Abstract
Objective
Stress is a known trigger for seizures in patients with epilepsy (PWE). However, the association between stress and seizures has not been thoroughly investigated. In December 2019, an outbreak of coronavirus disease (COVID‐19) occurred in Wuhan, Hubei province, China, causing tremendous collateral stress. This study was designed to evaluate the influence of the COVID‐19 outbreak on seizures in PWE in the most severely affected area, Wuhan, and its surrounding cities.
Methods
In this single‐center, cross‐sectional study, PWE were surveyed via online questionnaires between February 23 and March 5, 2020. Collected data included demographic information, epilepsy‐related characteristics (seizure type, frequency, antiepileptic drugs [AEDs], and medication management), direct and perceived threat of COVID‐19, and changes in seizures during the outbreak. Psychological comorbidities were evaluated by the Patient Health Questionnaire–9, Generalized Anxiety Disorder–7 items, and Insomnia Severity Index (ISI). Multivariate logistic regression was used to identify precipitants for seizure exacerbation.
Results
We received 362 completed questionnaires after excluding 12 duplicates (response rate = 63.51%). A total of 31 (8.56%) patients had increased seizures during the outbreak. Exposure history to COVID‐19 (P = .001), uncontrolled seizure after AED therapy (P = .020), seizure frequency of two or more times per month before the outbreak (P = .005), change of AED regimen during the outbreak (AED reduction, withdrawal, replacement, skipping altogether; P = .002), and worry about the adverse effect of the outbreak on overall seizure‐related issues (severity = moderate to critical; P = .038) were risk factors for increased seizures.
Significance
A minority of PWE experienced seizure exacerbation during the outbreak of COVID‐19. Stress, uncontrolled seizures, and inappropriate change in AED regimen were associated with increased seizures. Based on these findings, stress might be an independent precipitant for triggering seizures in some PWE.
Keywords: COVID‐19, epilepsy, precipitant, seizure exacerbation, stress
Key Points.
A small proportion of patients with epilepsy experienced seizure exacerbation during the outbreak of COVID‐19
Seizure exacerbation is associated with exposure history to COVID‐19, worry about a negative effect of COVID‐19 on seizure‐related issues, inappropriate change of AED treatment, and uncontrolled seizures, including unsuccessful AED treatment, as well as frequent seizures
Stress is an independent precipitant for triggering seizures in some patients with epilepsy
1. INTRODUCTION
Both objective and subjective stress are believed to exacerbate seizures in some patients with epilepsy (PWE). 1 , 2 Stress levels, 1 and major life events 3 have been reported to be associated with increased seizure frequency, which has been supported by experimental studies. 4 Unfortunately, direct clinical neurophysiological studies documenting the triggering of seizures by stress have been limited. One reason is that stress often coexists with other seizure triggers, such as sleep deprivation or fever, making it difficult to distinguish stress as an independent seizure‐promoting stimulus. Another reason is that stress is an ambiguous concept that can be challenging to measure or define empirically. Therefore, stress generally has been inferred either from the patient's subjective post hoc assessment or from other observable phenomena, behavioral or somatic, which are presumed to be of consequence. 5 One way of bypassing the problem of the definition of stress is to examine the effects on seizures of an event that is generally recognized as stressful. 6 Accordingly, the impact of traumatic events with widespread exposure, such as wars or natural disasters, on seizures offers an opportunity to study the role of stress as a precipitant for seizure occurrence. For example, Klein and van Passel examined the effect of 9/11 attack–related emotional stress on seizures among PWE in an area directly involved in one of the attacks 6 ; Bosnjak et al studied the occurrence of seizures in 72 children from war‐affected and 39 children from non–war‐affected areas during and after the 1991‐1992 war in Croatia 7 ; Neufeld et al recorded seizure frequency of 100 consecutive adult PWE who were under stress from the threat of Scud missile attacks during the 1991 Persian Gulf war 8 ; and Swinkels et al investigated the influence of the forced evacuation caused by a probable flood in the province of Gelderland in 1995 on seizure frequency of PWE. 9 However, the prevalence of increased seizures during these stressful events varied remarkably. 6 , 7 , 8 , 9 More studies are needed to further confirm the effect of stress as an independent precipitant for triggering seizures, and to investigate the prevalence of increased seizures during stressful events.
At the end of 2019, an outbreak of coronavirus disease 2019 (COVID‐19) caused by the novel coronavirus (SARS‐CoV2) emerged in Wuhan, Hubei Province, China, and has rapidly spread throughout the country and the rest of the world. 10 Almost 2 months later, the World Health Organization (WHO) declared the coronavirus outbreak a pandemic and predicted that the virus would likely spread to all countries on the globe. As of March 11, 2020, 114 countries have reported that 118 000 people have contracted COVID‐19; nearly 4300 people have died. 11 With uncertainty regarding the virulence and transmissibility of the disease in the early stages, the epicenter Wuhan and its surrounding cities, which accounted for the majority of confirmed cases and had a high fatality rate, were suddenly placed under unprecedented lockdowns. Public activities and transport were prohibited. Residents were generally isolated and inundated with often sensational reports from social media. There was widespread public fear, frustration, and loneliness. 12 The outbreak of COVID‐19 undoubtedly qualifies as a stressful crisis and provided an opportunity to investigate a possible independent role of stress in provoking seizures. Additionally, the outbreak of COVID‐19 also allowed us to identify other seizure precipitants related to daily life and medical care of PWE.
