Abstract
Kidney transplant recipients might be at higher risk for severe coronavirus disease 2019 (COVID-19). However, risk factors for relevant outcomes remain uncertain in this population. This is a multicentric kidney transplant cohort including 104 hospitalized patients between March 4 and April 17, 2020. Risk factors for death and acute respiratory distress syndrome (ARDS) were investigated, and clinical and laboratory data were analyzed. The mean age was 60 years. Forty-seven patients (54.8%) developed ARDS. Obesity was associated to ARDS development (OR 2.63; P = .04). Significant age differences were not found among patients developing and not developing ARDS (61.3 vs 57.8 years, P = .16). Seventy-six (73%) patients were discharged, and 28 (27%) died. Death was more common among the elderly (55 and 70.8 years, P < .001) and those with preexisting pulmonary disease (OR 2.89, P = .009). At admission, higher baseline lactate dehydrogenase (257 vs 358 IU/mL, P = .001) or ARDS conferred higher risk of death (HR 2.09, P = .044). In our cohort, ARDS was equally present among young and old kidney recipients. However, the elderly might be at higher risk of death, along with those showing higher baseline LDH at admission.
KEYWORDS: clinical research/practice, complication: infectious, epidemiology, infectious disease, kidney transplantation/nephrology, patient survival
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; ARDS, acute respiratory distress syndrome; CI, confidence interval; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; FIO2, fraction of inspired oxygen; HR, hazard ratio; KT, kidney transplant recipient; LDH, lactate dehydrogenase; MMF, mycophenolate mofetil; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin; rATG, rabbit antithymocyte globulin; rt-PCR, reverse transcriptase–polymerase chain reaction; SD, standard deviation; SpO2, blood oxygen saturation measured by pulse oximetry; TAC, tacrolimus
1. INTRODUCTION
In December 2019, severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2) emerged in China, which was shortly recognized as the pathogen of a new cluster of respiratory illness designated as coronavirus infectious disease 2019 (COVID-19).1
The clinical course and prognosis of COVID-19 have been thoroughly described, identifying older age and the presence of comorbidities as the main risk factors for mortality and acute respiratory distress syndrome (ARDS) development.2, 3, 4 Nevertheless, whether these clinical manifestations and risk factors are valid in renal transplant or other solid organ recipients remains unclear. The role of immunosuppressive therapies, its management during the course of the active viral infection and their potential interactions with currently compassionate treatments used for COVID-19 represent a unique clinical scenario that deserves to be characterized.
Among the 33 766 prevalent kidney transplant recipients (KTs) in Spain,5 433 COVID-19 infection cases had been reported up to April 25, 2020.6 The KT population constitutes a large group of patients considered to be at high risk of complications due to the maintenance of chronic immunosuppression. Single-center case-reports and small series of KT have been published7, 8, 9 with divergent results and recommendations. Actually, in those studies sample size was small and most patients remained admitted at the end of the follow-up.
Here, we report clinical data and outcomes of 104 consecutive KTs with confirmed COVID-19 infection hospitalized in 5 different Spanish kidney transplant units. Our main objectives were to evaluate main risk factors of ARDS and death and to delineate the clinical course and biological profile in this setting.
2. METHODS AND MATERIALS
This is a retrospective multicentric observational cohort study. Our study enrolled all KTs with COVID-19 infection and hospitalized between March 4 and April 17, 2020, in participant centers. All of them had confirmed SARS-CoV-2 infection by real-time reverse transcriptase–polymerase chain reaction (rt-PCR) analysis performed on nasal or pharyngeal swab samples. The hospital admission criteria were need of oxygen therapy, x-ray infiltrates, renal dysfunction, or those with recent (<7 days) symptom onset regardless of its severity (i.e., fever without pneumonia or diarrhea). All patients included had a complete follow-up until discharge (curation or clinical improvement) or death. COVID-19 KTs with exclusively outpatient care were excluded from the analysis because of the potential not-reported cases and the lack of follow-up data. Data were obtained and recorded in a common data collection form in all transplant centers. The study was approved by all hospital ethical review boards (PR173/20).
We collected the following baseline data: age, race/ethnicity, gender, time after KT, first or repeat transplant, type of transplant (kidney or combined pancreas with kidney or liver with kidney), type of donor (deceased or living), primary end-stage renal disease (ESRD), maintenance immunosuppression, induction therapy, basal graft function (serum creatinine and estimated glomerular filtration rate by CKD-EPI [eGFR]), comorbidities such as heart disease (heart failure, coronary artery disease, atrial fibrillation or valvular heart disease), hypertension (type of treatment before illness), obesity (body mass index ≥30), pulmonary disease (chronic obstructive pulmonary disease, bronchiectasis, asthma, or sleep apnea-hypopnea syndrome), active neoplasm, or lymphopenia before admission. Initial clinical symptoms (fever defined by a temperature >37.5°C, respiratory status recorded through the pulse oximetry saturation/fraction of inspired oxygen ratio [Spo 2/Fio 2]) and x-ray evaluation and analytical assessment that were carried out at admission and 3, 6, 9, 12, and 15 days after the admission were also recorded. Individuals considered to have a COVID-19 nosocomial infection were patients in these 2 clinical scenarios: a diagnosis of COVID-19 while being hospitalized due to a different clinical reason or COVID-19 infection in patients who had been discharged from the hospital within the preceding 14 days. Missing data were recovered and inconsistencies were corrected through online interaction.
The primary endpoints were death and ARDS defined by the World Health Organization interim guidance (bilateral opacities not explained by volume overload and Spo 2/Fio 2 ratio <315).10 The secondary endpoints were acute kidney injury (AKI) using KDIGO definition,11 number and type of immunosuppression withdrawal, use of anti–COVID-19 therapies, and associated adverse events (including gastrointestinal, cutaneous rash, QT prolongation [considered prolonged if QTc values are >450 milliseconds in males or >470 milliseconds in females], hepatitis [defined as an elevation of alanine transaminase and aspartate transaminase greater than twice the normal values], and tacrolimus intoxication defined by plasmatic levels of ≥20 ng/mL regardless of nephrotoxicity or neurotoxicity). Anti–COVID-19 protocols in all hospitals were similar and regularly updated according to newly published information. Generally, these included first hydroxychloroquine and lopinavir/ritonavir, darunavir/ritonavir, darunavir/cobicistat, and then remdesivir, interferon-β1a, intravenous steroid therapy, and tocilizumab in case of clinical deterioration.
