Abstract
Breast cancer is a highly heterogeneous disease at the molecular level and >90% of mortalities are due to metastasis and its associated complications. The present study determined the impact of molecular subtypes on metastatic behavior and overall survival (OS) of patients with metastatic breast cancer. The influence of molecular subtypes on the sites and number of metastases in 166 patients with metastatic breast cancer from a single center were assessed; and the influence of molecular subtypes on the sites and number of metastases and OS in 15,322 metastatic cases among 329,770 patients with primary breast cancer from the Surveillance, Epidemiology and End Results database were assessed. Analysis of both datasets revealed that different molecular subtypes exhibited differences in the prevalence of different metastatic sites and number of metastases. A larger proportion of bone metastasis was observed in the hormone receptor (HR)+/human epidermal growth factor receptor 2 (HER2)+ subtype than in other subtypes, more lung metastasis was observed in the HR-/HER2+ subtype and more liver metastasis occurred in the HR+/HER2+ and HR-/HER2+ subtypes. Single-site metastasis was more common for the HR+/HER2− subtype than in other subtypes, while 2–3 sites of metastases were more common for the HR+/HER2+ subtype and ≥4 sites of metastases were more frequent in the HR-/HER2+ and HR-/HER2- subtypes. The mean OS of patients with primary breast cancer in the HR+/HER2− subtype group was the longest (78.5 months), while the HR-/HER2- group had the shortest mean OS (69.1 months). The mean OS of the metastatic HR+/HER2+ group was the longest (46.0 months), while the mean OS of the metastatic HR-/HER2- group was the shortest (18.5 months). In conclusion, the results of the present study suggested that different molecular subtypes of breast cancer have different metastatic behavior, as well as mean OS.
Keywords: breast cancer, molecular subtypes, metastatic sites, number of metastatic sites, overall survival
Introduction
Breast cancer is the most common type of malignancy in females and >90% of mortalities are due to metastasis-associated complications (1). An estimated 5–10% of patients have metastasis at the time-point of diagnosis and 20–40% of those who did not have any metastasis at the time-point of diagnosis eventually experience recurrence after treatment and metastasis; once recurrence and metastasis occur, the overall prognosis is generally poor (2,3).
The status of hormone receptors (HRs) is closely linked to the treatment and prognosis of breast cancer. Certain studies have explored the association between molecular subtypes and sentinel lymph node metastasis and risk factors of metastasis (4–14). However, breast cancer is a highly heterogeneous disease at the molecular level and the metastatic behavior of different subtypes of breast cancer, including metastatic sites and the number of metastases, as well as the prognosis difference between primary and metastatic breast cancer patients of different subtypes, have remained to be fully defined.
In the present study, the possible association between molecular subtypes and metastatic behavior was retrospectively explored in a single-center sample of 166 patients with metastatic breast cancer from Hunan Provincial People's Hospital (Changsha, China) between January 2012 and December 2018, and the results were further supported by analysis of a large dataset from the Surveillance, Epidemiology, and End Results (SEER) database with 15,322 cases of metastatic breast cancer among 329,770 patients with primary breast cancer. Furthermore, differences in prognosis regarding overall survival (OS) of patients with primary and with metastatic breast cancer with different molecular subtypes were determined. The results of the present study may be valuable to inform improved monitoring for metastasis sites during follow-up. An understanding of the patterns of metastatic spread will allow the clinician to make more efficient surveillance decisions and select appropriate examinations and therapies.
Materials and methods
Patients
The present study was a retrospective analysis. First, 166 patients with metastatic breast cancer treated at Hunan Provincial People's Hospital (Changsha, China) between January 2012 and December 2018 were collected. All patients underwent primary tumor resection and the tumors were pathologically confirmed as breast cancer. The age at diagnosis was 25–79 years. Each patient included had a complete medical record and preserved pathological specimens. The study protocol was approved by the Ethics Committee of Hunan Provincial People's Hospital and The First Affiliated Hospital of Hunan Normal University (Changsha, China).
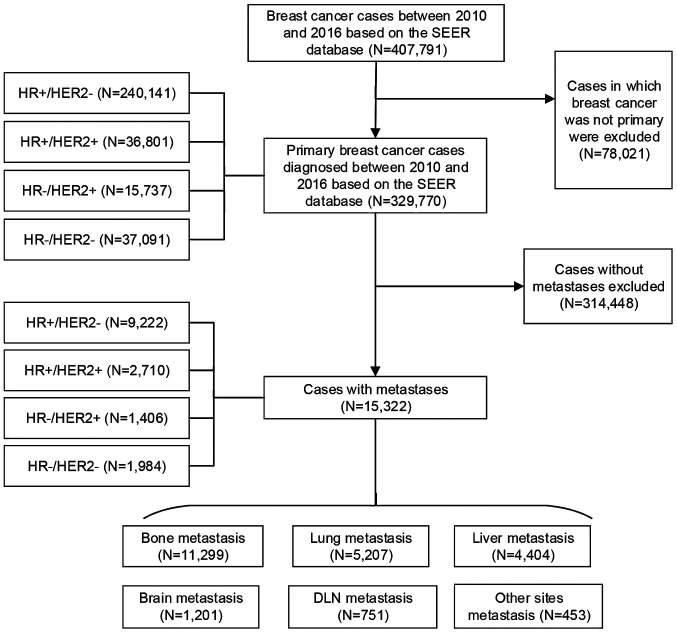
Furthermore, data were extracted from the SEER database (https://seer.cancer.gov/) on 407,791 patients who were diagnosed with malignant breast cancer between 2010 and 2016 and had known HR and human epidermal growth factor receptor 2 (HER2) statuses. After excluding non-primary breast cancer cases, there were 329,770 primary breast cancer patients, of which 15,322 cases had metastatic breast cancer. The number of HR+/HER2− metastatic cases was 9,222, compared with 2,710 HR+/HER2+ metastatic cases, 1,406 HR-/HER2+ metastatic cases and 1,984 HR-/HER2- metastatic cases. Bone metastasis was present in 11,299 cases, as compared with 5,207 cases with lung metastasis, 4,404 with liver metastasis, 1,201 with brain metastasis and 751 with distant lymph node (DLN) metastasis, as well as 453 cases that involved other metastatic sites (Fig. 1).