Therefore, we conducted a questionnaire survey–based, cross‐sectional study, in which we aimed to assess the clinical status of PWE in Wuhan and its surrounding cities during the COVID‐19 outbreak, and more importantly, to explore the effect of the viral epidemic on seizure incidence.
2. MATERIALS AND METHODS
2.1. Study design and participants
The study was a single‐center, cross‐sectional survey, covering PWE who were registered in the epilepsy outpatient clinic social media group (Tongji Epileptic Doctors‐Patients WeChat Group) of Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology. The study was conducted between February 2 and March 5, 2020, 1 month after the authorities in Wuhan suspended all public transport and activities on January 23. After excluding 225 patients who (1) were unable to read, (2) had a history of psychiatric illness, or (3) were younger than 10 years in terms of understanding the illness, 13 570 patients received self‐reported questionnaires individually by researchers via WeChat. The questionnaire was generated through an online survey platform (Sojump) and consists of five parts: online informed consent, sociodemographic information, epilepsy‐related characteristics before and during the epidemic, direct and perceived threat related to the COVID‐19 outbreak, and the rating scales Patient Health Questionnaire–9 (PHQ‐9), Generalized Anxiety Disorder–7 items (GAD‐7), and Insomnia Severity Index (ISI).
Each patient was allowed to participate in the survey once. The senior investigators performed quality control by checking the collected questionnaires daily. Two researchers entered data into the database double‐blinded using EpiData 3.0 to guarantee accuracy. The study was approved by the institutional ethics board of Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology (ID: TJ‐IRB20200321). The data analyses were done on unidentified datasets.
2.2. Measures
The questionnaire contained 88 items. Most variables were dichotomized for analyses. The variables are summarized briefly below.
2.2.1. Key demographic variables
Age data were grouped as defined by the WHO 14 , 15 as adolescent (10~19 years), adult (20~60 years), and elderly (≥60 years). Other variables were as follows: gender, habitation (Wuhan/non‐Wuhan), level of education, employment status, and raising children (yes/no). Exercise habits were defined as meeting the WHO physical activity recommendations for adults. 16
2.2.2. Direct and perceived threat of COVID‐19
Questions regarding direct threat related to COVID‐19 included diagnosis of COVID‐19 (yes+/no−) and exposure history, defined as patients in Wuhan or patients outside of Wuhan having direct or suspected contact with confirmed COVID‐19 cases (yes+/no−). In regard to having COVID‐19–like symptoms, multiple choices for all recognized symptoms associated with COVID‐19 were provided (yes+/no−). Questions regarding perceived stress related to COVID‐19 included two questions with 5‐point Likert scale formats 17 that were converted to numerical values ranging from 1 (presented as −) to 5 (presented as ++++) for the analyses. For example, the main question was provided as (1) Have you worried about the bad effect of COVID‐19 on overall seizure‐related issues? To assess worries, patients were asked to rate scale as follows: none−/mild+/moderate++/severe+++/critical++++. The detailed questions addressing worry were asked as (1) Have you worried about another seizure episode during the outbreak? (none−/mild+/moderate++/severe+++/critical++++); and (2) What is the idea you worried about most: Difficulty in acquiring antiseizure drugs? (yes+/no−); Lacking of medical consultation? (yes+/no−); Drug side effect? (yes+/no−); or Other concerns. Data were dichotomized for analysis; moderate‐to‐critical severity was combined.
2.2.3. Epilepsy‐related characteristics before and during the outbreak
Patients were asked questions ascertaining their epilepsy status, including seizure type (symptom of seizure episode), seizure frequency before the outbreak, number of seizures during the month of the outbreak, and use of antiepileptic drugs (AEDs; type and dosage before and during the outbreak). Specific questions concerning seizure and drug change were also asked: (1) Have you experienced an increased number of seizures? (yes+/no−); (2) Have you changed your AED regimen? (yes+/no−); (3) How often did you skip your AEDs before the outbreak?; and (4) How often did you skip your AEDs during the outbreak? For inconsistent answers, we confirmed with individual patients via telephone call. Patients who gave a definite answer to the question of seizure increase and showed an increase in number of seizures during the month of the outbreak as compared to seizure frequency before the outbreak were considered to be PWE with seizure exacerbation during the outbreak of COVID‐19. Data on AED regimen changes resulting in declined dosage of original drugs, including AED reduction, withdrawal, replacement, and newly started skipping of AED during the outbreak for more than three times per week, were grouped for analyses.