2.1. Statistical analysis
Continuous variables were expressed as mean ± SD or median and IQR and categorical variables as number of total (n) and percentage (%). Comparison between groups was performed using Pearson’ χ2 test for categorical data or Fisher exact test was applied when the number of cases was < 5. One-way analysis of variance and t tests were used for normally continuous distributed data, and nonparametric Kruskal–Wallis test and Mann–Whitney U test were used for nonnormally distributed variables.
Both univariate and multivariate logistic regression models were performed to examine the risk factors associated with ARDS. To explore the risk factors associated with patient survival, a Cox regression model was used to estimate hazard ratios in an univariate and multivariate analyses, missing data were excluded listwise. The analyses of patient’s survival were censored for death (death certificate date) or recovery (day of discharge and clinical recovery). Due to the relatively small number of death (25) events to avoid overfitting in the model, just 4 variables were chosen for multivariable analysis on the basis of previous findings and clinical constraints.
All P-values were 2-tailed and statistical significance level was fixed at P < .05. SPSS 20.0 software (SPSS Inc), STATA16, and GraphPad Prism version 6.0 (GraphPad Software) were used for data management and analysis.
3. RESULTS
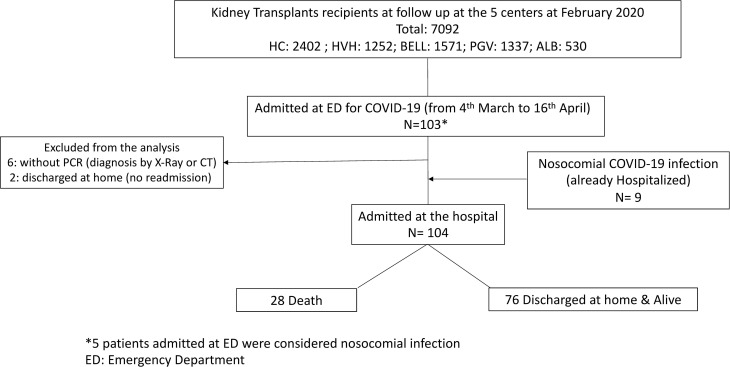
We followed the STROBE Guidelines to report this observational study. Data from 5 Spanish kidney transplant units were obtained (Hospital Universitari de Bellvitge, Hospital Clínic, Fundació Puigvert, Complejo Hospitalario Universitario de Albacete, and Hospital de Vall d’Hebron). Among the 7092 KTs followed in these units, 112 had COVID-19 infection during the study period, 109 patients required hospitalization, and 104 fulfilled inclusion and exclusion criteria ( Figure 1). Considering the prevalent KT population followed in the 5 hospitals, the admission for COVID-19 rate was 0.23 per 1000 patient-days. The median follow-up of the entire cohort was 14.5 (IQR 8-96) days.
FIGURE 1.
Flowchart of the study population. We excluded nonhospitalized and nonconfirmed by real-time RT-PCR COVID-19 kidney recipients. ED, emergency department
Baseline characteristics of the study population are shown in Table 1. The mean age was 59.7 ± 12.48 years. The majority of the patients were male (55.7%) with a high prevalence of hypertension (85.6%); 35.6% of the cohort were treated with angiotensin -converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs). Diabetes was present in 30.8%, and 15.4% of patients had previous pulmonary disease. The most frequent immunosuppressive drug used was tacrolimus in 85.5% of the cohort, and 19.3% of patients were maintained using a mTOR inhibitor–based strategy. The mean serum creatinine levels before admission were 158.6 ± 79.1 µmol/L.
TABLE 1.
Demographic and clinical characteristics of kidney transplant patients with coronavirus disease 2019 pneumonia
| Patient characteristics | |
| Age (y, mean ± SD) | 59.7 ± 12.48 |
| Sex: male/female (n, %) | 60/44 (55.7/42.3) |
| Race (n, %) | |
| Caucasian | 90 (86.5) |
| African/African American | 4 (3.8) |
| Latin American | 9 (8.7) |
| Asian | 1 (1) |
| Primary end-stage renal disease (n, %): | |
| Nephroangiosclerosis | 12 (11.5) |
| Diabetic nephropathy | 17 (16.3) |
| Glomerulonephritis | 30 (28.8) |
| Polycystic kidney disease | 13 (12.5) |
| Other | 11 (10.6) |
| Uncertain | 21 (20.2) |
| Comorbidities (n, %) | |
| Diabetes | 32 (30.8) |
| Arterial hypertension | 90 (86.5) |
| Obesity | 28 (26.9) |
| Pulmonary disease | 16 (15.4) |
| Heart disease | 31 (29.8) |
| Active neoplasm | 8 (7.7) |
| Lymphopenia before admission | 45 (43.3) |
| ACEI/ARB use (n, %) | 37 (35.6) |
| Nosocomial COVID-19 infection (n, %) | 15 (14.4) |
| Transplant characteristics | |
| Time after transplant <6 mo (n, %) | 15 (14.4) |
| Time (mo, median, IQR) | 59 (18-130) |
| Type of transplant (n, %) | |
| KT/combined transplanta | 100/4 (96.2/3.8) |
| First KT/repeat transplant | 88/16 (84.6/15.4) |
| Type of donor (n, %) | |
| Deceased/living | 90/14 (86.5/13.5) |
| Standard criteria/expanded criteriab | 48/42 (46.1)/ (40.3) |
| Induction therapy (n, %) | |
| None | 11 (10.6) |
| Rabbit antithymocyte globulin | 37 (35.6) |
| Basiliximab | 56 (53.8) |
| Maintenance therapy (n, %) | |
| TAC use | 89 (85.5) |
| Cyclosporine use | 3 (2.88) |
| mTOR inhibitor use | 20 (19.28) |
| MMF/MPA use | 87 (83.6) |
| Prednisone use | 96 (92.3) |
| Basal serum creatinine (µmol/L) (mean ± SD) | 158.6 ± 79.1 |
| Basal eGFR CKD-EPI (mL/min/1.73 m2) (mean ± SD) | 48.2 ± 21.9 |
| Initial clinical symptoms | |
| Cough (n, %) | 71 (68.3) |
| Dyspnea (n, %) | 38 (36.5) |
| Diarrhea (n, %) | 32 (30.8) |
| Myalgias (n, %) | 34 (32.7) |
| Fever (n, %) | 81 (77.9) |
Abbreviations: CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; MMF/MPA, mycophenolate mofetil or mycophenolic acid; mTOR, mammalian target of rapamycin; TAC, tacrolimus.
