Abstract
Background
Infectious diseases (ID) physicians perform a pivotal role in directing the response to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2).
Aim
To assess the impact of SARS‐CoV‐2 on workload and the perceptions of ID physicians regarding the national response in Australia and New Zealand in the pre‐pandemic.
Methods
A survey of ID physicians in Australia and New Zealand was undertaken from 3 to 10 March 2020. Respondents were asked to estimate time spent on SARS‐CoV‐2‐related activities in February and report their agreement with statements on a 5‐point Likert scale ranging from ‘strongly agree’ to ‘strongly disagree’. We also asked about the intended use of investigational agents.
Results
There were 214 respondents (36% of 600 eligible participants). The median workload due to SARS‐CoV‐2‐related activities was 34% of one full‐time equivalent (interquartile range 18–68%). Less than a quarter (50, 23%) of respondents had experience managing cases, while 33% (70) had experience preparing during similar pandemics. Nevertheless, 88% (188/213) believed they were well informed when giving testing and management advice, and 45% (95/212) believed their national response was well coordinated. Additionally, 41% (88/214) were worried about becoming infected through occupational exposure. Over half (116, 54%) the respondents intended to use lopinavir/ritonavir in confirmed cases of COVID‐19 with severe disease.
Conclusions
ID physicians spent a large proportion of time on SARS‐CoV‐2‐related activities. Increased staffing is required to avoid burnout. Importantly, ID physicians feel well informed when giving advice. A national body should be established to co‐ordinate response. Treatment efficacy trials are needed to clarify the utility of unproven treatments.
Keywords: survey, infectious diseases physicians, COVID‐19, workload, research, psychosocial
Introduction
From its initial emergence in Wuhan City, Hubei province of China, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and the associated clinical syndrome, COVID‐19, has spread swiftly across the globe. This is the third significant emergence in the 21st century of a novel human coronavirus.1, 2 SARS‐CoV‐2 has already had a substantially wider impact in terms of cases and both social and economic disruption. At the time of submission of this paper, there have been over 3 750 000 confirmed cases and 260 000 deaths. 3 The rising number of cases has necessitated a robust response at a national, regional and local level.
Effective utilisation of the time between identifying the risk and the subsequent outbreak is fundamental to mitigating the short‐ and long‐term challenges of a pandemic. It allows the opportunity to strengthen pre‐existing surveillance and reporting systems and establish surge capacity. 4 Infectious disease (ID) physicians, through their training and experience, perform a pivotal role in directing this response. 5 To be successful, such a response requires the gathering and assessment of rapidly changing information to produce guidelines and recommendations, in order to build capacity within healthcare systems and formulate an overarching public health response. An effective response also helps to safeguard healthcare workers, buffering the long‐term psychological and occupational effects of a prolonged outbreak. 6 However, the recognition of the importance of this interval and the urgent nature of the task has magnified the associated pressure on ID physicians.
Furthermore, several agents have been suggested as potential treatment options for patients. 7 However, perceptions of efficacy may be influenced by limited knowledge of the natural history of the pathogen coupled with physicians' desires to treat their patient with any available tools. This creates a tension between clinicians who advocate conducting research to identify truly efficacious treatments and those who advocate empirical therapy as part of routine care. 8
We aimed to assess the impact of SARS‐CoV‐2 on the workload and psychosocial outlook of ID physicians in Australia and New Zealand at the beginning of this pandemic, including their treatment intentions and research equipoise.
Methods
We conducted a survey of ID physicians in Australia and New Zealand. Respondents were contacted through an established Australasian Society for Infectious Diseases (ASID) online mailing list, OZbug. 9 ASID is the professional body for ID physicians and clinical microbiologists in Australasia. Approximately 600 ID physicians, including advanced specialty trainees, are members of ASID. A web‐based data collection tool (Survey Monkey, Palo Alto, CA USA) was utilised to collect de‐identified data from ID physicians in Australia and New Zealand over the period from 3 to 10 March 2020 (full survey available in Supporting Information Data S1). The adapted CHERRIES checklist for the reporting of electronic surveys, 10 including additional details of this survey's design, piloting and administration are available in Data S2. Data collected included speciality (adult infectious diseases vs paediatric infectious diseases vs clinical microbiology with infectious diseases), country, size and setting (metropolitan vs regional) of hospital, years of experience post‐commencement of advanced speciality training, full time equivalent (FTE) worked in medicine and FTE fraction worked in ID, and membership of relevant outbreak‐related committees at the hospital, health district, state, and national level. FTE was employed as a marker of time worked, with 1.0 FTE equating to a 40‐h working week.