Figure 1.
Flowchart of patient inclusion in the present cohort study based on the SEER database. SEER, Surveillance, Epidemiology and End Results; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; DLN, distant lymph node.
Characterization of specimens
Tumor specimen samples were processed by routine detection methods. First, tumor samples were fixed, after which they were consecutively sliced to a thickness of 4 µm and then stained with H&E, followed by immunohistochemistry (IHC) staining. Fluorescence in situ hybridization (FISH) was performed when necessary. After staining, the HR and HER2 statuses of all specimens were assessed by two senior pathologists based on the same criteria as those used by the hospital.
For evaluation, the following specific standards were used: A total of 5 high-power fields were randomly selected to count >500 cells and the number of positive cells and staining intensity were used for scoring. Samples were considered HR+ if >1% of cells exhibited IHC staining (15). HER2 expression was only present on the cell membrane or in the cell plasma, and samples scored 3+ (overexpression) via IHC were classified as HER2+. For those samples scoring as HER2 1–2+, 2+ and 2–3+ on IHC, FISH was further performed by hybridization of fluorescent DNA probes to the HER2 gene and chromosome 17. The result was considered HER2+ status when the HER2/centromeric probe for chromosome 17 (Cep17) ratio was ≥2 on average for 60 cells and detection of gene amplification was interpreted as HER2+ status (16).
Study design
The patients were divided into four groups according to molecular subtypes: HR+/HER2−, HR+/HER2+, HR-/HER2+ and HR-/HER2-. First, 166 patients with metastatic breast cancer with HR and HER2 expression data were assessed. The associations between molecular subtypes and the sites of and the number of metastases were examined.
Furthermore, 15,322 metastatic cases among 329,770 patients with primary breast cancer from the SEER database were analyzed. The possible impact of molecular subtypes on the sites and number of metastases was studied based on data from the SEER database. In addition, the possible impact of molecular subtypes on the OS of patients with primary and with metastatic breast cancer was evaluated. OS was defined as the time from the date of diagnosis to death from any cause.
Statistical analysis
All data were analyzed by using SPSS version 22.0 (IBM Corp.), where ratio variables were compared using the χ2 test or Fisher's exact test as appropriate. Survival rates were calculated with the Kaplan-Meier method and survival curves were drawn. The log-rank test was performed to perform univariate analysis. The statistical analyses were two-sided and P<0.05 was considered to indicate statistical significance.
Results
Major characteristics of patients with different molecular subtypes of breast cancer
A total of 166 patients with metastatic breast cancer from the Hunan Provincial People's Hospital (Changsha, China) and 15,322 metastatic breast cancer cases from the SEER database were identified. The major baseline features of the breast cancer molecular subtypes are presented in Tables I and II. In 166 patients with metastases, the HR+/HER2−, HR+/HER2+, HR-/HER2+ and HR-/HER2- cases accounted for 55.4, 15.7, 12.6 and 16.3%, respectively; all of the patients were female, with a mean age of 51.4 years (range, 25–79 years). For the 15,322 metastatic breast cancer cases from the SEER database, the mean age was 60.7 years (range, 19–103 years); the HR+/HER2−, HR+/HER2+, HR-/HER2+ and HR-/HER2- cases accounted for 60.2, 17.7, 9.2 and 12.9%, respectively. In both datasets, HR+/HER2− accounted for the largest proportion of molecular subtypes, while HR-/HER2+ had the smallest proportion. There was no statistically significant difference in the proportion of molecular subtypes between the two datasets (P=0.196; Table III).
Table I.
Major characteristics of the patients with metastatic breast cancer from the present cohort study (n=166).
| Main characteristics | HR+/HER2− (n=92) | HR+/HER2+ (n=26) | HR-/HER2+ (n=21) | HR-/HER2- (n=27) |
|---|---|---|---|---|
| Age (years) | ||||
| <40 | 19 (20.6) | 5 (19.2) | 2 (9.5) | 1 (3.7) |
| 40-49 | 26 (28.3) | 8 (30.8) | 3 (14.3) | 9 (33.3) |
| 50-59 | 26 (28.3) | 6 (23.1) | 9 (42.9) | 11 (40.8) |
| ≥60 | 21 (22.8) | 7 (26.9) | 7 (33.3) | 6 (22.2) |
| Menopausal status | ||||
| Premenopause | 41 (44.6) | 12 (46.2) | 5 (23.8) | 9 (33.3) |
| Postmenopause | 51 (55.4) | 14 (53.8) | 16 (76.2) | 18 (66.7) |
| Tumor stage | ||||
| T1 | 9 (9.8) | 5 (19.2) | 4 (19.1) | 8 (29.6) |
| T2 | 55 (59.8) | 11 (42.3) | 8 (38.1) | 15 (55.6) |
| T3 | 18 (19.6) | 9 (34.6) | 7 (33.3) | 3 (11.1) |
| T4 | 10 (10.8) | 1 (3.9) | 2 (9.5) | 1 (3.7) |
| TNM stage | ||||
| I | 7 (7.6) | 4 (15.4) | 0 (0) | 3 (11.1) |
| II | 34 (37.0) | 7 (26.9) | 6 (28.6) | 12 (44.4) |
| III | 51 (55.4) | 15 (57.7) | 15 (71.4) | 12 (44.4) |
| Lymph node status | ||||
| Positive | 65 (70.7) | 19 (73.1) | 19 (90.5) | 16 (59.3) |
| Negative | 27 (29.3) | 7 (26.9) | 2 (9.5) | 11 (40.7) |
| Histopathological type | ||||
| Invasive ductal carcinoma | 75 (81.5) | 23 (88.5) | 18 (85.7) | 25 (92.6) |
| Invasive lobular carcinoma | 4 (4.3) | 0 (0) | 0 (0) | 1 (3.7) |
| Ductal carcinoma in situ | 2 (2.2) | 2 (7.7) | 1 (4.8) | 0 (0) |
| Other | 11 (12.0) | 1 (3.8) | 2 (9.5) | 1 (3.7) |
Values are expressed as n (%). HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Table II.