Multiorgan transplant: 1 pancreas–kidney and 3 liver–kidney.
Expanded criteria donor refer to older kidney donors (≥60 years old) or donors who are aged 50-59 years and have 2 of the following 3 features: hypertension, terminal serum creatinine >1.5 mg/dL, or death from cerebrovascular accident.
There were 14 nosocomial COVID-19 infections. Clinical characteristics and outcomes of individuals with nosocomial COVID-19 infection are presented in detail in Table S1.
The median time between appearance of symptoms and admission was 5 (IQR 2-10) days. The most frequent initial clinical manifestation was fever (77.9%), followed by: cough (68.3%), dyspnea (36.5%), myalgia (32.7%), and diarrhea (30.8%) (Table 1, Figure 2). Analytical parameters at the admission showed a general inflammatory status with elevation of lactate dehydrogenase (LDH) with a mean of 317.46 ± 147.44 IU/mL, C-reactive protein (CRP) of 78.7 mg/L (IQR 31.9-137.15), D-dimer of 614 ng/mL (IQR 400.75-1344.5), ferritin levels of 574.5 µg/L (IQR 309.75-933.5), and lymphopenia with a median of 650 cells/mm3 (IQR 400-1000). Seventeen patients (16%) were admitted without oxygen requirement or x-ray abnormalities.
FIGURE 2.
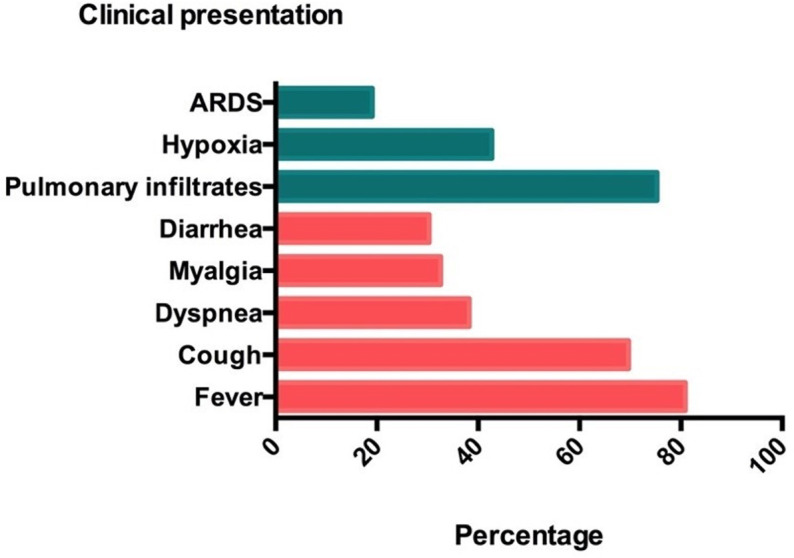
Clinical presentation of coronavirus disease 2019 pneumonia. Figure shows proportion of pulmonary and extrapulmonary manifestations at admission. ARDS, acute respiratory distress syndrome [Color figure can be viewed at wileyonlinelibrary.com]
3.1. ARDS
Oxygen supply was required at any time point in 85.6% of all the included patients, 54.8% met ARDS criteria, and 16.3% were treated with invasive and/or noninvasive ventilation (13.6% and 15.3%, respectively). The median time of appearance was 3 (IQR 3-6) days after admission (7 days after symptoms onset). Those who died presented ARDS before those who were alive at the end of follow-up (mean difference −1.44 days, P = .04). Patients with ARDS showed 11.4 times higher death risk than those without ARDS (95% CI 3.181-41.26, P < .001). Thirty-two of 58 patients who developed ARDS survived; among them, the mean time to resolve ARDS was 20.5 (IQR 14.2-30.7) days. The analysis of clinical and biological characteristics among patients with or without ARDS is shown in Table 2. By univariate analysis, we found an increased odd for obesity (OR 2.63, 95% CI 1.034-6.714, P = .04) and LDH at admission (OR 1.006, 95% CI 1.001-1.011, P = .01) and a decreased odds for PaFI/Spo2 (partial pressure of oxygen/fraction of inspired oxygen ratio) (OR 0.991, 95% CI 0.985-0.997, P = .005). No differences were found in terms of age, type of maintenance immunosuppression use, prevalence of previous lymphopenia, pulmonary disease, baseline graft function, or AKI for ARDS. The antiviral therapy did not impact on ARDS outcomes either.
TABLE 2.
Main clinical characteristics associated with patient death and acute respiratory disease distress syndrome
| Mortality | ARDS | |||||
|---|---|---|---|---|---|---|
| Alive (n = 76) | Death (n = 28) | P-value | No (n = 47) | ARDS (n = 57) | P-value | |
| Clinical characteristics | ||||||
| Age (y, mean ± SD) | 55 ± 11.4 | 70.8 ± 9.4 | <.001 | 57.8 ± 12.4 | 61.3 ± 13.2 | .16 |
| Sex (n, %): female/male | 31/45 (40.8/59.2) | 13/15 (46.4/53.6) | .60 | 21/26 (40.7/55.3) | 23/34 (40.4/59.6) | .65 |
| Race (n, %): Caucasian/other | 64/12 (84.2/15.8) | 27/1 (96.4/3.6) | .17 | 40/7 (85.1/14.9) | 51/6 (89.5/ 10.5) | .5 |
| Comorbidities (n, %) | ||||||
| Hypertension (n) (no/ACEI/ARB/other) | 10/6/21/38 (13/8/28/50) | 4/2/8/14 (14/7/28/50) | .98 | 7/5/14/20 (15/10/30.4/43.5) | 7/3/15/32 (12.3/5.3/23.3/56.1) | .51 |
| Diabetes | 21 (27) | 11 (39) | .25 | 12 (25.5) | 20 (35.1) | .29 |
| Obesity | 17 (22.4) | 11 (39.3) | .08 | 8 (17) | 20 (35.1) | .03 |
| Cardiac disease | 20 (19.4) | 11 (39.3) | .2 | 16 (34.8) | 15 (26.3) | .35 |