Respondents were asked to estimate how many hours they spent in the month of February on various categories of SARS‐CoV‐2 related activities (Table 2), and whether they had to work outside normal hours. Five‐point Likert scale questions, with response options ranging from ‘strongly agree’ to ‘strongly disagree’, were utilised to assess respondents' agreement with a range of statements (Fig. 1).
Table 2.
Time spent on SARS‐CoV‐2 activities by category
| Category | Median hours (IQR) |
|---|---|
| Direct provision of care to confirmed cases | 0 (0–0) |
| Advice or assessment on testing, infection control and/or clinical management of suspected cases | 4 (1–10) |
| Attending face to face planning and preparedness meetings (at any level – hospital through to national) | 3 (1.5–6) |
| Attending teleconferences or webinars relevant to SARS‐CoV‐2 planning and preparedness | 1 (0–4) |
| Providing updates and education to other clinicians | 2 (0.38–3) |
| Providing updates and education to the general public (other than media) | 0 (0–1) |
| Media interviews (including preparation time) | 0 (0–0) |
| Reading and/or responding to emails relevant to SARS‐CoV‐2 | 5 (3–10) |
| Self‐education about SARS‐CoV‐2 by, for example, reading medical literature | 6 (4–10) |
| Planning or implementing SARS‐CoV‐2‐related research projects | 0 (0–1) |
| Total hours spent per month | 27 (17–50) |
IQR, interquartile range.
Figure 1.
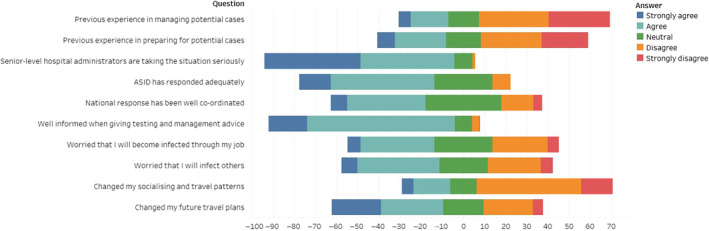
Demonstrates the percentage of agreement, ranging from strongly agree to strongly disagree, to several statements.
In addition, respondents were asked to report their intended usage of lopinavir/ritonavir for proven SARS‐CoV‐2 infection. They were asked whether they plan to prescribe it to all confirmed cases; confine its use to patients with severe disease (e.g. those requiring non‐invasive ventilation or ICU admission); use it only as part of a clinical trial; or not use it altogether. Respondents could also supply a free‐text response; these were regrouped where appropriate. Respondents were asked whether they would have equipoise to randomise usage of lopinavir/ritonavir and chloroquine among all hospitalised patient and among those with severe disease.
Data were analysed using Excel® 2013 (Microsoft, Redmond, WA, USA) and Stata/IC 11.2 (StataCorp, College Station, TX, USA). Excel® 2013 was used for data collation, cleaning and descriptive statistics. Stata/IC 11.2 was used for all other statistical analyses. Ordinal logistic regression was used to determine factors associated with working overtime (with the number of days in which they worked outside normal hours grouped as 0, 1–2, 2–3 and >4), and Likert responses. In the regression analysis, the explanatory variables were made binary: years of experience (‘less experienced’ and ‘more experienced’, with ≤15 and >15 years‐experience post‐commencement of advanced specialty training, respectively), location (Australia vs New Zealand), hospital size (‘small’ and ‘large’, with ≤500 vs > 500 beds, respectively) and committee membership (member vs non‐member) (Fig. 2). P‐values <0.05 were considered statistically significant.
Figure 2.
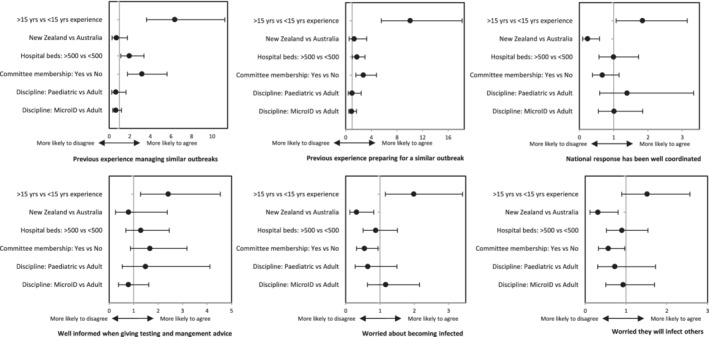
Ordinal regression analysis comparing Likert responses (comparator vs reference group) to key statements. A higher odds ratio in this figure indicates an increased likelihood that the comparator group, compared with the reference group, agrees with the statement. A lower odds ratio indicates an increased likelihood that the comparator group disagrees with the statement.