2.2.4. Psychological and insomnia assessment
The psychological response of PWE was assessed using three established assessment tools. The PHQ‐9 scale was used to measure depression symptoms. A cutoff of 10 has been recommended for diagnosis of major depression, which provides adequate sensitivity (88.0%) and specificity (88.0%). 18 The GAD‐7 scale was used to identify anxiety disorders. A cutoff score 8 is recommended to identify clinically important anxiety symptoms, with adequate specificity (82.0%) and sensitivity (77.0%). 19 The ISI was used to detect insomnia. Total scores on the ISI range from 0 to 27; a cutoff of ≥8 on the ISI achieved high discriminant validity (area under the curve > 0.85) in identifying individuals with insomnia based on both Diagnostic and Statistical Manual of Mental Disorders, 5th edition and International Classification of Sleep Disorders, 3rd edition criteria. 20 The selected three questionnaires had good internal consistency, with Cronbach α coefficients of >0.80. 20 , 21
2.3. Statistical analysis
Continuous variables were divided into categorical groups, and all variables were shown as the counts and percentages. Categorical variables were analyzed by Pearson chi‐squared test or Fisher exact test. Variables with P values < .05 in univariate analyses were subjected to multivariate logistic regression analysis with a stepwise backward elimination procedure. Multivariate logistic regression analysis was used to determine the risk factors associated with increased seizures during the outbreak of COVID‐19. Two‐sided values of P < .05 were considered statistically significant. Statistical analyses were performed using Statistical Package for the Social Sciences for Windows 22.0 (IBM).
2.4. Role of the funding source
The funders had no role in the design and conduct of the study; collection, analysis, management, and interpretation of the data; and preparation, review, or approval of the manuscript. Suiqiang Zhu had full access to the raw data and did the statistical analyses. With approval from all authors, Suiqiang Zhu had the final decision to submit.
3. RESULTS
3.1. Sociodemographic characteristics of epilepsy patients
A total of 570 PWE were surveyed, and 374 individuals completed the online questionnaire. Three hundred sixty‐two patients were included in the final analysis after excluding the 12 duplicates. The overall response rate was 63.51%. Table 1 summarizes the sociodemographic characteristics of the sample. A proportion of 67.40% PWE was in the age interval of 20‐60 years old, whereas only six (1.66%) patients were 60 years old or older. Males accounted for 54.14% of the total subjects. Eighty‐eight (24.31%) patients were from Wuhan, the epicenter of COVID‐19.
TABLE 1.
Sociodemographic characteristics of patients with epilepsy (N = 362)
| Variable | n (%) |
|---|---|
| Age | |
| 10‐19 y | 112 (30.94%) |
| 20‐60 y | 244 (67.40%) |
| ≥60 y | 6 (1.66%) |
| Gender | |
| Male | 196 (54.14%) |
| Female | 166 (45.86%) |
| Habitation | |
| Non‐Wuhan | 274 (75.69%) |
| Wuhan | 88 (24.31%) |
| Level of education | |
| Below university | 233 (64.36%) |
| University and above | 129 (35.64%) |
| Exercise habit | |
| No | 334 (92.27%) |
| Yes | 28 (7.73%) |
| Employment status | |
| Unemployed | 41 (11.33%) |
| Employed, including students | 321 (88.67%) |
| Raising children | |
| No | 235 (64.92%) |
| Yes | 127 (35.08%) |
3.2. Epilepsy‐related characteristics before and during the outbreak
An overview of epilepsy‐related characteristics of the overall 362 patients is presented in Figure 1. The most common seizure type was focal onset (80.11%). Seizure frequency of 78.18% of patients was less than once per month, and only 28 (7.73%) patients suffered more than four seizures per month before the outbreak. Two hundred sixty‐two (72.38%) patients were treated with AED polytherapy. One hundred eight (29.83%) patients were on treatment with a combination of three or four AEDs. A proportion of 45.86% patients had achieved complete seizure control after AED therapy prior to the outbreak. During the outbreak, 31 patients (8.56%) experienced an increased number of seizures. Nineteen of them had focal to bilateral tonic‐clonic seizures. No patients who had focal seizure with awareness experienced seizure exacerbation. Among 163 (45.86%) patients who achieved seizure freedom after AED treatment, three patients experienced a relapse. Among 20 (5.52%) patients who showed poor response to AEDs, 12 of them suffered more seizures. Twenty‐eight (7.73%) patients changed their AED regimen, resulting in reduced AED dosage of their original drugs (includes reduction, withdrawal, replacement, and newly started skipping of AEDs more than three times per week during the outbreak), and experienced seizure worsening. In addition, data showed all the changes were made involuntarily and could be counted as lack of medication services. A majority of patients had no change in seizure frequency (86.19%) as well as drug regimen (91.16%).
FIGURE 1.