| Pulmonary disease | 6 (7.9) | 10 (35.7) | <.001 | 5 (10.6) | 11 (19.3) | .28 |
| Active cancer | 3 (3.9) | 5(17.9) | .03 | 3 (6.4) | 5 (8.8) | .64 |
| Lymphopenia before admission | 31 (41.3) | 14 (50) | .43 | 22 (47.8) | 23 (44.4) | .44 |
| Days from symptoms onset to admission (median, IQR)a | 7 (3-10) | 6 (4-10) | .76 | 7 (3-10) | 6 (4-10.7) | .77 |
| Initial symptoms (n, %) | ||||||
| Fever | 60 (78.9) | 21 (75) | .66 | 39 (83.1) | 42 (73.7) | .3 |
| Cough | 52 (68.4) | 19 (67.9) | .95 | 30 (63.8) | 41 (71.9) | .29 |
| Dyspnea | 21 (27.6) | 17 (60.7) | .002 | 9 (19) | 29 (50.9) | <.001 |
| Myalgia | 25 (32.9) | 9 (31.1) | .94 | 14 (29.8) | 20 (35.1) | .56 |
| Diarrhea | 22 (28.9) | 10 (35.7) | .50 | 12 (25.5) | 20 (35.1) | .29 |
| Nosocomial COVID-19 infection (n, %) | 6 (7.9) | 8 (28.6) | .01 | 6 (12.8) | 9 (15.8) | .66 |
| Initial Spo2 (%, mean ± SD) | 96.4 ± 2.4 | 94.8 ± 3.6 | .12 | 96.6 ± 2.2 | 95.3 ± 3.3 | .03 |
| Initial Spo2/Fio2 (mean ± SD) | 407.3 ± 97.3 | 353.2 ± 123.4 | .03 | 432.1 ± 76.6 | 357.4 ± 118.5 | .001 |
| Any radiography infiltrate initially (n, %) | 53 (69.7) | 23 (82.1) | .23 | 33 (70.2) | 43 (75.4) | .55 |
| Transplant characteristics | ||||||
| Type of transplant (n, %) | ||||||
| First kidney transplant | 65 (85.5) | 23 (82.1) | .76 | 41 (87.2) | 47 (82.5) | .50 |
| Type of donor (n, %) | ||||||
| Cadaveric | 62 (81.6) | 28 (100) | .01 | 37 (78.7) | 53 (93) | .04 |
| ECD | 22 (37.9) | 16 (66.7) | .02 | 13 (35.1) | 25 (55.6) | .06 |
| Induction therapy (n, %) | ||||||
| None | 8 (10.5) | 3 (10.7) | .88 | 4 (8.5) | 7 (12.3) | .73 |
| rATG | 26 (34.2) | 11 (39.3) | 16 (34) | 21 (36.8) | ||
| Basiliximab | 42 (55.3) | 14 (50) | 27 (57.4) | 29 (50.9) | ||
| Maintenance therapy (n, %) | ||||||
| TAC use | 66 (86.8) | 23 (82.1) | .54 | 40 (85.1) | 49 (86) | .9 |
| mTORi use | 22 (21.6) | 4 (3.9) | .19 | 15 (31.9) | 11 (20) | .1 |
| MMF/MPA use | 61 (80.3) | 26 (92.9) | .14 | 38 (80.9) | 49 (86) | .48 |
| Prednisone use | 71 (93.4) | 24 (85.7) | .21 | 44 (93.6) | 51 (89.5) | .4 |
| Time after transplant | ||||||
| <6 mo (n, %) | 6 (11.8) | 6 (21.4) | .21 | 5 (10.6) | 10 (17.5) | .40 |
| Time (mo, median, IQR) | 56.5 (20-130.5) | 71.6 (6-135) | .8 | 65 (24-133) | 57 (12.5-127) | .33 |
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; Fio2, fraction of inspired oxygen; MMF/MPA, mycophenolate mofetil or mycophenolic acid; mTORi, mammalian target of rapamycin inhibitors; rATG, rabbit antithymocyte globulin; SD, standard deviation; Spo2, blood oxygen saturation measured by pulse oximetry; TAC, tacrolimus.
Those who were hospitalized because of other reasons were excluded from the analysis.
3.2. Mortality
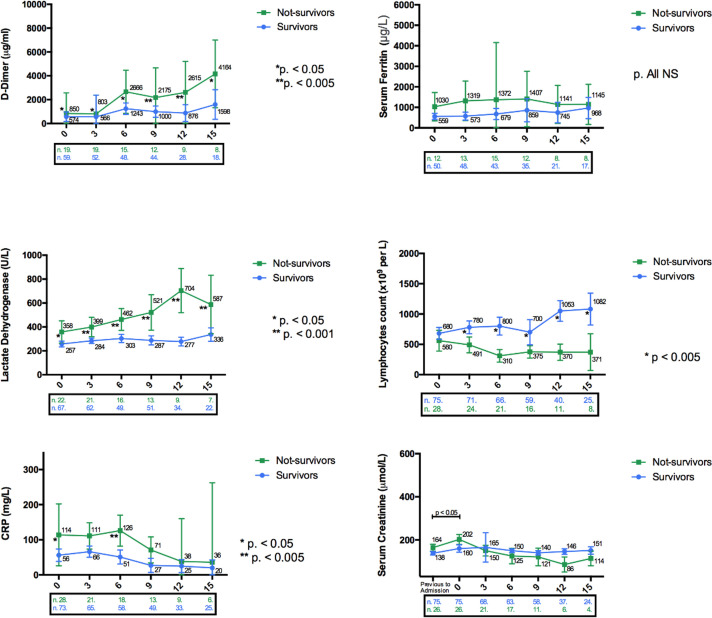
The overall mortality was 26.9%. All deaths were due to ARDS except one that was due to sudden death and another one that occurred after an aspiration pneumonia. We found that age was related to mortality with an HR of 1.101 (95% CI 1.057-1.157, P < .001). The mean age for those who survived was 55 ± 11.4 years, and for those who died, 70.8 ± 9.4 years (P < .001) (Table 2). There was also an increased risk of mortality for patients presenting ARDS at admission (HR 3.923, 95% CI 1.641-3.942, P = .002), patients with previous pulmonary disease, increased levels of LDH, CRP, and ferritin, and low lymphocyte count ( TABLE 2, TABLE 3, TABLE 4). Other significant differences in the evolution of analytical parameters between survivors and nonsurvivors are shown in Figure 3. In the multivariate Cox regression model, we found that age, ARDS, and higher baseline LDH were associated with increased risk of death (Table 4). No differences in terms of patient survival were found depending on baseline graft function, time after transplant, presence or absence of AKI, and type of maintenance immunosuppression used. None of the antiviral therapies used had any impact on patient survival.
TABLE 3.