Results
We received 302 responses (50% of 600 eligible participants). Eighty‐eight of these responses were excluded: 80 due to incomplete data, four were from non‐ID physicians, and four were located outside of Australasia. Data were, thus, available for 214 respondents (36% of 600 eligible participants).
Respondent characteristics
Respondent characteristics are shown in Table 1. The majority of physicians were located in Australia (92%) and worked in metropolitan settings (84%). Adult ID was the most commonly reported subspecialty (66%). Ten percent were paediatric ID physicians. Paediatric ID physicians worked in smaller hospitals than their adult ID counterparts, with 17 of 18 (94%) working in hospitals with 200–500 beds. Membership of a COVID‐19 related committee was high (59%), but this figure was lower for physicians earlier in their career (<5 years; 15/45, 33%).
Table 1.
Demographic details of participants
| Total | |
|---|---|
| Respondents, n (%) | 214 (100) |
| Country, n (%) | |
| Australia | 196 (92) |
| New Zealand | 18 (8) |
| Speciality, n (%) | |
| Adult ID | 141 (66) |
| Paediatric ID | 21 (10) |
| ID with clinical microbiology | 52 (24) |
| Hospital setting, n (%) | |
| Metropolitan | 179 (84) |
| Regional | 35 (16) |
| Number of inpatient beds, n (%) | |
| <200 | 16 (8) |
| 200–500 | 94 (44) |
| 500–1000 | 95 (44) |
| >1000 | 9 (4) |
| Years of experience post‐commencement of advanced specialty training, n (%) | |
| <5 years | 45 (21) |
| 5–15 years | 95 (44) |
| >15 years | 74 (35) |
| FTE, median (IQR) | |
| Total | 1 (0.8–1) |
| ID FTE | 0.5 (0.3–0.8) |
| Committee membership, n (%) | |
| Any | 127 (59) |
| National | 18 (8) |
| State† | 14 (7) |
| District | 37 (17) |
| Hospital | 113(53) |
| None of the above | 87 (41) |
State committee membership only in Australia. FTE, full‐time equivalent; ID, infectious diseases; IQR, interquartile range.
Workload impact
A total of 8850 h was spent on SARS‐CoV‐2 over the month of February. This is equivalent to a median of 27 (17–50) h per physician. A detailed breakdown of SARS‐CoV‐2‐related activities by category is shown in Table 2. Physicians spent a median 34% (interquartile range 18–68%) of their FTE in ID on SARS‐CoV‐2‐related activities.
Nearly three‐quarters (159/214, 74.3%) of respondents stayed late at work more than 1 day/week, with 33% (70/214) working late more than 3 days a week. New Zealand employment (odds ratio (OR) 3.16, P = 0.012 (95% confidence interval (CI) 1.29–7.79)) and committee membership (OR 2.41, P = 0.001 (95% CI 1.42–4.09)) were significantly associated with spending more time working outside of normal hours.
Experience and risk perception
Of the respondents, 23.4% (50/214) agreed that they have previous extensive involvement in managing potential cases in an outbreak of similar scale. More experienced physicians (OR 6.37, P < 0.001 (95% CI 3.63–11.24)), those who worked in a hospital with >500 beds (OR 1.95, P = 0.017 (95% CI 1.13–3.38) and members of a committee (OR 3.18, P < 0.001 (95% CI 1.79–5.63)) were significantly more likely to agree with this statement.
Previous extensive involvement in preparing for potential cases in an outbreak of similar scale was reported by 33% (70/214). Variables significantly associated with agreement were being a more experienced physician (OR 10.05, P < 0.001 (95% CI 5.56–18.18)), working in a large hospital (OR 3.02, P = 0.042 (95% CI 1.02–3.02)) and committee membership (OR 2.75, P < 0.001 (95% CI 1.57–4.81)).
The majority of respondents (192/214, 90%) stated that their senior level hospital administrators are taking COVID‐19 seriously. Almost two‐thirds also agree that ASID has responded adequately (64%, 137/214).