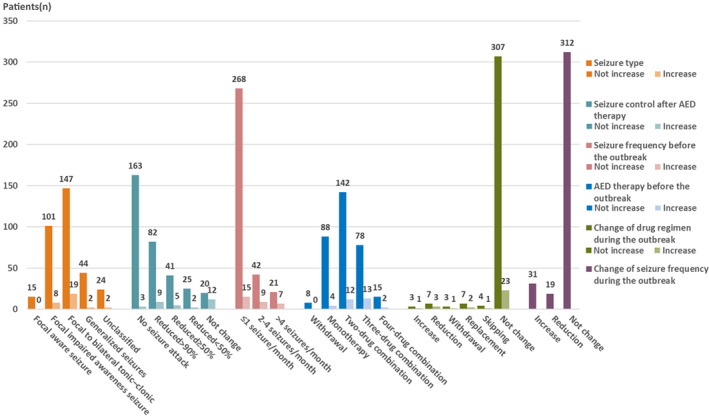
Bar chart illustrating epilepsy‐related characteristics before and during the outbreak reported by patients with epilepsy. AED, antiepileptic drug. "No increase" indicates that seizures did not increase during the month of the outbreak of coronavirus disease 2019 (COVID‐19). "Increase" indicates that seizures increased during the month of the outbreak of COVID‐19
3.3. Direct and perceived threat of COVID‐19
Figure 2 shows direct and perceived threat of COVID‐19 reported by patients. Due to the contagious property and potential lethality of COVID‐19, both exposure history and undergoing COVID‐19–like symptoms during the epidemic outbreak were defined as a direct threat of COVID‐19, an objective stressor. Ninety‐three (25.69%) patients had exposure history. Ten percent of patients showed COVID‐19–like symptoms during the epidemic. Only two patients were confirmed cases with mild symptoms. Moderate‐to‐critical worries about the adverse effect of the outbreak on overall epilepsy‐related issues rose in 19.61% patients. Eighty‐seven patients (24.03%) showed moderate‐to‐critical worries about unanticipated seizures during the epidemic; 41.16% and 48.62% worried about lack of professional consultation and medication supply, respectively.
FIGURE 2.
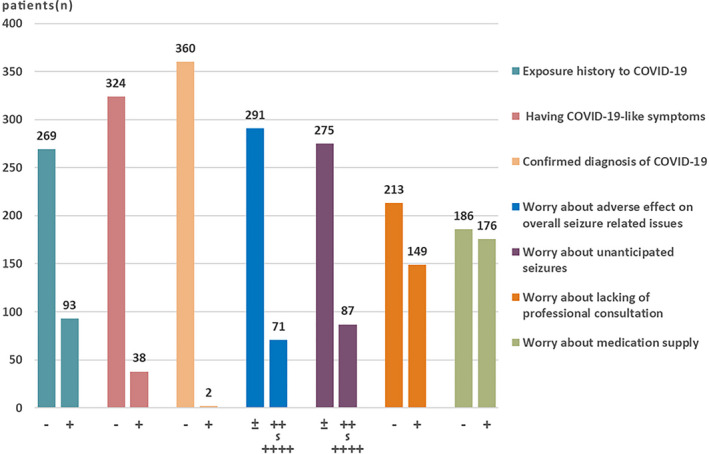
Reported direct and perceived threat of coronavirus disease 2019 (COVID‐19) by patients with epilepsy. −, no; +, yes. The number of + signs represents the severity of worry
3.4. Characteristics of patients with seizure exacerbation
As shown in Table 2, a total of 31 (8.56%) patients had seizure exacerbation during the outbreak. Age was significantly different between groups with and without seizure exacerbation (P = .010). Compared with the seizure nonexacerbation group, the following were also significantly different in the seizure exacerbation group: location of Wuhan (41.94% vs 22.66%, P = .026), exposure history to COVID‐19 (51.61% vs 23.26%, P = .001), worry about the adverse effect of the outbreak on overall seizure‐related issues (35.38% vs 18.13%, P = .031), high seizure frequency prior to the outbreak (51.61% vs 19.03%, P < .001), and uncontrolled epilepsy after AED treatment (90.32% vs 50.76%, P < .001). In addition, change of drug regimen during the outbreak was significantly different between the two groups (P = .005). Meanwhile, distribution of depression, anxiety, and insomnia among patients with or without increased number of seizures was 22.58% versus 12.08%, 19.35% versus 8.46%, and 25.81% versus 19.03%, respectively. We did not see significant differences in psychological and sleep responses between the groups with and without seizure exacerbation.
TABLE 2.