Major characteristics of patients with metastatic breast cancer from the Surveillance, Epidemiology and End Results dataset (n=15,322).
| Main characteristics | HR+/HER2− (n=9,222) | HR+/HER2+ (n=2,710) | HR-/HER2+ (n=1,406) | HR-/HER2- (n=1,984) |
|---|---|---|---|---|
| Age (years) | ||||
| <40 | 553 (6.0) | 298 (11.0) | 169 (12.0) | 160 (8.1) |
| 40-49 | 1,193 (12.9) | 430 (15.9) | 227 (16.2) | 289 (14.6) |
| 50-59 | 2,178 (23.6) | 765 (28.2) | 411 (29.2) | 512 (25.8) |
| ≥60 | 5298 (57.5) | 1,217 (44.9) | 599 (42.6) | 1,023 (51.5) |
| Sex | ||||
| Female | 9,107 (98.8) | 2,673 (98.6) | 1,403 (99.8) | 1969 (99.2) |
| Male | 115 (1.2) | 37 (1.4) | 3 (0.2) | 15 (0.8) |
| Tumor stage | ||||
| T0 | 110 (1.2) | 23 (0.8) | 15 (1.1) | 26 (1.3) |
| T1 | 819 (8.9) | 224 (8.3) | 99 (7.0) | 133 (6.7) |
| T2 | 2,287 (24.8) | 668 (24.6) | 278 (19.8) | 416 (21.0) |
| T3 | 1,188 (12.9) | 345 (12.7) | 190 (13.5) | 301 (15.2) |
| T4 | 2,244 (24.3) | 713 (26.3) | 449 (31.9) | 617 (31.1) |
| Unknown | 2,574 (27.9) | 737 (27.3) | 375 (26.7) | 491 (24.7) |
| Lymph node status | ||||
| Positive | 8,919 (96.7) | 2,563 (94.6) | 1,296 (92.2) | 1,819 (91.7) |
| Negative | 303 (3.3) | 147 (5.4) | 110 (7.8) | 165 (8.3) |
| Grade | ||||
| I | 894 (9.7) | 62 (2.3) | 7 (0.5) | 23 (1.2) |
| II | 3,855 (41.8) | 891 (32.9) | 310 (22.0) | 296 (14.9) |
| III | 2,604 (28.2) | 1,326 (48.9) | 839 (59.7) | 1,333 (67.2) |
| IV | 34 (0.4) | 16 (0.6) | 11 (0.8) | 25 (1.3) |
| Unknown | 1,835 (19.9) | 415 (15.3) | 239 (17.0) | 307 (15.4) |
| Radiation therapy | ||||
| Prior to surgery | 146 (1.6) | 37 (1.4) | 20 (1.4) | 22 (1.1) |
| After surgery | 1,343 (14.6) | 409 (15.1) | 209 (14.9) | 315 (15.9) |
| Prior to and after surgery | 39 (0.4) | 8 (0.3) | 7 (0.5) | 7 (0.4) |
| None | 7,678 (83.3) | 2,250 (83.0) | 1,166 (82.9) | 1,636 (82.4) |
| Other | 16 (0.1) | 6 (0.2) | 4 (0.3) | 4 (0.2) |
| Chemotherapy | ||||
| No | 5,058 (54.8) | 748 (27.6) | 288 (20.5) | 553 (27.9) |
| Yes | 4,164 (45.2) | 1,962 (72.4) | 1,118 (79.5) | 1,431 (72.1) |
Values are expressed as n (%). HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Table III.
Similarities and differences of molecular subtypes between the two datasets.
| Molecular subtype | Single-centerdataset (n=166) | Surveillance, Epidemiology and End Results dataset (n=15,322) | χ2 | P-value |
|---|---|---|---|---|
| HR+/HER2− | 92 (55.4) | 9,222 (60.2) | 4.605 | 0.196 |
| HR+/HER2+ | 26 (15.7) | 2,710 (17.7) | ||
| HR-/HER2+ | 21 (12.6) | 1,406 (9.2) | ||
| HR-/HER2− | 27 (16.3) | 1,984 (12.9) |
Values are expressed as n (%). HR, hormone receptor; HER2, human epidermal growth factor receptor 2. P-value pertains to comparison among all groups.
Molecular subtypes and distribution of metastatic sites in the two datasets
Among the 166 cases of the present single-center study, the molecular subtypes were significantly associated with the prevalence of DLN metastasis (P=0.010), but not significantly associated with the prevalence of any other sites of metastasis. Bone metastasis was more common in the HR+/HER2+ and HR-/HER2+ subtypes compared to the other subtypes and their rates of occurrence were 50.0 and 42.9%, respectively. Lung metastasis was more common in the HR+/HER2− and HR-/HER2+ subtypes, and their rates of occurrence were 23.9 and 23.8% respectively; furthermore, pleural metastasis was more common in the HR+/HER2− and HR-/HER2-subtypes, and their rates of occurrence were 19.6 and 18.5%, respectively. Liver metastasis was more common in the HR-/HER2+ and HR+/HER2+ subtypes and their rates of occurrence were 33.3 and 30.8%, respectively. Brain metastasis was more common in the HR+/HER2+ and HR+/HER2− subtypes and their rates of occurrence were 11.5 and 5.4% respectively; DLN metastasis was also more common in the HR-/HER2- and HR-/HER2+ subtypes and their rates of occurrence were 44.4 and 23.8%, respectively (Table IV).
Table IV.