When asked about their national response, 95 of the 212 responses (45%) agreed that it was well coordinated. Australian respondents were four times more likely to agree that their national response was well co‐ordinated compared to New Zealand respondents (OR 4.16, P = 0.002, 95% CI 1.71–10.15). In this metric, 7 of the 18 (39%) New Zealand respondents reported that they disagreed with this statement. Of the remainder, 10 were neutral and one did not respond.
When giving testing and management advice, 88% (188/213) believe they are well informed. Concerning infection with SARS‐CoV‐2, 41% (88/214) of respondents were worried they would become infected through occupational exposure. More experienced physicians were twice as likely to agree with this statement (OR 1.99, P = 0.013 (95% CI 1.16–3.41). Physicians in New Zealand were significantly less worried about becoming infected (OR 0.32, P = 0.017 (95% CI 0.12–0.82). Similarly, being a member of a committee was also significantly associated with less concern (OR 0.55, P = 0.03 (95% CI 0.32–0.94). A similar proportion (99/214, 46%) was worried about passing infection onto family and friends. This was less of a concern for physicians in New Zealand (OR 0.31, P = 0.017 (95% CI 0.12–0.81)) and physicians who were committee members (OR 0.56, P = 0.039 (95% CI 0.33–0.97)).
Of the respondents, 23% (49/214) agreed that they have changed their socialising and travel patterns. A higher percentage, 53% (113/214), agreed that they have changed future travel plans.
Research equipoise
Around half (116/214, 54%) of respondents intended to reserve the use of lopinavir/ritonavir for those with severe COVID‐19 (defined as requiring intensive care or non‐invasive ventilation). Only 7% (14/214) of respondents intended to use lopinavir/ritonavir in all patients with confirmed COVID‐19 with a small number (7, 3%) intending to follow a unit lead decision and 2% (6/214) gave an uncategorised response.
The majority of respondents indicated there was clinical equipoise to conduct a randomised trial of lopinavir/ritonavir although there was differing opinion with regards to severity of disease as inclusion criteria (Fig. 3). The majority were undecided with regards to a clinical trial of chloroquine.
Figure 3.
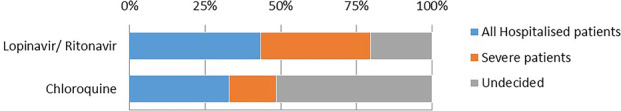
Percentage of respondents that would have clinical equipoise to randomise usage in confirmed severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) by clinical severity.
Discussion
On a day‐to‐day level, ID physicians provide a wide range of services, including guiding judicious antibiotic usage, directing infection control practice and providing direct patient management. 11 In an outbreak setting, this role expands to meet the increased need, focussed on developing a multifaceted response. During this critical time, ID physicians in Australia and New Zealand spent a significant amount number of hours on SARS‐CoV‐2‐related activities, equating a median of 34% of all their FTE in ID for that month. Unsurprisingly, physicians who were members of SARS‐CoV‐2‐related committees devoted more time to this endeavour.
Increasing the number of hours worked, especially outside the normal hours, is associated with burn‐out, even given the protective effect of a professional interest. 12 Nearly three‐quarters of ID physicians spent more time working outside of normal working at least one additional day per week during the month of February. Furthermore, almost one‐third stayed late more than 3 days a week. New Zealand respondents were more likely to stay late, reflecting the smaller number of specialists available. The FTE a physician had allotted to ID did not significantly alter this finding. As a consequence, New Zealand ID physicians and those with smaller amounts of FTE are at higher risk of burning out. It is important to note that our survey was carried out in the preparatory stage of the pandemic in Australasia, and ID physician workloads are likely to have since increased substantially. This has clear implications for human resources management and staff well‐being.
Experience and training are crucial when guiding outbreak preparation and management. ID physicians later in their career reported more experience with similar outbreaks, such as SARS‐CoV‐1, MERS and 2009 pandemic influenza. The majority of respondents felt well‐informed, with those further in their career tending to respond even more favourably. Furthermore, the majority of later‐career ID physicians, including those with direct pandemic experience, were members of committees. ID physicians early in their career were less likely to be part of a committee, potentially missing out on an important opportunity to gain invaluable experience. An important fact to note is that the majority of ID physicians in our sample, especially those with experience with outbreaks of similar nature, work in large metropolitan hospitals, clustering knowledge and experience away from smaller regional centres. It is important that these physicians continue to offer guidance and support to their less experienced and poorer‐resourced colleagues. Facilitating such links will also be of benefit even after the COVID‐19 pandemic is over, by improving collegiality and ensuring sustainability of ID services in smaller hospitals.