Laboratory findings at the time of hospital admission among patient deaths and patients with or without acute respiratory disease distress syndrome
| No. of patients tested | Mortality | P-value | ARDS | P-value | |||
|---|---|---|---|---|---|---|---|
| Alive (n = 76) | Death (n = 28) | No (n = 47) | Yes (n = 57) | ||||
| Basal laboratory findings | |||||||
| Basal serum creatinine (µmol/L, mean ± SD) | 95 | 152.8 ± 77 | 170.5 ± 86 | .35 | 159.4 ± 74.2 | 155.1 ± 85.2 | .84 |
| Basal eGFR (mL/min/1.73 m2, mean ± SD) | 95 | 50 ± 19.8 | 48.3 ± 23.5 | .45 | 47.7 ± 23 | 48.9 ± 20 | .57 |
| Initial laboratory findings | |||||||
| Serum creatinine (µmol/L, median, IQR) | 95 | 160 (120-221.2) | 202 (143-164) | .08 | 167 (104-232) | 164.5 (124.5-164.5) | .67 |
| CK (IU/mL, median, IQR) | 32 | 59 (38.7-169.5) | 49.5 (31.7-129.5) | .54 | 50 (30-169) | 59 (38-140) | .77 |
| White blood cells (×103/cmm, mean ± SD) | 103 | 6 ± 2.6 | 6.9 ± 3.4 | .18 | 5.5 ± 2.4 | 6.8 ± 3.2 | .032 |
| Hemoglobin (g/dL) | 103 | 12.2 ± 1.94 | 11.5 ± 2.0 | .1 | 12.1 ± 1.9 | 12.0 ± 2.0 | .97 |
| Platelets (×103/cmm) | 103 | 172 ± 68 | 186 ± 75 | .39 | 168 ± 64 | 182 ± 73 | .32 |
| Lymphocytes (cells/mm, median, IQR) | 103 | 680 (400-1000) | 560 (325-711) | .14 | 690 (400-910) | 600 (400-1000) | .68 |
| D-dimer (ng/mL, median, IQR) | 78 | 574 (324-1081) | 850 (610-2599) | .004 | 606.5 (288-1337.5) | 626.5 (424.2-1375.7) | .25 |
| ALT (IU/mL, median, IQR) | 94 | 23.5 (15-35.5) | 18.5 (11.5-27.5) | .06 | 21 (16-31) | 22 (13-39) | .73 |
| LDH (IU/mL, median, IQR) | 89 | 257 (212-332) | 358.5 (258-522.5) | .001 | 255 (203-317.5) | 278.5 (242.2-448.2) | .007 |
| CRP (mg/L, median, IQR) | 101 | 56 (27.3-132) | 114-2 (62.5-199.5) | .006 | 62.8 (22.5-114.8) | 87 (44.5-153.7) | .07 |
| Serum ferritin (pg/L, median, IQR) | 62 | 559.5 (301.7-812.7) | 1030 (350.5-1952) | .13 | 478 (301.7-932) | 631 (330.5-1140) | .47 |
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; CK, creatinine kinase; eGFR, estimated glomerular filtration rate measured by CKD-EPI; LDH, lactate dehydrogenase; cmm, per cubic millimeter of whole blood; CRP, C-reactive protein.
TABLE 4.
Risk factors associated with mortality in kidney transplant patients hospitalized for COVID-19
| Univariate HR (95% CI) | P-value | Multivariate HR (95% CI) | P-value | |
|---|---|---|---|---|
| Age | 1.101 (1.057-1.157) | <.001 | 1.103 (1.048-1.162) | <.001 |
| ARDS day 0 | 3.923 (1.641-3.942) | .002 | 2.091 (1.031-8.233) | .044 |
| Pulmonary disease | 2.891 (1.311-6.392) | .009 | 1.544 (0.592-4.026) | .375 |
| LDH day 0 | 1.004 (1.002-1.006) | <.001 | 1.003 (1-1.005) | .024 |
| Day 3 | 1.003 (1.000-1.006) | .016 | ||
| Day 9 | 1.002 (1.000-1.004) | .03 | ||
| Day 15 | 1.004 (1.000-1.007) | .026 | ||
| CRP day 0 | 1.003 (1.002-1.005) | <.001 | — | |
| Ferritin day 0 | 1.001 (1.000-1.001) | .056 | — | |
| Lymphocytes | ||||
| Day 6 | 0.998 (0.996-1) | .018 | — | |
| Day 9 | 0.997 (0.995-0.999) | .007 | ||
| Day 12 | 0.997 (0.995-0.999) | .014 | ||
Note: Adjusted and nonsignificant for sex; race; repeat transplant; induction therapy; maintenance immunosuppression without mTOR inhibitors; heart disease; AKI stage 3 vs others; AKI 2 and 3 stages vs others; hypertension; use of ACEi/ARB; diabetes; obesity; basal lymphopenia; lymphocyte days 0, 3, and 15; serum creatinine; white blood cells; hemoglobin; D-dimer; ALT; LDH days 6-12; ferritin rest of the days; platelets; tacrolimus levels; diarrhea at admission; fever at admission; cough at admission; myalgia at admission; anticoagulation; time after transplant; days from symptoms onset to admission; nosocomial infection.
Abbreviations: ARDS, acute respiratory distress syndrome; CRP, C-reactive protein; CI, confidence interval; LDH, lactate dehydrogenase; HR, hazard ratio.
FIGURE 3.
Dynamic profile of laboratory markers in kidney transplant recipients with COVID-19. Differences between survivors and non survivors are shown; significant differences are indicated. Box below each graph detail the number of patient at risk and/or availability of the test. CRP, C-reactive protein [Color figure can be viewed at wileyonlinelibrary.com]
Mortality was different depending on patient baseline characteristics. Of the total cohort, 23.1% of patients needed to be admitted to the intensive care unit and 15 of 24 (62.5%) died. Of the 17 patients who were admitted without oxygen requirement and no x-ray abnormalities, 8 (47%) progressed to ARDS, 4 (23%) needed ICU admission, 6 (46%) developed AKI, and 1 patient (5.9%) died. Of these 17 patients, those eventually requiring ICU admission presented a significant rapid increase in their CRP levels until day 6 after admission, unlike patients who did not need ICU admission (Figure S1). Furthermore, patients with COVID-19 nosocomial infection had a high mortality rate of 57.14% (8/14) (Table S1).