Molecular subtypes and metastatic sites in the patients with metastatic breast cancer from the present cohort study (n=166).
| Metastatic site | HR+/HER2− (n=92) | HR+/HER2+ (n=26) | HR-/HER2+ (n=21) | HR-/HER2- (n=27) | P-value |
|---|---|---|---|---|---|
| Bone | 0.165 | ||||
| Metastasis | 31 (33.7) | 13 (50.0) | 9 (42.9) | 6 (22.2) | |
| No metastasis | 61 (66.3) | 13 (50.0) | 12 (57.1) | 21 (77.8) | |
| Lung | 0.779 | ||||
| Metastasis | 22 (23.9) | 4 (15.4) | 5 (23.8) | 5 (18.5) | |
| No metastasis | 70 (76.1) | 22 (84.6) | 16 (76.2) | 22 (81.5) | |
| Pleural | 0.330 | ||||
| Metastasis | 15 (19.6) | 1 (3.8) | 2 (9.5) | 5 (18.5) | |
| No metastasis | 77 (80.4) | 25 (96.2) | 19 (90.5) | 22 (81.5) | |
| Liver | 0.438 | ||||
| Metastasis | 18 (19.6) | 8 (30.8) | 7 (33.3) | 6 (22.2) | |
| No metastasis | 74 (80.4) | 18 (69.2) | 14 (66.7) | 21 (77.8) | |
| Brain | 0.425 | ||||
| Metastasis | 5 (5.4) | 3 (11.5) | 0 (0.0) | 1 (3.7) | |
| No metastasis | 87 (94.6) | 23 (88.5) | 21 (100.0) | 26 (96.3) | |
| DLN | 0.010 | ||||
| Metastasis | 18 (19.6) | 2 (7.7) | 5 (23.8) | 12 (44.4) | |
| No metastasis | 74 (80.4) | 24 (92.3) | 16 (76.2) | 15 (55.6) |
Values are expressed as n (%). Pleural refers to the pleural space or sac. HR, hormone receptor; HER2, human epidermal growth factor receptor 2; DLN, distant lymph node. P-value pertains to comparison among all groups.
Among the 15,322 metastatic cases from the SEER database, the metastasis sites were significantly different among the different molecular subtypes (bone, lung, liver, brain and DLN; P<0.001). Bone metastasis was more common in the HR+/HER2− and HR+/HER2+ than in the other subgroups, with rates of occurrence of 82.0 and 71.3%, respectively. Lung metastasis was more common in the HR-/HER2+ and HR-/HER2- subtypes and the rates of occurrence were 40.8 and 47.7%, respectively. Liver metastasis was more common in the HR-/HER2+ and HR+/HER2+ subtypes, with rates of occurrence of 51.6 and 39.2%, respectively; brain metastasis was more common in the HR-/HER2+ and HR-/HER2- subtypes and the rates of occurrence were 12.7 and 12.9%, respectively. DLN metastasis was more common in the HR-/HER2- and HR-/HER2+ subtypes, with rates of occurrence of 6.4 and 6.9%, respectively (Table V).
Table V.
Molecular subtypes and metastatic sites in patients with metastatic breast cancer from the Surveillance, Epidemiology and End Results dataset (n=15,322).
| Metastatic sites | HR+/HER2− (n=9,222) | HR+/HER2+ (n=2,710) | HR-/HER2+ (n=1,406) | HR-/HER2- (n=1,984) | P-value |
|---|---|---|---|---|---|
| Bone | <0.001 | ||||
| Metastasis | 7,563 (82.0) | 1,931 (71.3) | 762 (54.2) | 1,043 (52.6) | |
| No metastasis | 1,659 (18.0) | 779 (28.7) | 644 (45.8) | 941 (47.4) | |
| Lung | <0.001 | ||||
| Metastasis | 2,787 (30.2) | 901 (33.2) | 573 (40.8) | 946 (47.7) | |
| No metastasis | 6,435 (69.8) | 1,809 (66.8) | 833 (59.2) | 1,038 (52.3) | |
| Liver | <0.001 | ||||
| Metastasis | 1,965 (21.3) | 1,062 (39.2) | 726 (51.6) | 651 (32.8) | |
| No metastasis | 7,257 (78.7) | 1,648 (60.8) | 680 (48.4) | 1,333 (67.2) | |
| Brain | <0.001 | ||||
| Metastasis | 542 (5.9) | 226 (8.3) | 178 (12.7) | 255 (12.9) | |
| No metastasis | 8,680 (94.1) | 2,484 (91.7) | 1,228 (87.3) | 1,729 (87.1) | |
| DLN | <0.001 | ||||
| Metastasis | 363 (3.9) | 165 (6.1) | 97 (6.9) | 126 (6.4) | |
| No metastasis | 8,859 (96.1) | 2,545 (93.9) | 1,309 (93.1) | 1,858 (93.6) |
Values are expressed as n (%). HR, hormone receptor; HER2, human epidermal growth factor receptor 2; DLN, distant lymph node. P-value pertains to comparison among all groups.
In summary, bone metastasis was more likely to occur in the HR+/HER2+ subtype patients according to the single-center data as well as the dataset from the SEER database. Lung metastasis was more likely to occur in patients of the HR-/HER2+ subtype in both datasets. Liver metastasis was more likely to occur in the HR-/HER2+ and HR+/HER2+ patients in both datasets. DLN metastasis was more likely to occur in the HR-/HER2- and HR-/HER2+ patients in both datasets.
Molecular subtypes and the number of metastatic sites in both datasets
In the 166 cases from the single-center cohort, molecular subtypes were not significantly associated with the prevalence of the number of metastatic sites (P=0.221). A single site of metastasis was more frequent in the HR+/HER2− and HR-/HER2+ patients than in the other subgroups, with frequencies of 59.8 and 57.1%, respectively. Furthermore, two sites of metastasis were more common in the HR-/HER2- and HR+/HER2+ patients and the rates of occurrence were 37.0 and 30.8%, respectively. In addition, three sites of metastasis were more frequent in HR+/HER2+ and HR+/HER2−patients, with rates of occurrence of 19.2 and 10.9%, respectively. Finally, ≥4 sites of metastasis were more frequent in the HR-/HER2- and HR-/HER2+ subtypes and the rates of occurrence were 14.8 and 14.3%, respectively (Table VI).
Table VI.