Leadership and support are needed at both local and national levels to facilitate effective preparation. 4 ID physicians mostly agree that their senior hospital administrators are taking SARS‐CoV‐2 seriously and that ASID, the Australasian specialist society for ID physicians and medical microbiologists, had responded appropriately.
The lower positive report rate of 45% regarding the effectiveness of the national response is not unexpected. It is difficult to respond swiftly to an outbreak with multiple unknowns and rapidly evolving information. It is expected that this metric will improve as policies and approaches become more clearly delineated. Experienced physicians were more likely to assess their government response favourably, recognising the difficulty of the situation and their role in preparation. ID physicians in New Zealand responded particularly unfavourably to this question. This is likely multi‐factorial, but principally influenced by the limited pool of specialists to guide their national approach and the consequent immensity of this task. One step that may strengthen outbreak responses is the establishment of a national body, such as a Centre for Disease Control. This central coordinating and advisory body, as a repository of epidemiological and clinical expertise, would allow for more efficient dissemination of guidelines and policies, reducing the current duplication of effort and, importantly, allow a more consistent regional and national approach. This body would need to be adequately funded and politically supported to be effective. The importance of this backing is underlined by the contrasting performance of similar bodies in other jurisdictions at this stage of the pandemic, such as the United States, New Zealand and South Korea. Additionally, in Australia, it would require legislative change as many powers are currently decentralised to the state level.
The SARs‐CoV‐1 outbreak in the early 2000s highlighted physicians' concern about getting infected through their work. In our study, 41% were worried about being infected, while 46% were worried about infecting friends and family. ID physicians further in their career were more concerned about getting infected. This finding may be explained by the older ages of more experienced physicians, and the fact that age is a risk factor for adverse outcomes. 13
The proportion of ID physicians with concerns about being infected and infecting friends and family is considerably lower than those reported by paediatricians in Australia and New Zealand. 14 In comparison, 86% of paediatricians were worried about becoming infected SARS‐CoV‐2 through their work and 92% transmitting the infection to others. The variation in concern may be partially explained by differing levels of exposure risk and knowledge. This is supported by our finding that membership of a committee was associated with less concern about being infected. Interestingly, ID physicians in New Zealand tended to be less worried about infection, despite their concerns about the adequacy of their national response. This may reflect a high degree of trust in their healthcare system, 15 and is an issue that could be explored further in their local context.
As demonstrated in the Ebola outbreak in West Africa, balancing research and medical care during an outbreak can pose an ethical quandary. 16 The Declaration of Helsinki states that research should be conducted ‘only to the extent that (it) is justified by its potential preventive, diagnostic, or therapeutic value and if the physician has good reason to believe that participation in the research study will not adversely affect the health of the patients who serve as research subjects’.16, 17 The evidence for treatments for COVID‐19 remains limited. Building on a potential benefit in SARS‐CoV‐1 18 and MERS, 19 as well as early case reports, 20 lopinavir/ritonavir was put forward as a potential treatment option. Despite a lack of robust evidence, over 50% of ID physicians in our sample intended to use lopinavir/ritonavir in severe disease. At the time of the survey, there were no published data on the efficacy of lopinavir/ritonavir for COVID‐19. Since then, Cao et al. published a randomised controlled trial of lopinavir/ritonavir among 199 participants that did not demonstrate a benefit for lopinavir/ritonavir. 21 The rapid release and publication of data may mean that the respondent answers to this question about lopinavir/ritonavir may be different at the time of writing.
There was a similar level of equipoise for further investigational work. It is unclear at this juncture which groups will potentially benefit from intervention, and which interventions are most efficacious. The number of unknowns is significant but this survey supports the appetite ID physicians have for randomised clinical trials to determine the best possible therapies.
Our study had several limitations. Firstly, it was conducted at a single time point and is a simplification of a complex situation. Secondly, there may have been a degree of response bias as more motivated physicians are more likely to respond. In addition, physicians that were heavily involved in SARS‐CoV‐2‐related activities, may not have had time to complete the survey. Nevertheless, the response and completion rates were high and there were respondents from multiple jurisdictions, hospital settings, and levels of experience, which increases the representativeness of the sample.
