Abstract
Patients waitlisted for and recipients of solid organ transplants (SOT) are perceived to have a higher risk of contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and death; however, definitive epidemiological evidence is lacking. In a comprehensive national cohort study enabled by linkage of the UK transplant registry and Public Health England and NHS Digital Tracing services, we examined the incidence of laboratory-confirmed SARS-CoV-2 infection and subsequent mortality in patients on the active waiting list for a deceased donor SOT and recipients with a functioning SOT as of February 1, 2020 with follow-up to May 20, 2020. Univariate and multivariable techniques were used to compare differences between groups and to control for case-mix. One hundred ninety-seven (3.8%) of the 5184 waitlisted patients and 597 (1.3%) of the 46 789 SOT recipients tested positive for SARS-CoV-2. Mortality after testing positive for SARS-CoV-2 was 10.2% (20/197) for waitlisted patients and 25.8% (154/597) for SOT recipients. Increasing recipient age was the only variable independently associated with death after positive SARS-CoV-2 test. Of the 1004 transplants performed in 2020, 41 (4.1%) recipients have tested positive for SARS-CoV-2 with 8 (0.8%) deaths reported by May 20. These data provide evidence to support decisions on the risks and benefits of SOT during the coronavirus disease 2019 pandemic.
KEYWORDS: clinical research/ practice, complication: infectious, epidemiology, health services and outcomes research, infection and infectious agents - viral, infectious disease, organ transplantation in general, patient survival, registry/ registry analysis
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; NHSBT, National Health Service Blood and Transplant; ONS, Office of National Statistics; PHE, Public Health England; SARS-CoV-2, severe acute respiratory syndrome coronavirus-type 2 pathogen; SOT, solid organ transplant; UK, United Kingdom
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus-type 2 pathogen (SARS-CoV-2), the causative agent for coronavirus disease 2019 (COVID-19), was first diagnosed in the United Kingdom (UK) on January 29, 2020. By May 16, >240 000 had tested positive for infection with more than 34,000 deaths due to COVID-19.1 Demographic factors such as sex, age, ethnicity, and comorbidity (eg, diabetes, obesity) were associated with higher mortality in the UK population.2, 3, 4
Solid organ transplant (SOT) recipients or patients waitlisted for such transplants were considered to be at high risk of infection or poor outcomes following COVID-19 due to proportionately higher rate of underlying comorbidities, the need for frequent contact with medical care settings, and requirement for systemic immunosuppression. On March 22, 2020, Public Health England (PHE) issued guidance classifying SOT recipients as “clinically extremely vulnerable” and advised additional nonpharmaceutical interventions to reduce the risk of SARS-CoV-2 infection such as “shielding,” where patients undergo strict self-isolation even within multiperson households.5
Single-center or regional experiences describing COVID-19 mortality rates in transplant recipients have been published.6, 7, 8, 9, 10, 11, 12, 13, 14, 15 While such reports provide a limited assessment of the impact of COVID-19 on SOT recipients, inclusion of a large “at risk” population with a high degree of data completeness is essential to more accurately quantify risk. Furthermore, there is a paucity of data on the impact of the pandemic on patients waitlisted for organ transplantation. This is the clinically most appropriate risk comparator group for SOT recipients, rather than the general population, because they share more similar comorbidity and risk profiles for SARS-CoV-2 infection and associated mortality. Availability of high-quality data on outcomes following SARS-CoV-2 infection in waitlisted vs SOT recipients is essential for clinicians and patients to make informed decisions, especially as transplant centers consider re-opening or restoring transplant activity back to prepandemic activity levels.
We report on the first national registry-based cohort analysis describing the incidence of SARS-CoV-2 infection and all-cause mortality in both patients waitlisted for transplants and SOT recipients.