Epilepsy‐related characteristics of patients during the outbreak
| Characteristic | Total, N = 362, n (%) | With seizure exacerbation, n = 31, n (%) | Without seizure exacerbation, n = 331, n (%) | P |
|---|---|---|---|---|
| Age | ||||
| 10‐19 y | 112 (30.94%) | 3 (9.68%) | 109 (32.93%) | .010 |
| 20‐60 y | 244 (67.40%) | 27 (87.10%) | 217 (65.56%) | |
| ≥60 y | 6 (1.66%) | 1 (3.22%) | 5 (1.51%) | |
| Gender | ||||
| Male | 196 (54.14%) | 18 (58.06%) | 178 (53.78%) | .709 |
| Female | 166 (45.86%) | 13 (41.94%) | 153 (46.22%) | |
| Habitation | ||||
| Non‐Wuhan | 274 (75.69%) | 18 (58.06%) | 256 (77.34%) | .026 |
| Wuhan | 88 (24.31%) | 13 (41.94%) | 75 (22.66%) | |
| Level of education | ||||
| Below university | 233 (64.36%) | 21 (67.74%) | 212 (64.05%) | .702 |
| University and above | 129 (35.64%) | 10 (32.26%) | 119 (35.95%) | |
| Exercise habit | ||||
| No | 334 (92.27%) | 28 (90.32%) | 306 (92.45%) | .943 |
| Yes | 28 (7.73%) | 3 (9.68%) | 25 (7.55%) | |
| Employment status | ||||
| Unemployed | 41 (11.33%) | 5 (16.13%) | 36 (10.88%) | .558 |
| Employed, including students | 321 (88.67%) | 26 (83.87%) | 295 (89.12%) | |
| Raising children | ||||
| No | 235 (64.92%) | 17 (54.84%) | 218 (65.86%) | .240 |
| Yes | 127 (35.08%) | 14 (45.16%) | 113 (34.14%) | |
| Seizure type | ||||
| Focal onset | 290 (80.11%) | 27 (87.10%) | 263 (79.46%) | .592 |
| Generalized onset | 46 (12.71%) | 2 (6.45%) | 44 (13.29%) | |
| Unknown onset | 26 (7.18%) | 2 (6.45%) | 24 (7.25%) | |
| Seizure control after AED therapy | ||||
| Controlled | 166 (45.86%) | 3 (9.68%) | 163 (49.24%) | <.001 |
| Uncontrolled | 196 (54.14%) | 28 (90.32%) | 168 (50.76%) | |
| Seizure frequency before the outbreak | ||||
| ≤1 seizure/mo | 283 (78.18%) | 15 (48.39%) | 268 (80.97%) | <.001 |
| ≥2 seizures/mo | 79 (21.82%) | 16 (51.61%) | 63 (19.03%) | |
| AED therapy before the outbreak | ||||
| Withdrawal or monotherapy | 100 (27.62%) | 4 (12.90%) | 96 (29.00%) | .060 |
| Combination therapy | 262 (72.38%) | 27 (87.10%) | 235 (71.00%) | |
| AED therapy since the outbreak | ||||
| Withdrawal or monotherapy | 102 (28.18%) | 5 (16.13%) | 97 (29.31%) | .145 |
| Combination therapy | 260 (71.82%) | 26 (83.87%) | 234 (70.69%) | |
| Change of drug regimen during the outbreak | ||||
| No change | 330 (91.16%) | 23 (74.19%) | 307 (92.75%) | .005 |
| Increased | 4 (1.10%) | 1 (3.23%) | 3 (0.91%) | |
| Reduction/withdrawal/replacement/skipping | 28 (7.73%) | 7 (22.58%) | 21 (6.34%) | |
| Exposure history to COVID‐19 | ||||
| No | 269 (74.31%) | 15 (48.39%) | 254 (76.74%) | .001 |
| Yes | 93 (25.69%) | 16 (51.61%) | 77 (23.26%) | |
| Having COVID‐19–like symptoms | ||||
| No | 324 (89.50%) | 27 (87.10%) | 297 (89.73%) | .880 |
| Yes | 38 (10.50%) | 4 (12.90%) | 34 (10.27%) | |
| Worried about adverse effect on overall seizure‐related issues | ||||
| None to mild | 291 (80.39%) | 20 (64.52%) | 271 (81.87%) | .031 |
| Moderate to critical | 71 (19.61%) | 11 (35.38%) | 60 (18.13%) | |
| Worried about unanticipated seizures | ||||
| None to mild | 275 (75.97%) | 20 (64.52%) | 255 (77.04%) | .127 |
| Moderate to critical | 87 (24.03%) | 11 (35.48%) | 76 (22.96%) | |
| Worried about lack of professional consultation | ||||
| No | 213 (58.84%) | 18 (58.06%) | 195 (58.91%) | .711 |
| Yes | 149 (41.16%) | 13 (41.94%) | 136 (41.09%) | |
| Worried about medication supply | ||||
| No | 186 (51.38%) | 17 (54.84%) | 169 (51.06%) | >.99 |
| Yes | 176 (48.62%) | 14 (45.16%) | 162 (48.94%) | |
| GAD‐7 | ||||
| No anxiety, score < 8 | 328 (90.61%) | 25 (80.65%) | 303 (91.54%) | .096 |
| Anxiety, score ≥ 8 | 34 (9.39%) | 6 (19.35%) | 28 (8.46%) | |
| PHQ‐9 | ||||
| No depression, score < 10 | 315 (87.02%) | 24 (77.42%) | 291 (87.92%) | .167 |
| Depression, score ≥ 10 | 47 (12.98%) | 7 (22.58%) | 40 (12.08%) | |
| ISI | ||||
| No insomnia, score < 8 | 291 (80.39%) | 23 (74.19%) | 268 (80.97%) | .477 |
| Insomnia, score ≥ 8 | 71 (19.61%) | 8 (25.81%) | 63 (19.03%) | |
Abbreviations: AED, antiepileptic drug; COVID‐19, coronavirus disease 2019; GAD‐7, Generalized Anxiety Disorder–7 items; ISI, Insomnia Severity Index; PHQ‐9, Patient Health Questionnaire–9.