Molecular subtypes and the number of metastatic sites in patients with metastatic breast cancer from the present cohort study (n=166).
| Number of metastatic sites | HR+/HER2− (n=92) | HR+/HER2+ (n=26) | HR-/HER2+ (n=21) | HR-/HER2- (n=27) | χ2 | P-value |
|---|---|---|---|---|---|---|
| 1 | 55 (59.8) | 12 (46.2) | 12 (57.1) | 13 (48.2) | 11.328 | 0.221 |
| 2 | 20 (21.7) | 8 (30.8) | 4 (19.1) | 10 (37.0) | ||
| 3 | 10 (10.9) | 5 (19.2) | 2 (9.5) | 0 (0) | ||
| ≥4 | 7 (7.6) | 1 (3.8) | 3 (14.3) | 4 (14.8) |
Values are expressed as n (%). HR, hormone receptor; HER2, human epidermal growth factor receptor 2. P-value pertains to comparison among all groups.
Among the 15,322 metastatic cases from the SEER database, the molecular subtypes were significantly associated with the prevalence of the number of metastatic sites (P<0.001). A single site of metastasis was more common in the HR+/HER2− and HR-/HER2- patients than in the other subgroups, with rates of occurrence of 65.5 and 60.5%, respectively. Furthermore, two sites of metastasis were more frequent in the HR+/HER2+ and HR-/HER2+ patients and the rates of occurrence were 28.6 and 28.4%, respectively. In addition, three sites of metastasis were more common in the HR-/HER2+ and HR+/HER2+ subtypes, with rates of occurrence of 13.0 and 11.5%, respectively. Finally, ≥4 sites of metastasis were more common for the HR-/HER2+ and HR-/HER2-subtypes (4.5 and 3.5%, respectively; Table VII).
Table VII.
Molecular subtypes and the number of metastatic sites in patients with metastatic breast cancer from the Surveillance, Epidemiology and End Results dataset (n=15,322).
| Number of metastatic sites | HR+/HER2− (n=9,222) | HR+/HER2+ (n=2,710) | HR-/HER2+ (n=1,406) | HR-/HER2- (n=1,984) | χ2 | P-value |
|---|---|---|---|---|---|---|
| 1 | 6,042 (65.5) | 1,546 (57) | 761 (54.1) | 1,200 (60.5) | 160.329 | <0.001 |
| 2 | 2,289 (24.8) | 774 (28.6) | 399 (28.4) | 541 (27.3) | ||
| 3 | 722 (7.8) | 312 (11.5) | 183 (13.0) | 173 (8.7) | ||
| ≥4 | 169 (1.8) | 78 (2.9) | 63 (4.5) | 70 (3.5) |
Values are expressed as n (%). HR, hormone receptor; HER2, human epidermal growth factor receptor 2. P-value pertains to comparison among all groups.
The results of both datasets all suggested that the number of metastatic sites was diverse across the molecular subtypes. A single site of metastasis was more likely in HR+/HER2−patients, while 2–3 sites of metastases were more likely in HR+/HER2+ patients. Furthermore, ≥4 sites of metastasis were more likely in patients with the HR-/HER2+ and HR-/HER2- subtype.
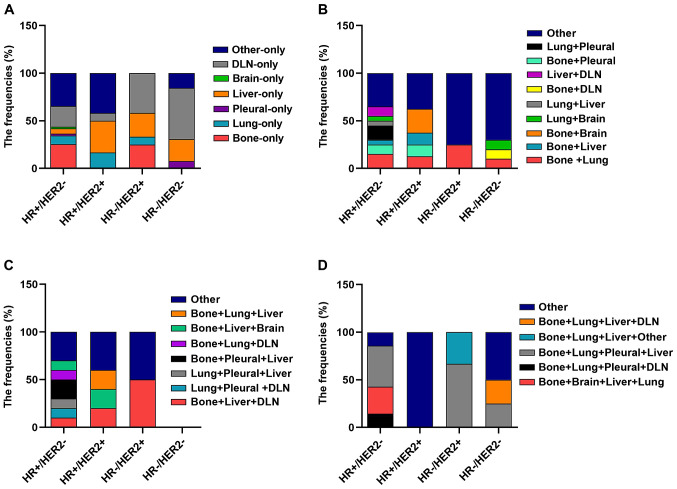
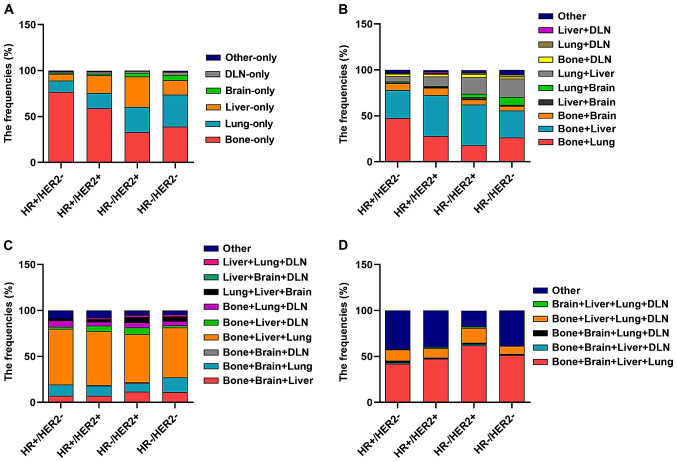
Subsequently, the information on all combinations of different distant metastases was further analyzed in the subtypes of patients in both datasets. In both datasets, it was indicated that regarding single distant metastasis, bone-only metastasis was more common in the HR+/HER2−subtype (P<0.05) and liver-only metastasis was more common in the HR+/HER2+ and HR-/HER2+ subtypes (P<0.05), while DLN-only metastasis was more common in HR-/HER2- patients (P<0.05). Regarding the combinations of distant metastases to two different sites, bone + liver metastases were more common in the HR+/HER2+ subtype and bone + brain metastases were also more common in the HR+/HER2+ subtype, while lung + brain metastases were more common in the HR-/HER2- subtype. For combinations of metastasis to three different distant sites, bone + lung + DLN metastases were more common in the HR+/HER2− subtype (Figs. 2 and 3; Tables SI and SII).
Figure 2.
Frequencies of metastasis locations in 166 patients with metastatic breast cancer of the present cohort study according to molecular subtype. Frequencies of different metastatic sites in patients with breast cancer with involvement of (A) one, (B) two, (C) three and (D) four metastatic sites by each breast cancer subtype. DLN, distant lymph node; HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Figure 3.