3.3. Acute kidney injury
AKI was present in 47% of the included cohort ( Table 5). Four patients were excluded from this analysis. The majority of patients presented with AKI stage 1 (30%). No differences in terms of age or antiviral use were found. Interestingly, AKI stage 3 presented a higher median tacrolimus through levels compared with other AKI stage patients (P < .001). Mortality was higher in AKI stage 3 patients compared with the rest of the cohort (P < .05), although in Cox regression analysis, the presence of AKI at any stage or AKI stage 3 compared to no AKI was not a risk factor associated with death or ARDS. There were no acute graft rejection episodes during the follow-up.
TABLE 5.
Acute kidney injury stages according to KDIGO definition and clinical outcomes
| NO AKI (n = 53) | AKI stage 1 (n = 30) | AKI stage 2 (n = 7) | AKI stage 3 (n = 10) | |
|---|---|---|---|---|
| Age (y, mean ± SD) | 60.4 ± 13 | 56.3 ± 13 | 63.4 ± 10 | 64 ± 11 |
| Tacrolimus levels day 6 (ng/mL, median, IQR)a | 5.6 (3.3-8.5) | 6.6 (4.7-10.1) | 10.4 (6.7-22.8) | 24.3 (16.9-44)** |
| Antiviral use (n, %) | 23 (43.4) | 18 (60) | 3 (42.9) | 6 (60) |
| ARDS (n, %) | 25 (47.2) | 18 (60) | 6 (85.7) | 6 (60) |
| Death (n, %) | 12 (22.6) | 6 (20) | 3 (42.9) | 6 (60)*** |
Note: Antiviral use included lopinavir/ritonavir-darunavir/ritonavir or darunavir/cobicistat use. Four patients were excluded from the analysis: 2 with delayed graft function in dialysis after kidney transplant and 2 because were their basal eGFR was inferior to 10 mL/min before admission (one pending to start hemodialysis and the other with obstructive AKI due to lymphocele).
Abbreviations: AKI, acute kidney injury; AKI stage 1, rise in serum creatinine ≥26.5 μmol/L in 48 h or rise 1.5-1.9 times from baseline; AKI stage 2, rise in serum creatinine 2.0-2.9 times from baseline; AKI stage 3, rise in serum creatinine 3 times from baseline or increase in serum creatinine to ≥353.6 μmol/L or initiation of renal replacement therapy irrespective of serum creatinine; ARDS, acute respiratory distress syndrome.
Number of patients with data of tacrolimus levels available: no AKI = 20, AKI stage 1 = 18, AKI stage 2 = 5; AKI stage 3 = 4.
P < .001 no AKI vs AKI stage 1.
P < .05 no AKI vs AKI stage 1.
3.4. Immunosuppression, other treatments, and safety endpoints
At least 1 immunosuppressive drug was withdrawn in 91.3% of patients (Table S2). Intravenous steroid treatment (methylprednisolone 0.5-1 mg/kg/d) was used in 52.9% of cases. CNI withdrawal was higher in patients who developed ARDS (P = .018), as well as in patients taking an mTOR inhibitors (P = .028). We did not find any relationship between type of immunosuppression modification and mortality.
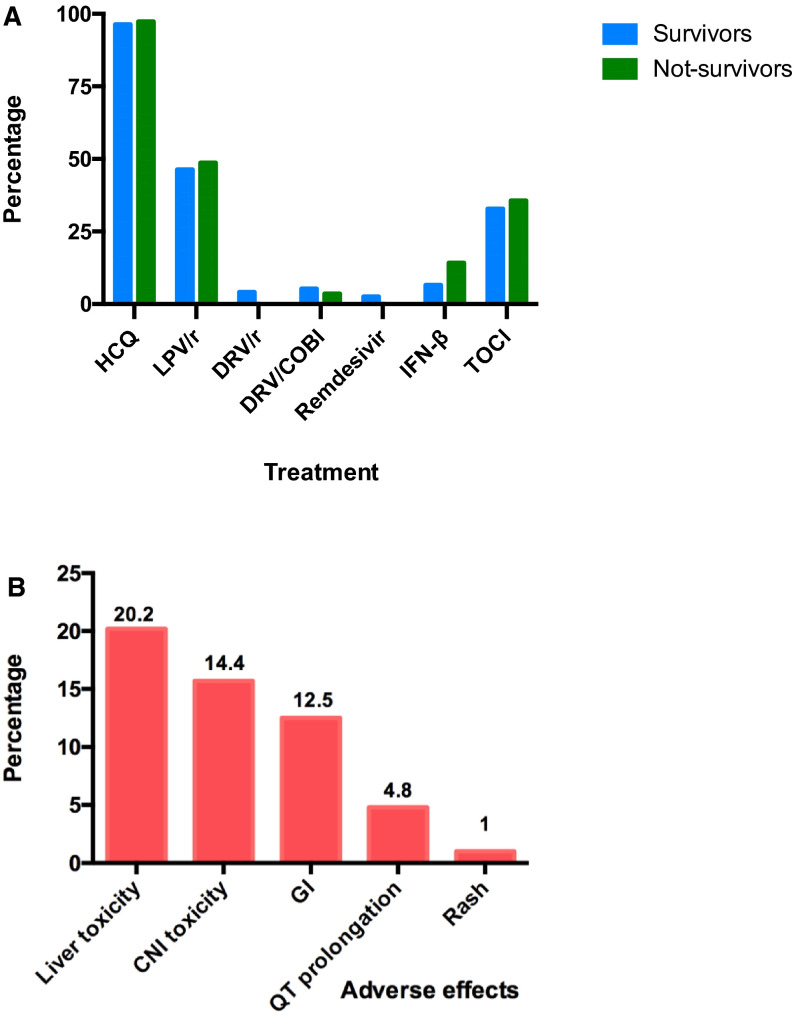
Regarding anti–COVID-19 therapies, different drugs were used ( Figure 4A). Hydroxychloroquine was given to 97.1% and lopinavir/ritonavir to 48.1% of patients. Azythromycin was used in 63.5% of patients. None of these strategies showed any impact on mortality or ARDS, except interferon-β1a or tocilizumab, which were associated with worse outcomes for ARDS (Table S2). Importantly, these investigational treatments were related to 28.8% incidence of adverse effects such as hepatitis (20.2%), tacrolimus toxicity (14.4%), QT prolongation (observed in 5 patients), or gastrointestinal (12.5%) (Figure 4B).
FIGURE 4.
