/2015-04 PANGEA-Breast. Ann Oncol 2018; 29 (Suppl. 10): X32. [Google Scholar]
- 25. Spira AI, Hamid O, Bauer TM, et al. Efficacy/safety of epacadostat plus pembrolizumab in triple-negative breast cancer and ovarian cancer: phase I/II ECHO-202 study. J Clin Oncol 2017; 35 (Suppl. 15): 1103. [Google Scholar]
- 26. Anders CK, Moore D, Sambade M, et al. LCCC 1525: a phase II study of a priming dose of cyclophosphamide prior to pembrolizumab to treat metastatic triple negative breast cancer (mTNBC). Cancer Res 2019; 79 (Suppl. 4): Abstract P2-09-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weiss GJ, Waypa J, Blaydorn L, et al. A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br J Cancer 2017; 117: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vinayak S, Tolaney SM, Schwartzberg L, et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol 2019; 5: 1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loi S, Giobbie-Hurder A, Gombos A, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b–2 trial. Lancet Oncol 2019; 20: 371–382. [DOI] [PubMed] [Google Scholar]
- 30. Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol 2016; 34: 2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 2019; 30: 397–404. [DOI] [PubMed] [Google Scholar]
- 32. Emens LA, Esteva F, Beresford M, et al. Results from KATE2, a randomized phase 2 study of atezolizumab (atezo) plus trastuzumab emtansine (T-DM1) versus placebo (pbo)+T-DM1 in previously treated HER2+advanced breast cancer (BC). Cancer Res 2019; 79 (Suppl. 4): Abstract PD3-01. [Google Scholar]
- 33. Quintela-Fandino M, Sanchez LMM, Martin EH, et al. Addition of durvalumab (Dur) upon progression to bevacizumab (Bev) maintenance in advanced HER2-negative (HERNEG) breast cancer (BC): safety, efficacy and biomarkers. Ann Oncol 2018; 29 (Suppl. 9): ix24. [Google Scholar]
- 34. Domchek SM, Postel-Vinay S, Im SA, et al. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): updated results in patients with germline BRCA-mutated (gBRCAm) metastatic breast cancer (MBC). Cancer Res 2019; 79 (Suppl. 4): Abstract PD5-04. [Google Scholar]
- 35. Veitch ZWN, Cescon DW, Elston S, et al. Phase II (INSPIRE) trial of pembrolizumab (pembro) with serial immune and genomic profiling in patients (pts) with metastatic triple negative breast cancer (mTNBC). J Clin Oncol 2018; 36 (Suppl. 15): 1094. [Google Scholar]
- 36. Jiang DM, Fyles A, Nguyen LT, et al. Phase I study of local radiation and tremelimumab in patients with inoperable locally recurrent or metastatic breast cancer. Oncotarget 2019; 10: 2947–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res 2010; 16: 3485–3494. [DOI] [PubMed] [Google Scholar]
- 38. Santa-Maria CA, Kato T, Park JH, et al. A pilot study of durvalumab and tremelimumab and immunogenomic dynamics in metastatic breast cancer. Oncotarget 2018; 9: 18985–18996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cimino-Mathews A, Thompson E, Taube JM, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol 2016; 47: 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharma P, Allison JP. The future of immune checkpoint therapy. Science (New York, NY) 2015; 348: 56–61. [DOI] [PubMed] [Google Scholar]
- 41. Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol 2009; 27: 83–117. [DOI] [PubMed] [Google Scholar]
- 42. Pasquali S, Chiarion-Sileni V, Rossi CR, et al. Immune checkpoint inhibitors and targeted therapies for metastatic melanoma: a network meta-analysis. Cancer Treat Rev 2017; 54: 34–42. [DOI] [PubMed] [Google Scholar]
- 43. Iacovelli R, Ciccarese C, Bria E, et al. Immunotherapy versus standard of care in metastatic renal cell carcinoma. A systematic review and meta-analysis. Cancer Treat Rev 2018; 70: 112–117. [DOI] [PubMed] [Google Scholar]
- 44. Zhou Y, Chen C, Zhang X, et al. Immune-checkpoint inhibitor plus chemotherapy versus conventional chemotherapy for first-line treatment in advanced non-small cell lung carcinoma: a systematic review and meta-analysis. J Immunother Cancer 2018; 6: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Polk A, Svane IM, Andersson M, et al. Checkpoint inhibitors in breast cancer – current status. Cancer Treat Rev 2018; 63: 122–134. [DOI] [PubMed] [Google Scholar]