Frequencies of metastasis locations in patients with metastatic breast cancer from the Surveillance, Epidemiology and End Results dataset (n=15,322) according to molecular subtype. Frequencies of different metastatic sites in patients with breast cancer with involvement of (A) one, (B) two, (C) three and (D) four metastatic sites by each breast cancer subtype. DLN, distant lymph node; HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
Molecular subtypes and the OS of patients with primary and metastatic breast cancer based on the SEER database
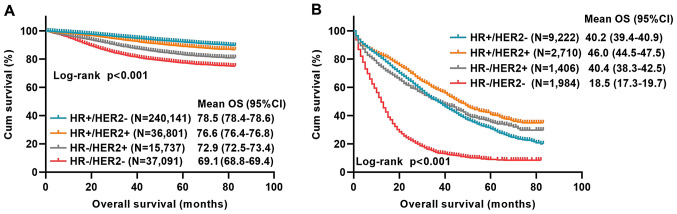
Based on the results discussed in the previous section, the influence of the molecular subtype on the OS of 329,770 primary breast cancer patients from the SEER database between 2010 and 2016 was first analyzed. There was a statistical difference in OS among the molecular subtypes with primary breast cancer (P<0.001). The mean OS period of HR+/HER2− patients was 78.5 months and the mean OS period of HR+/HER2+, HR-/HER2+ and HR-/HER2- patients was 76.6, 72.9 and 69.1 months, respectively (Fig. 4A).
Figure 4.
Influence of molecular subtypes on the OS of patients with primary breast cancer and with metastatic breast cancer based on the SEER database. (A) OS of 329,770 patients with primary breast cancer by molecular subtype; Log-rank P<0.001. (B) OS of 15,322 patients with metastatic breast cancer by molecular subtype; Log-rank P<0.001. OS, overall survival; SEER, Surveillance, Epidemiology and End Results; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; Cum, cumulative survival. The definition of any censored datapoints: alive that their total time until death could not be determined; or dead of other cause; or missing.
Furthermore, the association between molecular subtypes of the 15,322 metastatic breast cancer patients from the SEER database between 2010 and 2016 and OS was determined. There was a statistically significant difference in OS among the different molecular subtypes with metastasis (P<0.001). The mean OS of patients with metastasis of the HR+/HER2−type was 40.2 months, while that of the HR+/HER2+, HR-/HER2+ and HR-/HER2-types was 46.0, 40.4 and 18.5 months, respectively (Fig. 4B).
Discussion
In most cases, the generation of breast cancer metastatic lesions may last for months, years or even decades prior to becoming a clinically detectable metastasis (17). While the underlying mechanisms remain to be fully elucidated, it is known that metastasis is a process that begins with the detachment of tumor cells from the primary tumor (18). According to the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer in 2011 (19), given the limitation of molecular profiling studies in routine clinical practice, the combination of the HR and HER2 status with or without an accompanying Ki-67 proliferation index has been recently used as an indicator for molecular subtypes. Different molecular subtypes have distinctive biological features and clinical outcomes (20). Molecular subtypes remain the most important prognostic determinants in breast cancer (21). The goal of the present study was to explore the possible impact of molecular subtypes on metastatic behavior and OS in a single-center study combined with a large cohort study from the SEER database. Monitoring the biological behavior of breast cancer may benefit a patient by allowing for the implementation of a personalized treatment strategy (22).
The present study indicated that bone metastasis is more frequent in HR+/HER2+ patients, while metastasis to the lung is more frequent in the HR-/HER2+ subtype as compared to the other molecular subtypes of patients with breast cancer. Smid et al (6) also determined that bone metastasis is most abundant in the luminal subtypes. Largely in accordance with these observations, tissue microarray analysis suggested that the HER2 subtype exhibited higher rates of lung metastasis compared with luminal A cancers (7). The luminal B subtype was less frequently associated with lung metastasis than the HER2 subtype (23). The liver was a common organ involved in breast cancer metastasis. A previous study reported that liver metastasis was more frequently observed in the HER2 subtype than the luminal A and triple-negative breast cancer (TNBC) subtypes (23). Similarly, the present study indicated that liver metastasis was more likely to be present in the HR-/HER2+ and HR+/HER2+ subtypes from both datasets. However, only a small number of previous studies have investigated pleural metastases. One previous study reported that the luminal A and B subtypes were both less frequently associated with pleural metastasis than the TNBC subtype (23). In the present study, the HR+/HER2− and HR-/HER2- subtypes had an increased likelihood to have pleural metastasis in the single-center cohort. Since the metastatic sites recorded in the SEER database did not include the pleura, it was not possible to use those big data to further support this result. The brain metastasis of breast cancer is not common (6); the present study indicated that metastasis to the brain was less common than metastasis to other organs. In the present study, the HR-/HER2+ and HR-/HER2-subtypes were observed to have relatively more brain metastasis than the other two subtypes. This result is consistent with a previous study, which indicated that the HR-/HER2+ subtype had a higher risk of brain metastasis (5).
There may be various reasons why different molecular subtypes of breast cancer exhibit differences in metastatic sites. A previous study reported that downregulation of E-cadherin was crucial to the dissemination and invasion of cancer cells, which may augment breast cancer metastasis to the bone (24). Zhang et al (25) recently identified differentially expressed DEGs and signaling pathways that may make a contribution towards the understanding of the pathological mechanisms of bone metastasis from breast cancer. For example, integrin binding sialoprotein, matrix metallopeptidase, TNF α-induced protein 6, dehydrogenase/reductase 3, receptor interacting serine/threonine kinase 4, and CD200 had a diagnostic value for patients with breast cancer bone metastasis. There was evidence that the ability of breast cancer cells to activate osteoclasts is similar to that of normal glandular tissue of mammary epithelial cells during lactation; therefore, breast cancer cells have intrinsic properties that allow them to metastasize to bone tissue (26). Sphingosine kinase 1 (SPHK1) promoted lung metastasis by transcriptionally upregulating the expression of the metastasis-promoting gene fascin actin-building protein 1 via NF-κB activation, and targeting SPHK1 and NF-κB using clinically-applicable inhibitors significantly inhibited aggressive mammary tumor growth and spontaneous lung metastasis in orthotopic syngeneic HR-/HER2- subtype mouse models (27). The mechanism for the tendency of HER2+ tumors to appear in the liver remains elusive. C-X-C motif chemokine receptor 4, a chemokine receptor enhanced by HER2 activation, has been proposed to be involved in promoting invasion of these cells to visceral organs (28). In addition, a previous study indicated that hepatic steatosis may serve as an independent factor to decrease liver metastasis in patients with breast cancer (29). The biological mechanisms of brain metastasis are currently unclear in breast cancer. A study reported that the WNT pathway was associated with relapse of breast cancer or metastasis to the brain (6). Future studies should investigate the metastatic mechanism in breast cancer across the different subtypes and develop strategies of how to reduce the overall risk of metastasis.