A, Proportion of antiviral therapies use and associated adverse effects. Distribution among survivors and nonsurvivors is shown. B, Associated adverse effects. AR, acute graft rejection; CNI, calcineurin inhibitor; DRV/r, darunavir/ritonavir; DRV/COBI, darunavir/cobicistat; GI, gastrointestinal; HCQ, hydroxychloroquine; IFN-β, interferon-beta; LPV/r, lopinavir/ritonavir; TOCI, tocilizumab [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In early 2020, Spain emerged as one of the most affected countries by the COVID-19 pandemic.12 This situation forced the discontinuation of many transplant programs worldwide.13 Transplant units faced a significant number of infected recipients without evidence-based strategies and many uncertainties regarding the clinical course and prognosis of this novel infection. Here, we report the clinical characteristics and risk factors associated with the development of ARDS and death in a cohort of 104 consecutive kidney transplant patients hospitalized for COVID-19 infection in 5 different Spanish centers.
In agreement with previous reports of an immunocompetent infected population, the most common symptom reported at admission was fever,3 although one-third of patients were admitted with gastrointestinal complaints, as already described in other transplant reports.14 X-ray abnormalities preceded hypoxemia onset, which accounts for the natural history of pulmonary involvement on the general population.4
ARDS is considered a severe form of COVID-19 infection and entails greater mortality risk,2 which was also confirmed in our cohort. Half of our COVID-19 cohort progressed to ARDS, and 50% of them had a fatal outcome. Recently, case report series of in-hospital kidney and other solid organ transplants described similar ARDS incidence.14 , 15 Early observations among hospitalized general population reported a 41.8% ARDS incidence,2 which is in line with our results with KTs. However, in our cohort no age differences were described among patients with and without ARDS, contrary to immunocompetent published cohorts.2
It has been suggested that KTs encompass a susceptible group for aggressive manifestations of COVID-19 infection7 , 16 due to the ongoing immunosuppression. In our current study, we report an overall mortality rate of 26.9% in consonance with recent reports on kidney and other solid organ transplant patients showing similar fatality rates, ranging from 6% to 30%.7 , 9 , 14 , 16 General population fatality rates were initially described as 2.3% in China,17 whereas in Spain, it has reached around 10%.12 It should be emphasized, though, that these figures relate to both hospitalized and nonhospitalized infected patients. Hence, since published kidney transplant cohorts are mainly composed of hospitalized individuals, these comparisons might be inaccurate. Furthermore, admission criteria are likely to differ between solid organ recipients and the general population (in fact, 16% of our patients were admitted without pneumonia or hypoxia in our cohort). Nonetheless, recently accepted for publication OpenSAFELY trial suggests a higher HR for mortality among solid organ recipients.18
In terms of AKI, nearly half of our patients developed renal dysfunction, according to recently published kidney transplant cohorts.15 , 19 AKI occurrence in general population studies ranges from 5% to 10%20 , 21; therefore, KTs entail a group of risk for this complication.
The etiology of AKI in patients with COVID-19 remains elusive, and several conditions might act as major contributors, beyond the virus in itself.22 As a matter of fact, AKI severity was related to tacrolimus trough levels, especially in those with the most severe disfunction (AKI stage 3). No relationship was found between COVID-19 severity and AKI in our cohort, and no associations with mortality were identified.
A relevant concern derived from SARS-CoV-2 transmissibility is the presymptomatic disease stage,23 thereby resulting in health care professionals’ contagion and nosocomial patient infection. Fourteen patients were infected within our facilities, with 8 deaths in this group. Most nosocomial infections occurred at the beginning of the pandemic, and all these patients were admitted before the implementation of measures of isolation. Taking into account the inherent limitations of this sample size, these outcomes might be explained by the intrinsic morbidity associated with the ongoing admission in itself.24 It is of utmost importance to assess the benefits and potential consequences of admission amid this pandemic, which have become one of the reasons for decreased transplant activity in our country in the past months.13
The vast majority of our patients had at least 1 of their immunosuppressants withdrawn, in consonance with already published works.9 , 16 Mycophenolate mofetil was the most frequently withdrawn medication, regardless of infection severity. In contrast, CNI and mTOR inhibitors were withheld more frequently in the ARDS group, restricting this strategy to those patients with severe pulmonary involvement.
Steroid withdrawal was, however, exceptional, and its administration as intravenous treatment was used in more than half (52%) of patients. Our study reports cases detected in the early phase of the COVID-19 pandemic, when the efficacy of anti-inflammatory therapies such as steroids was speculative. Thus, in our cohort, steroidal use was mainly reactive to clinical worsening, to ensure immunosuppression after CNI, mTOR inhibitor, and antimetabolite withdrawal. However, recently published results from the RECOVERY trial25 have shown that dexamethasone use reduced mortality in severe COVID-19 cases in the general population, which might support, to some extent, our adopted strategy.
In terms of antiviral treatments, the World Health Organization10 claimed that there is no existing evidence to recommend any treatment in this regard. However, the use of compassionate treatments has become a widespread practice during the pandemic. Accordingly, a high proportion of our cohort was treated with some of these drugs (Table S1). We did not find any differences in terms of outcomes among different treatments, although our study does not allow, by nature, this type of analysis.
Initial reports suggested that the combination of hydroxychloroquine and azithromycin might provide superior viral clearance and improved clinical outcomes, despite significant limitations in its design.26 However, one of the major concerns about these therapies combinations is cardiotoxicity.27 In fact, QT prolongation was recorded in 5 individuals, of whom 1 had sudden death while presenting with a mildly symptomatic COVID-19 case that was treated with hydroxychloroquine and azithromycin. Moreover, recently published data from large trials show the absence of clinical benefit from the use of hydroxychloroquine in COVID-19 patients. Because of the current available data,25 , 28 along with the adverse effects reported in our cohort, we advise against the use of hydroxychloroquine in COVID-19 KTs.
On the other hand, more than half of our patients received protease inhibitors as adjunctive therapy, resulting in 15.7% of tacrolimus intoxications. Additionally, severe AKI were significantly prevalent among those patients exhibiting tacrolimus overexposure. Thus, given the lack of evidence supporting its use28 and the concurrent risk of the above-mentioned adverse effects, we support the idea that the use of investigational anti–COVID-19 therapies must be restricted to randomized controlled trials, as it has recently shown in a randomized controlled trial of remdesivir that resulted in U.S. Food and Drug Administration approval.29
At present, there are no available data in terms of risk stratification in KTs affected by COVID-19. As aforementioned, significant rates of COVID-19 progression among patients without pneumonia nor hypoxemia at admission were observed. Therefore, given the unpredictable clinical course of this infection, discharge criteria should differ from the general population at early stages regardless of age, and a strict follow-up must be provided if an outpatient approach is agreed on.