- 46. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017; 9: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol 2016; 2: 1354–1360. [DOI] [PubMed] [Google Scholar]
- 48. Thomas A, Routh ED, Pullikuth A, et al. Tumor mutational burden is a determinant of immune-mediated survival in breast cancer. Oncoimmunology 2018; 7: e1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luen S, Virassamy B, Savas P, et al. The genomic landscape of breast cancer and its interaction with host immunity. Breast 2016; 29: 241–250. [DOI] [PubMed] [Google Scholar]
- 50. Lim B, Woodward WA, Wang X, et al. Inflammatory breast cancer biology: the tumour microenvironment is key. Nat Rev Cancer 2018; 18: 485–499. [DOI] [PubMed] [Google Scholar]
- 51. Wagner J, Rapsomaniki MA, Chevrier S, et al. A Single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell 2019; 177: 1330–1345.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zheng S, Zou Y, Xie X, et al. Development and validation of a stromal immune phenotype classifier for predicting immune activity and prognosis in triple-negative breast cancer. Int J Cancer 2020; 147: 542–553.32285442 [Google Scholar]
- 53. Matikas A, Zerdes I, Lovrot J, et al. Prognostic implications of PD-L1 expression in breast cancer: systematic review and meta-analysis of immunohistochemistry and pooled analysis of transcriptomic data. Clin Cancer Res 2019; 25: 5717–5726. [DOI] [PubMed] [Google Scholar]
- 54. Jia YQ, Yang B, Wen LL, et al. Prognostic value of immune checkpoint molecules in head and neck cancer: a meta-analysis. Aging 2019; 11: 501–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang G, Fu X, Chang Y, et al. B7-CD28 gene family expression is associated with prognostic and immunological characteristics of diffuse large B-cell lymphoma. Aging 2019; 11: 3939–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lu S, Stein JE, Rimm DL, et al. Comparison of biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade: a systematic review and meta-analysis. JAMA Oncol 2019; 5: 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ghate K, Amir E, Kuksis M, et al. PD-L1 expression and clinical outcomes in patients with advanced urothelial carcinoma treated with checkpoint inhibitors: a meta-analysis. Cancer Treat Rev 2019; 76: 51–56. [DOI] [PubMed] [Google Scholar]
- 58. Roviello G, Corona SP, Nesi G, et al. Results from a meta-analysis of immune checkpoint inhibitors in first-line renal cancer patients: does PD-L1 matter? Ther Adv Med Oncol 2019; 11: 1758835919861905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2014; 2: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pardee AD, Butterfield LH. Immunotherapy of hepatocellular carcinoma: unique challenges and clinical opportunities. Oncoimmunology 2012; 1: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, et al. The yin and yang of evasion and immune activation in HCC. J Hepatol 2015; 62: 1420–1429. [DOI] [PubMed] [Google Scholar]
- 62. Tumeh PC, Hellmann MD, Hamid O, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 2017; 5: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. John B, Crispe IN. Passive and active mechanisms trap activated CD8+ T cells in the liver. J Immunol 2004; 172: 5222–5229. [DOI] [PubMed] [Google Scholar]
- 64. Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013; 14: 996–1006. [DOI] [PubMed] [Google Scholar]
- 65. Wang JC, Livingstone AM. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J Immunol 2003; 171: 6339–6343. [DOI] [PubMed] [Google Scholar]
- 66. Sun C, Sun H, Zhang C, et al. NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cell Mol Immunol 2015; 12: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol 2013; 31: 4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee HH, Wang YN, Xia W, et al. Removal of N-Linked glycosylation enhances PD-L1 detection and predicts anti-PD-1/PD-L1 therapeutic efficacy. Cancer Cell 2019; 36: 168–178.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Materials_3 for Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: a systematic review and meta-analysis by Yutian Zou, Xuxiazi Zou, Shaoquan Zheng, Hailin Tang, Lijuan Zhang, Peng Liu and Xiaoming Xie in Therapeutic Advances in Medical Oncology