2. MATERIALS AND METHODS
2.1. Study population
The at-risk cohorts of interest were all patients resident in England who were either active on the wait-list for any deceased donor SOT, or were a SOT recipient with a functioning graft, as of February 1, 2020. Patients who were suspended on the wait-list on that date or recipients of tissue or cell transplants (eg, cornea, sclera, bone, bone marrow, hepatocyte) were not included. Patients who were on the wait-list on February 1 but were subsequently transplanted before May 20, 2020 were classified within the SOT recipient group. Patients on the wait-list for, or recipients of, islet transplants were reported jointly with solid organ pancreas-alone transplants. Any recipient of more than 1 simultaneous organ transplant (other than simultaneous pancreas-kidney transplants) was defined as receiving a multi-organ transplant.
In both cohorts, the outcome variables of interest were (1) laboratory-confirmed SARS-CoV-2 infection and (2) among those positive for SARS-CoV-2, vital status, and date of death if applicable. Patients were followed for outcomes of interest from February 1 to May 20, 2020.
Testing for SARS-CoV-2 was performed using PHE-validated, real time-polymerase chain reaction test kits with a probe specific for B-betacoronavirus (target E gene) and SARS-CoV-2 (target S gene). This enabled parallel detection of B-betacoronavirus and SARS-CoV-2-specific RNA.
2.2. Study design and data sources
This was a national cohort study enabled by linkage of 3 national registries in England. National Health Service Blood and Transplant (NHSBT) database is the central repository that holds records, including identifiable information and demographics, on all patients waitlisted for a deceased donor SOT or in receipt of a SOT in the United Kingdom. The NHSBT database includes data on patients from all 4 nations in the United Kingdom (population ≈66 million) with the majority of the population in England (population ≈56 million). Access to a valid NHS number, a unique identifier for all patients in England with care provided on the NHS, enabled accurate data linkage. Forty-seven (0.9%) of the 5 184 waitlisted patients and 803 (1.7%) of the 46 789 SOT recipients had a missing NHS number and were excluded from outcome analyses. The at-risk cohorts were identified from the NHSBT database.
COVID-19 is designated as a “notifiable disease” and Public Health England (PHE) centrally collects reports on all patients living in England who test positive for SARS-CoV-2 under the Health Protection Regulations 2010, with a statutory requirement to report within 3 days. Merger of the at-risk cohorts identified from the NHSBT dataset with the PHE database was performed using 2 unique identifiers (NHS number and date of birth).
The NHS Digital Tracing Service records the vital status of all patients under the care of the NHS in England. The service records date of death either from hospital admission care episodes (for in-hospital deaths) or by linkage with the UK Office for National Statistics for deaths occurring outside the hospital. The NHS Digital Tracing Service only records date of death and does not collect information on cause of death. The death data reported in this study describes all-cause mortality and does not exclusively describe COVID-19-associated mortality.
The merged NHSBT and PHE dataset comprising all laboratory confirmed SARS-CoV-2 infected waitlisted patients or SOT recipients in England was securely linked with the NHS Digital Tracing Service using 3 unique identifiers (NHS number, date of birth, and sex). The final dataset therefore had near real-time, complete mortality information on all waitlisted and transplanted patients who tested positive for SARS-CoV-2 in England.
2.3. Statistical analysis
Demographic characteristics (type of organ awaited/received, sex, age, ethnicity, and NHS region) were summarized for waitlisted patients, SARS-CoV-2-positive wait-list patients, SARS-CoV-2-positive wait-list patient deaths, SOT recipients, SARS-CoV-2-positive SOT recipients, and SARS-CoV-2-positive SOT recipient deaths. In addition, type of donor and year of transplant were also summarized for SOT recipients, SARS-CoV-2-positive SOT recipients, and SARS-CoV-2-positive SOT recipient deaths.
Differences in characteristics for SOT recipients vs waitlisted patients were tested univariately using the χ2 and Kruskal-Wallis tests.
Cumulative number of SOT recipients and waitlisted patients testing positive for SARS-CoV-2 and subsequent all-cause mortality in England was compared to the general English population using data provided by the UK Government (https://coronavirus.data.gov.uk/).