Despite this, we were able to identify certain risk factors for ARDS and death among KTs. We found that obesity was independently associated with ARDS. Likewise, in the 2009 H1N1 pandemic, an association between hospitalization and obesity was described.30 Interestingly, although we did not identify older age as a risk factor for ARDS, it was certainly associated with mortality. These data suggest that ARDS might develop indistinctly among young and old KTs; however, once it is established, the elderly would be at most risk for death. Likewise, the preexisting pulmonary disease did not confer additional risk for ARDS development in our cohort, but it was associated (in the univariate analysis) with mortality.
Among laboratory markers, our analysis showed that higher LDH levels at admission were associated with increased odds for both ARDS and death, which might be useful to identify the KTs at higher risk from the admission.19
We have to acknowledge some limitations in our study. First, our cohort is not representative of the whole kidney transplant population, because outpatient individuals were not included. Second, we did not consider postdischarge follow-up data; therefore, long-term conclusions cannot be drawn. On the other hand, biochemical data (CRP, D-dimer, ferritin) were not available for all established time-points, which may undervalue their association with the main outcomes. Last, our findings might be limited and our results underpowered because of the small sample size. As far as we are concerned, however, this is one of the largest published cohorts of COVID-19 infection of a homogeneous cohort of KTs. Additionally, the exclusive inclusion of patients with definite outcomes in our analysis provides more reliable and clearer information regarding these population outcomes.
In conclusion, older age, obesity, and pulmonary disease, along with high baseline LDH levels at presentation and ARDS, were associated with poorer outcomes in KTs affected by COVID-19. Half of our population developed ARDS, even those without pneumonia at admission. In terms of pharmacologic strategies, steroids arose as the most commonly used antirejection drug during the infection, especially in severe forms, whereas compassionate anti–COVID-19 treatments lead to remarkable rates of adverse effects. A larger study with a longer-term follow-up for COVID-19 transplant recipients could answer some of the remaining questions, particularly concerning the treatment, long-term prognosis, and most suitable strategy in terms of immunosuppression management in this scenario.
ACKNOWLEDGMENTS
We thank CERCA Program/Generalitat de Catalunya and the ISCIII RETICS RedinRen RD16/0009/0003 for institutional support. We are grateful to all health coworkers from the Hospitals involved in this study. As the first line defense against COVID-19 pandemic, they faced a very stressful situation in an environment made of uncertainty. We are aware that without their efforts this study could not be realized. Our thoughts are with all transplant recipients affected by COVID-19 and their families.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
AUTHOR CONTRIBUTIONS
FA: designed the study, collected the data, analyzed the data, interpreted the data, drafted the article and revised the article critically. MN: designed the study, collected the data, analyzed the data, interpreted the data, drafted the article and revised the article critically. CD: collected the data and revised the article critically. TN: collected the data and revised the article critically. CJ: collected the data and revised the article critically. VA: collected the data and revised the article critically. CA: collected the data and revised the article critically. MM: collected the data and revised the article critically. MA: collected the data and revised the article critically. SJ: collected the data and revised the article critically. TI: collected the data and revised the article critically. GR: collected the data and revised the article critically. FC: collected the data and revised the article critically. LI: collected the data and revised the article critically. VP: collected the data and revised the article critically. CF: collected the data and revised the article critically. TV: collected the data and revised the article critically. PM: revised the article critically. MF: revised the article critically. SD: revised the article critically. OF: revised the article critically. OB: revised the article critically. CJM: interpreted the data and revised the article critically. ME: designed the study, collected the data, analyzed the data, interpreted the data, drafted the article and revised the article critically.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information CERCA Program/Generalitat de Catalunya; ISCIII RETICS RedinRen, Grant/Award Number: RD16/0009/0003
Footnotes
Alexandre Favà and David Cucchiari contributed equally to this work.
Francesc Moreso and Edoardo Melilli are co-senior authors.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Supplementary Material
REFERENCES
- 1.Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Informe de Diálisis y Trasplante. 2018. https://www.senefro.org/contents/webstructure/SEN_2019_REER_modificada.pdf. Accessed May 2, 2020
- 6.Informe 5 (18 marzo - 25 abril) Registro COVID-19. https://mailchi.mp/senefro/registro-epidemiolgico-vhc-vhb-vih-1314562. Accessed May 2, 2020
- 7.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97(6):1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu L, Xu X, Ma KE, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20(7):1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Columbia University Kidney Transplant Program Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31(6):1150–1156. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical management of severe acute respiratory infection when COVID-19 is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed May 2, 2020
- 11.KDIGO Clinical Practice Guideline for Acute Kidney Injury. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf. Accessed May 2, 2020
- 12.Situación de COVID-19 o Coronavirus en España. https://covid19.isciii.es/. Accessed May 2, 2020
- 13.Domínguez-Gil B, Coll E, Fernández-Ruiz M, et al. COVID-19 in Spain: transplantation in the midst of the pandemic [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.15983 [DOI] [PMC free article] [PubMed]
- 14.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20(7):1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bossini N, Alberici F, Delbarba E, et al. Kidney transplant patients with SARS-CoV-2 infection: the brescia renal COVID task force experience [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.16176 [DOI] [PMC free article] [PubMed]
- 16.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 18.Williamson EJ, Walker AJ, Bhaskaran K, et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020;1-11: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed]
- 19.Cravedi P, Mothi SS, Azzi Y, et al. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium [published online ahead of print 2020]. Am J Transplant. 10.1111/ajt.16185 [DOI] [PMC free article] [PubMed]
- 20.Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31(6):1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delsante M, Rossi GM, Gandolfini I, Bagnasco SM, Rosenberg AZ. Kidney Involvement in COVID-19: need for better definitions. J Am Soc Nephrol. 2020. 10.1681/ASN.2020050630 [DOI] [PMC free article] [PubMed]
- 23.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 24.Pascual J, Melilli E, Jiménez-Martín C, et al. COVID-19–related mortality during the first 60 days after kidney transplantation. Eur Urol. 2020. 10.1016/j.eururo.2020.06.036 [DOI] [PMC free article] [PubMed]
- 25.Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. https://www.recoverytrial.net/files/recovery_dexamethasone_statement_160620_v2final.pdf. Accessed July 13, 2020.
- 26.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solidarity clinical trial for COVID-19 treatments. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments. Accessed July 13, 2020.
- 29.Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment | FDA. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed May 4, 2020.
- 30.Dietz W, Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity. 2020;28(6):1005. doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.