To date, the association of molecular subtypes with the number of metastatic sites in patients with breast cancer has been rarely investigated. In the present study, analysis of the single-center cohort and the SEER dataset both indicated that the the number of metastatic sites of the HR+/HER2− subtype patients was lower than in patients with the other three subtypes after those patients had experienced metastases, while the number of metastatic sites of the HR-/HER2+ and HR-/HER2- subtypes patients was higher. The present study lacks mechanistic evidence to explain why the molecular subtype affected the number of metastatic sites. Future work by our group will continue to explore the reasons for this and attempt to identify a possible mechanism.
Numerous studies have demonstrated different survival rates between molecular subtypes (30–32). However, the difference in prognosis between primary and metastatic tumors of different molecular subtypes remains to be fully elucidated. In the present study, there was a statistically significant difference in OS among the different molecular subtypes of patients with distant metastatic primary breast cancer between 2010 and 2016 from the SEER database. The patients of the HR+/HER2− subtype had the longest mean OS compared with the other three subtypes. HR-/HER2- was associated with a significantly poorer OS, whether in primary breast cancer or the subset of metastatic breast cancer patients. The Notch signaling pathway has emerged as a regulatory factor in the pathogenesis and tumor progression of TNBC (33). A previous study also reported that in a survival analysis of females diagnosed with de novo metastasis, the mortality risk relative to the HR+/HER2− subtype was twice as high for HR-/HER2- and slightly lower for HR+/HER2+; HER2+ metastatic breast cancers had relatively better survival in recent years (34). The present study also determined that the mean OS of metastatic patients of the HR+/HER2+ subtype was the longest (46.0 months). Previous studies have indicated that the median survival time of patients with metastatic breast cancer was ~2–4 years (3) and the 5-year survival rate was only ~25% (35). This was consistent with the present study.
As a limitation of the present study, no Kaplan-Meier analysis was performed for the present single-center cohort. As a proportion of metastatic breast cancer patients enrolled in the present single-center study between 2012 and 2016 were initially treated at Hunan Provincial People's Hospital (Changsha, China), when they were diagnosed with metastases later here, then they went back to their local hospitals for final treatment and some of them were lost to follow-up. In addition, some metastatic breast cancer patients enrolled in the present single-center study between 2017 and 2018 were still alive at the end of the follow-up that their total time until death could not be determined. Thus, considering the large amount of censored data in the present dataset, no corresponding prognostic survival analysis of the cases collected at Hunan Provincial People's Hospital (Changsha, China) was performed.
The present results may assist clinicians in the treatment of patients to select appropriate and standardized treatments. Although the results for the single-center cohort were not always consistent with the results obtained with the SEER data due to limited samples in the single-center cohort, the present results may be valuable for developing appropriate follow-up strategies and to guide personalized care.
In conclusion, the present study indicated that different molecular subtypes of breast cancer have different metastatic behaviors. The subtypes exhibited differences regarding the sites and number of metastases. The survival was different among the different molecular subtypes with metastasis. These results may assist clinicians in the prediction of metastatic behavior of breast cancers and develop more efficient follow-up monitoring strategies to further improve OS.
Supplementary Material
Acknowledgements
Not applicable.
Funding
This study was supported by The National Natural Science Foundation of China (grant no. 81572966).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
Study design and supervision, as well as data curation were performed by WW. The manuscript was drafted by HY. Pathological work was performed by JZ and WG. Data collection and processing were performed by HY, RW, FRZ, SLP, YYM, SYC, SJD and LHZ. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. It was approved by the Medical Ethical Committee of Hunan Provincial People's Hospital and The First Affiliated Hospital of Hunan Normal University (Changsha, China) and written informed consent was provided by all of the participating patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Santa-Maria CA, Nye L, Mutonga MB, Jain S, Gradishar WJ. Management of Metastatic HER2-positive breast cancer: Where are we and where do we go from here? Oncology (Williston Park) 2016;30:148–155. [PubMed] [Google Scholar]
- 3.Puglisi F, Rea D, Kroes MA, Pronzato P. Second-line single-agent chemotherapy in human epidermal growth factor receptor 2-negative metastatic breast cancer: A systematic review. Cancer Treat Rev. 2016;43:36–49. doi: 10.1016/j.ctrv.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Gerratana L, Fanotto V, Bonotto M, Bolzonello S, Minisini AM, Fasola G, Puglisi F. Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis. 2015;32:125–133. doi: 10.1007/s10585-015-9697-2. [DOI] [PubMed] [Google Scholar]