3.5. Risk factors associated with seizure exacerbation
Multivariate analysis results, summarized in Table 3, revealed five risk factors of PWE who suffered increased seizures: exposure history to COVID‐19 (odds ratio [OR] = 3.953, P = .001, 95% confidence interval [CI] = 1.713‐9.122), uncontrolled epilepsy after AED treatment (OR = 4.656, P = .020, 95% CI = 1.268‐17.092), two or more seizures per month before the outbreak (OR = 2.245, P = .005, 95% CI = 1.275‐3.952), change of AED regimen during outbreak (OR = 5.417, P = .002, 95% CI = 1.848‐15.886), and moderate‐to‐critical worries about the adverse effect of the outbreak on overall seizure‐related issues (OR = 2.539, P = .038, 95% CI = 1.053‐6.124).
TABLE 3.
Factors associated with epileptic frequency in multivariate logistic regression
| Characteristic | P | OR (95% CI) |
|---|---|---|
| Age | .055 | |
| Gender | .225 | |
| Exposure history to COVID‐19 | .001 | 3.953 (1.713‐9.122) |
| Having COVID‐19–like symptoms | .438 | |
| Seizure type | .410 | |
| Uncontrolled seizure after AED therapy | .020 | 4.656 (1.268‐17.092) |
| Seizure frequency before the outbreak ≥ 2 seizures/mo | .005 | 2.245 (1.275‐3.952) |
| Change of drug regimen during the outbreak | .003 | |
| No change | Ref | |
| Increased | .089 | 9.490 (0.712‐126.529) |
| Reduction/withdrawal/replacement/skipping | .002 | 5.417 (1.848‐15.886) |
| Worried about adverse effect on overall seizure‐related issues, moderate to critical | .038 | 2.539 (1.053‐6.124) |
Abbreviations: AED, antiepileptic drug; CI, confidence interval; COVID‐19, coronavirus disease 2019; OR, odds ratio; Ref, referent.
4. DISCUSSION
The epicenter Wuhan and its surrounding cities were the worst‐hit areas during the outbreak of COVID‐19. The dramatic rise in numbers of infected cases and deaths, the sudden city lockdowns, and the tremendous number of media reports together likely resulted in significant public fear and distress. This stress may have been augmented in those PWE. The outbreak allows us to objectively evaluate stress as an individual precipitant for seizures.
Our study found that a small proportion, 9% of all surveyed patients, experienced an increased number of seizures during the outbreak of COVID‐19. Interestingly, 7% of patients without a history of exposure to COVID‐19 underwent increased seizures, whereas a greater proportion of patients with a history of exposure to COVID‐19, 27%, experienced seizure exacerbation. Although the prevalence of increased seizures during a stressful event remarkably varies between 3% and 58% in previous studies, 6 , 7 , 8 , 9 our findings are very similar to two previous reports. Among 100 adult PWE exposed to missiles attacks in Israel during the 1991 Gulf War, 8% patients experienced increased seizure frequency. 8 During the terrorist attack of September 11, 2001, 12% of PWE exposed indirectly to 9/11 experienced seizure exacerbation, but 50% of patients directly affected by the event had seizure exacerbation. 6 This consistence suggested that stress can exacerbate seizures not in a majority but in a minority of PWE. Compared with other studies, the low prevalence in our study might be explained by (1) the differences in methodology, length of follow‐up, and sample size 4 ; (2) the magnitude of stress, which may be important in determining whether stress is associated with seizure exacerbation 6 ; and (3) the perception of stress varying across individuals, the same stimulus being relatively innocuous for some and a potential threat for others. 22 These individual variables could affect the power of influence of event‐related stress. Nevertheless, more studies, with larger populations of PWE, on exploring the effect of stress‐induced seizure worsening are needed in future. Our multiple logistic regression analysis then confirmed exacerbated seizure outcome was associated with exposure history to COVID‐19. Exposure history encompasses not only the likelihood of getting infected by a novel virus but also the possibility of facing death. Therefore, these findings are evidence to support the association of objective stress in triggering seizures.
In our study, we took advantage of the inherent characteristics of the outbreak of COVID‐19 and identified emotional stress as a precipitant for seizures. Moderate‐to‐critical worry thoughts about the adverse impact of COVID‐19 on overall seizure‐related issues were associated with seizure exacerbation. To define emotional stress is difficult, especially because well‐known precipitants of seizures such as sleep deprivation and fatigue usually coexist with stress. 23 , 24 For example, of the 400 patients participating in a survey‐based study, 30% of patients reported association of emotional stress with seizure exacerbation, but this variable was highly correlated with sleep deprivation and fatigue. 23 In another study of 89 patients, 64% of patients reported the belief that emotional stress increased the frequency of their seizures, independent of sleep deprivation 2 ; however, the evaluation relied on experiential judgment reported by patients. As in our study, the prevalence of anxiety, depression, and insomnia were consistently lower than those from the population‐based epidemiology studies on PWE. 25 The cause of discrepancy is complicated; it can be explained as (1) the sample selection bias attributed to either patients who declined to take the survey or the intrinsic characteristics of the patient population of the online‐based social media database, because not many elderly patients are in the group; (2) the unique lockdowns of cities extremely limiting the real contact‐based social activities of PWE, thus reducing the disease stigma, which could ameliorate psychological comorbidities; or (3) patients staying close to intimate family members and receiving continuous support during the epidemic, or other unknown reasons. Taken together, these results support that worry thoughts were independent of depression, anxiety, and insomnia, and served as a relatively isolated, epilepsy‐specific emotional stress precipitant promoting seizures.