Conclusion
SARS‐CoV‐2 has had, and continues to have, a significant impact on Australian and New Zealand ID physicians. They have risen to challenge, spending a significant amount of time on pandemic‐related activities. However, without support, this response may not be sustainable. Furthermore, there were concerns expressed about the national response in both Australia and New Zealand. We advocate for the establishment of a national body to coordinate future responses.
Supporting information
Data S1. Survey.
Data S2. CHERRIES checklist.
Funding: None.
Conflict of interest: None.
References
- 1. Gastanaduy PA. Update: severe respiratory illness associated with Middle East respiratory syndrome coronavirus (MERS‐CoV)—worldwide, 2012–2013. MMWR Morb Mortal Wkly Rep 2013; 62: 480. [PMC free article] [PubMed] [Google Scholar]
- 2. Cherry JD. The chronology of the 2002–2003 SARS mini pandemic. Paediatr Respir Rev 2004; 5: 262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Novel Coronavirus (COVID‐19) Situation. 2020. [cited 2020 Jun 5]. Available from URL: https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd
- 4. Hawryluck L, Lapinsky SE, Stewart TE. Clinical review: SARS – lessons in disaster management. Crit Care 2005; 9: 384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Srinivasan A, Jernign DB, Liedtke L, Strausbaugh L. Hospital preparedness for severe acute respiratory syndrome in the United States: views from a national survey of infectious diseases consultants. Clin Infect Dis 2004; 39: 272–4. [DOI] [PubMed] [Google Scholar]
- 6. Maunder RG, Lancee WJ, Balderson KE, Bennett JP, Borgundvaag B, Evans S et al Long‐term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerg Infect Dis 2006; 12: 1924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network‐based drug repurposing for novel coronavirus 2019‐nCoV/SARS‐CoV‐2. Cell Discov 2020; 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Academies of Sciences Engineering and Medicine . Health and Medicine Division, Board on Health Sciences Policy, Board on Global Health, Committee on Clinical Trials During the 2014–2015 Ebola Outbreak In: Busta ER, Mancher M, Cuff PA, McAdam K. and Keusch G, eds Integrating Clinical Research into Epidemic Response: The Ebola Experience. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 9. Watson DA. Ozbug: an email mailing list for physicians that works. Intern Med J 2003; 33: 532–4. [DOI] [PubMed] [Google Scholar]
- 10. Eysenbach G. Improving the quality of web surveys: the Checklist for Reporting Results of Internet E‐Surveys (CHERRIES). J Med Internet Res 2004; 6: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dickstein Y, Nir‐Paz R, Pulcini C, Cookson B, Beovic B, Tacconelli E et al Staffing for infectious diseases, clinical microbiology and infection control in hospitals in 2015: results of an ESCMID member survey. Clin Microbiol Infect 2016; 22: 812.e9–812.e17. [DOI] [PubMed] [Google Scholar]
- 12. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med 2018; 283: 516–29. [DOI] [PubMed] [Google Scholar]
- 13. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foley DA, Kirk M, Jepp C, Brophy‐Williams S, Tong SYC, Davis JS et al COVID‐19 and paediatric health services: a survey of paediatric physicians in Australia and New Zealand. J Paediatr Child Health 2020. doi:10.1111/jpc.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gauld R. A view from abroad: a New Zealand perspective on the English NHS health reforms In: Exworthy M, Mannion R, Powell M, editors. Dismantling the NHS?: Evaluating the impact of health reforms. England, UK: Bristol University Press; 2016; p. 343–64. [Google Scholar]
- 16. Masic I, Hodzic A, Mulic S. Ethics in medical research and publication. Int J Prev Med 2014; 5: 1073–82. [PMC free article] [PubMed] [Google Scholar]
- 17. Goodyear MD, Krleza‐Jeric K, Lemmens T. The declaration of Helsinki. BMJ 2007; 335: 624–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS et al Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004; 59: 252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Min CK, Cheon S, Ha NY, Sohn KM, Kim Y, Aigerim A et al Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep 2016; 6: 25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lim J, Jeon S, Shin HY, Kim MJ, Seong YM, Lee WJ et al Case of the index patient who caused tertiary transmission of COVID‐19 infection in Korea: the application of Lopinavir/ritonavir for the treatment of COVID‐19 infected pneumonia monitored by quantitative RT‐PCR. J Korean Med Sci 2020; 35: e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G et al A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med 2020; 382: 1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Survey.
Data S2. CHERRIES checklist.