- 5.Sihto H, Lundin J, Lundin M, Lehtimäki T, Ristimäki A, Holli K, Sailas L, Kataja V, Turpeenniemi-Hujanen T, Isola J, et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: A nationwide cohort study. Breast Cancer Res. 2011;13:R87. doi: 10.1186/bcr2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, Foekens JA, Martens JW. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 7.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 8.Berman AT, Thukral AD, Hwang WT, Solin LJ, Vapiwala N. Incidence and patterns of distant metastases for patients with early-stage breast cancer after breast conservation treatment. Clin Breast Cancer. 2013;13:88–94. doi: 10.1016/j.clbc.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Heitz F, Harter P, Lueck HJ, Fissler-Eckhoff A, Lorenz-Salehi F, Scheil-Bertram S, Traut A, du Bois A. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer. 2009;45:2792–2798. doi: 10.1016/j.ejca.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, Gelber RD, Castiglione-Gertsch M, Coates AS, Goldhirsch A, Cardoso F. Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: Results from international breast cancer study group trials VIII and IX. J Clin Oncol. 2013;31:3083–3090. doi: 10.1200/JCO.2012.46.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paluch-Shimon S, Ben-Baruch N, Wolf I, Zach L, Kopolovic J, Kruglikova A, Modiano T, Yosepovich A, Catane R, Kaufman B. Hormone receptor expression is associated with a unique pattern of metastatic spread and increased survival among HER2-overexpressing breast cancer patients. Am J Clin Oncol. 2009;32:504–508. doi: 10.1097/COC.0b013e3181967d72. [DOI] [PubMed] [Google Scholar]
- 12.Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115:423–428. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 13.Minisini AM, Moroso S, Gerratana L, Giangreco M, Iacono D, Poletto E, Guardascione M, Fontanella C, Fasola G, Puglisi F. Risk factors and survival outcomes in patients with brain metastases from breast cancer. Clin Exp Metastasis. 2013;30:951–956. doi: 10.1007/s10585-013-9594-5. [DOI] [PubMed] [Google Scholar]
- 14.Sperduto PW, Kased N, Roberge D, Chao ST, Shanley R, Luo X, Sneed PK, Suh J, Weil RJ, Jensen AW, et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol. 2013;112:467–472. doi: 10.1007/s11060-013-1083-9. [DOI] [PubMed] [Google Scholar]
- 15.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6:195–197. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 17.Kimbung S, Loman N, Hedenfalk I. Clinical and molecular complexity of breast cancer metastases. Semin Cancer Biol. 2015;35:85–95. doi: 10.1016/j.semcancer.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 19.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, Panel members Strategies for subtypes-dealing with the diversity of breast cancer: Highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Onco. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ongaro E, Gerratana L, Cinausero M, Pelizzari G, Poletto E, Giangreco M, Andreetta C, Pizzolitto S, Di Loreto C, Minisini AM, et al. Comparison of primary breast cancer and paired metastases: Biomarkers discordance influence on outcome and therapy. Future Oncol. 2018;14:849–859. doi: 10.2217/fon-2017-0384. [DOI] [PubMed] [Google Scholar]
- 21.Killelea BK, Gallagher EJ, Feldman SM, Port E, King T, Boolbol SK, Franco R, Fei K, Le Roith D, Bickell NA. The effect of modifiable risk factors on breast cancer aggressiveness among black and white women. Am J Surg. 2019;218:689–694. doi: 10.1016/j.amjsurg.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujii K, Watanabe R, Ando T, Kousaka J, Mouri Y, Yoshida M, Imai T, Nakano S, Fukutomi T. Alterations in three biomarkers (estrogen receptor, progesterone receptor and human epidermal growth factor 2) and the Ki67 index between primary and metastatic breast cancer lesions. Biomed Rep. 2017;7:535–542. doi: 10.3892/br.2017.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soni A, Ren Z, Hameed O, Chanda D, Morgan CJ, Siegal GP, Wei S. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol. 2015;143:471–478. doi: 10.1309/AJCPYO5FSV3UPEXS. [DOI] [PubMed] [Google Scholar]
- 24.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, He W, Zhang S. Seeking for correlative genes and signaling pathways with bone metastasis from breast cancer by integrated analysis. Front Oncol. 2019;9:138. doi: 10.3389/fonc.2019.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 27.Acharya S, Yao J, Li P, Zhang C, Lowery FJ, Zhang Q, Guo H, Qu J, Yang F, Wistuba II, et al. Sphingosine-kinase-1 signaling promotes metastasis of triple-negative breast cancer. Cancer Res. 2019;79:4211–4226. doi: 10.1158/0008-5472.CAN-18-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, Hung MC. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Wu W, Chen J, Ye W, Li X, Zhang J. Fatty liver decreases the risk of liver metastasis in patients with breast cancer: A two-center cohort study. Breast Cancer Res Treat. 2017;166:289–297. doi: 10.1007/s10549-017-4411-5. [DOI] [PubMed] [Google Scholar]
- 30.Park HS, Kim S, Kim K, Yoo H, Chae BJ, Bae JS, Song BJ, Jung SS. Pattern of distant recurrence according to the molecular subtypes in Korean women with breast cancer. World J Surg Oncol. 2012;10:4. doi: 10.1186/1477-7819-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carvalho ST, Stiepcich MM, Fregnani JH, Nonogaki S, Rocha R, Soares FA. Evaluation of prognostic factors in stage IIA breast tumors and their correlation with mortality risk. Clinics (Sao Paulo) 2011;66:607–612. doi: 10.1590/S1807-59322011000400014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C, Wang S, Israel HP, Yan SX, Horowitz DP, Crockford S, Gidea-Addeo D, Clifford Chao KS, Kalinsky K, Connolly EP. Higher locoregional recurrence rate for triple-negative breast cancer following neoadjuvant chemotherapy, surgery and radiotherapy. Springer Plus. 2015;4:386. doi: 10.1186/s40064-015-1116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giuli MV, Giuliani E, Screpanti I, Bellavia D, Checquolo S. Notch signaling activation as a hallmark for triple-negative breast cancer subtype. J Oncol. 2019;2019:8707053. doi: 10.1155/2019/8707053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Press DJ, Miller ME, Liederbach E, Yao K, Huo D. De novo metastasis in breast cancer: Occurrence and overall survival stratified by molecular subtype. Clin Exp Metastasis. 2017;34:457–465. doi: 10.1007/s10585-017-9871-9. [DOI] [PubMed] [Google Scholar]
- 35.Bonotto M, Gerratana L, Poletto E, Driol P, Giangreco M, Russo S, Minisini AM, Andreetta C, Mansutti M, Pisa FE, et al. Measures of outcome in metastatic breast cancer: Insights from a real-world scenario. Oncologist. 2014;19:608–615. doi: 10.1634/theoncologist.2014-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.