Besides two stress‐related precipitants, we also found three epilepsy‐specific risk factors associated with increased seizures: failed achievement of complete seizure control after AED treatment, two or more seizures per month before the outbreak, and inappropriate change of AED regimen (AED reduction, withdrawal, replacement, skipping altogether). Patients with uncontrolled epilepsy or poorly managed treatment generally have worse long‐term outcome and a high rate of recurrence of epilepsy and are prone to stress‐related exacerbation of epilepsy. 26 , 27 , 28 Appropriate medication management to achieve seizure freedom is important to reduce the risk of seizure exacerbation in a stressful event. This again stresses the significance of optimizing AED therapy to reduce seizure attacks and achieve seizure freedom in epilepsy treatment.
This study is a web‐based survey study; the overall response rate was 63.51%. Response rates for PWE participation in survey studies varied. The postal questionnaire‐based survey by Reuber et al achieved 32%. 29 The mail survey by Mills et al accomplished 66%. 30 The telephone or email survey by Patel et al reached 47%. 31 In the present context specifically, the response rate of our study would be sufficient to enable generalizing the results to the target population. However, our study has inherent limitations. First, this study used a cross‐sectional study design, which only allows for conclusions regarding correlation, not causation. Second, participants were from an online social media group, which allows the possibility of sample bias. These findings may not represent the population as a whole. In particular, elderly people with epilepsy were underrepresented in our study. However, it is possible that elderly patients are more likely to experience stress in the context of COVID‐19 outbreak, as elderly people tend to have higher morbidity and mortality when infected, which may cause worse seizure outcome. Third, natural fluctuations of individual seizure rates from month to month may affect the increase in seizure count during the month of the outbreak of COVID‐19. However, changes in individual monthly seizure rates are widely used in AED trials. 32 Comparing seizures 1 month after the event to seizure frequency before the event was also used in prior public disaster–related seizure exacerbation studies. 6 , 7 , 8 , 9 Fourth, the data for epilepsy‐related characteristics such as detailed symptoms of seizures rely on accurate recording by patients. It is probable that some seizures were not reported due to unawareness by patients. Lastly, some stress assessment scales that are used for evaluating stress in the general population were not used in our study. However, questionnaire‐based seizure and stress data collection has been widely used and demonstrated reliability in epilepsy. 33 , 34 Our study highlights the significant relationship between a stressful event and epileptic seizures, which could be used in counseling patients to avoid certain stressful situations or even to have additional medications as a contingency for patients who may unexpectedly encounter a stressful situation.
Overall, our study found that a small proportion of PWE experienced seizure exacerbation during the outbreak of COVID‐19. Stress is an independent precipitant for triggering seizures in these patients.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
This research was supported by funds from the National Key R&D Program of China (grant 2017YFC1310000) and the Fundamental Research Funds for the Central Universities (grant 2019kfyXKJC075). We are grateful for help with data collection by Xiaoyan Song and Ping Li, Huazhong University of Science and Technology.
Huang S, Wu C, Jia Y, et al. COVID‐19 outbreak: The impact of stress on seizures in patients with epilepsy. Epilepsia. 2020;61:1884–1893. 10.1111/epi.16635
Contributor Information
Furong Wang, Email: wangfurong.china@163.com.
Suiqiang Zhu, Email: zhusuiqiang@163.com.
REFERENCES
- 1. Temkin NR, Davis GR. Stress as a risk factor for seizures among adults with epilepsy. Epilepsia. 1984;25:450–6. [DOI] [PubMed] [Google Scholar]
- 2. Haut SR, Vouyiouklis M, Shinnar S. Stress and epilepsy: a patient perception survey. Epilepsy Behav. 2003;4:511–4. [DOI] [PubMed] [Google Scholar]
- 3. Webster A, Mawer GE. Seizure frequency and major life events in epilepsy. Epilepsia. 1989;30:162–7. [DOI] [PubMed] [Google Scholar]
- 4. Becker C, Bouvier E, Ghestem A, et al. Predicting and treating stress‐induced vulnerability to epilepsy and depression. Ann Neurol. 2015;78:128–36. [DOI] [PubMed] [Google Scholar]
- 5. McKee HR, Privitera MD. Stress as a seizure precipitant: identification, associated factors, and treatment options. Seizure. 2017;44:21–6. [DOI] [PubMed] [Google Scholar]
- 6. Klein P, van Passel L. Effect of stress related to the 9/11/2001 terror attack on seizures in PWE. Neurology. 2005;64:1815–6. [DOI] [PubMed] [Google Scholar]
- 7. Bosnjak J, Vukovic‐Bobic M, Mejaski‐Bosnjak V. Effect of war on the occurrence of epileptic seizures in children. Epilepsy Behav. 2002;3:502–9. [DOI] [PubMed] [Google Scholar]
- 8. Neufeld MY, Sadeh M, Cohn DF, Korczyn AD. Stress and epilepsy: the Gulf War experience. Seizure. 1994;3:135–9. [DOI] [PubMed] [Google Scholar]
- 9. Swinkels WA, Kasteleijn‐Nolst Engelsman M, Trenité DG, Baal MG, de Haan GJ, Oosting J. Influence of an evacuation in February 1995 in the Netherlands on the seizure frequency in PWE: a controlled study. Epilepsia. 1998;39:1203–7. [DOI] [PubMed] [Google Scholar]
- 10. Jernigan DB. Update public health response to the coronavirus disease 2019 outbreak—United States, February 24, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Helen Branswell AJ.WHO declares the coronavirus outbreak a pandemic. [cited 2020 Mar 11]. Available from https://www.statnews.com/2020/03/11/who‐declares‐the‐coronavirus‐outbreak‐a‐pandemic/
- 12. Xiang Y‐T, Yang Y, Li W, et al. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7:228–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vacik HW, Nagy MC, Jessee PO. Children's understanding of illness: students' assessments. J Pediatr Nurs. 2001;16:429–37. [DOI] [PubMed] [Google Scholar]
- 14. Sacks D. Age limits and adolescents. Paediatr Child Health. 2003;8:577–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Proposed working definition of an older person in Africa for the MDS Project. Available at: https://www.who.int/healthinfo/survey/ageingdefnolder/en/ .
- 16. Wicker P, Frick B. Intensity of physical activity and subjective well‐being: an empirical analysis of the WHO recommendations. J Public Health (Oxf). 2017;39:e19 –26. [DOI] [PubMed] [Google Scholar]
- 17. Hazzard A, Hutchinson SJ, Krawiecki N. Factors related to adherence to medication regimens in pediatric seizure patients. J Pediatr Psychol. 1990;15:543–55. [DOI] [PubMed] [Google Scholar]
- 18. Kroenke K, Spitzer RL, Williams JB. The PHQ‐ 9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kroenke K, Spitzer RL, Williams JBW, Monahan PO, Löwe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146:317–25. [DOI] [PubMed] [Google Scholar]
- 20. Wong ML, Lau KNT, Espie CA, Luik AI, Kyle SD, Lau EYY. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep Med. 2017;33:76–81. [DOI] [PubMed] [Google Scholar]
- 21. Kroenke K, Spitzer RL, Williams JBW, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345–59. [DOI] [PubMed] [Google Scholar]
- 22. Novais A, Monteiro S, Roque S, Correia‐Neves M, Sousa N. How age, sex and genotype shape the stress response. Neurobiol Stress. 2017;6:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frucht MM, Quigg M, Schwaner C, Fountain NB. Distribution of seizure precipitants among epilepsy syndromes. Epilepsia. 2000;41:1534–9. [DOI] [PubMed] [Google Scholar]
- 24. Malow BA. Sleep deprivation and epilepsy. Epilepsy Curr. 2004;4:193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(Suppl 1):i45–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sillanpää M, Schmidt D. Early seizure frequency and aetiology predict long‐term medical outcome in childhood‐onset epilepsy. Brain. 2009;132:989–98. [DOI] [PubMed] [Google Scholar]
- 27. Lamberink HJ, Otte WM, Geerts AT, et al. Individualised prediction model of seizure recurrence and long‐term outcomes after withdrawal of antiepileptic drugs in seizure‐free patients: a systematic review and individual participant data meta‐analysis. Lancet Neurol. 2017;16:523–31. [DOI] [PubMed] [Google Scholar]
- 28. Moon H‐J, Seo J‐G, Park S‐P. Perceived stress and its predictors in people with epilepsy. Epilepsy Behav. 2016;62:47–52. [DOI] [PubMed] [Google Scholar]
- 29. Reuber M, Jamnadas‐Khoda J, Broadhurst M, et al. Psychogenic nonepileptic seizure manifestations reported by patients and witnesses. Epilepsia. 2011;52:2028–35. [DOI] [PubMed] [Google Scholar]
- 30. Mills N, Bachmann M, Harvey I, McGowan M, Hine I. Patients' experience of epilepsy and health care. Fam Pract. 1997;14:117–23. [DOI] [PubMed] [Google Scholar]
- 31. Patel A, Pyzik PL, Turner Z, Rubenstein JE, Kossoff EH. Long‐term outcomes of children treated with the ketogenic diet in the past. Epilepsia. 2010;51:1277–82. [DOI] [PubMed] [Google Scholar]
- 32. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011–20. [DOI] [PubMed] [Google Scholar]
- 33. Pham T, Sauro KM, Patten SB, et al. The prevalence of anxiety and associated factors in persons with epilepsy. Epilepsia. 2017;58:e107 –10. [DOI] [PubMed] [Google Scholar]
- 34. Salpekar J. Seizures, nonepileptic events, trauma, anxiety, or all of the above. Epilepsy Curr. 2019;19:29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]