Kaplan-Meier estimates of patient survival for those testing positive for SARS-CoV-2 were compared for SOT recipients vs waitlisted patients using the log-rank test.
Multiple logistic regression analyses were used to identify factors associated with testing positive for SARS-CoV-2 in the SOT cohort and factors associated with SARS-CoV-2-positive recipient deaths in the SARS-CoV-2-positive SOT cohort. Variables included in the multivariable models were type of organ received, sex, age, ethnicity, NHS region, type of donor, and year of transplant. P values of <0.1 were judged to be significant. The multivariable analysis excluded 25 recipients with missing sex data.
Analyses were undertaken using SAS version 9.4 (SAS Institute Inc, Cary, NC).
2.4. Ethical approval
The analyses described in this article were carried out at NHSBT, who maintain the UK Transplant registry on behalf of transplant centers in the United Kingdom. NHSBT are reliant on the General Data Protection Regulation Article 6(1)(e) – Performance of a public task. This lawful basis requires specific exemptions under Article 9(2)(h) and also (i) and (j). This allows NHSBT to use patient identifiable information for service evaluation without the consent of patients.
3. RESULTS
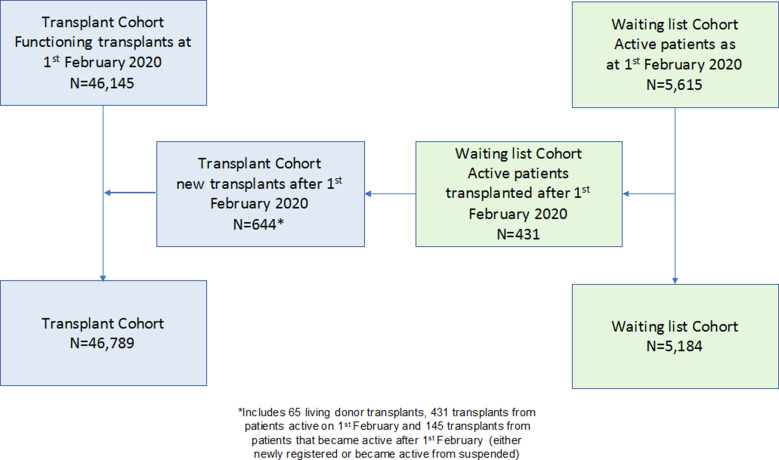
On February 1, 2020 there were 46 145 SOT recipients alive with a functioning graft and 5 615 patients on the active wait-list for a deceased donor SOT. Six hundred forty-four patients received a SOT between February 1 and May 20, 2020, 431 of whom were active on the wait-list on February 1, 2020, leaving 46 789 SOT recipients and 5 184 waitlisted patients for analysis ( Figure 1). The majority of recipients had received a kidney-only transplant (69.5%), and most patients were waiting for a kidney-only transplant (79.0%) ( Table 1). The demographic characteristics of SOT recipients and waitlisted patients are also shown in Table 1. The median (interquartile range [IQR]) age of recipients was 56 (43-65) years vs 53 (41-61) years in the waitlisted cohort. SOT recipients were more likely to be older (P = .001), white (P < .001), and have a history of liver disease (P = .001) than those patients on the wait-list (Table 1). The majority (66.9%) of recipients had their transplants prior to 2016, with 1004 (2.1%) being transplanted in 2020 up to May 20 ( Table 2).
FIGURE 1.
Flowchart of study cohorts [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Organ type and demographic characteristics of solid organ transplant recipients with a functioning graft and patients on the active waiting list on February 1, 2020 in England, and SARS-CoV-2 infections and subsequent mortality between February 1 and May 20, 2020
| Recipients | SARS-CoV-2 + recipients | SARS-CoV-2 + recipient deaths | Waiting list patients | SARS-CoV-2 + wait-list patients | SARS-CoV-2 + wait-list patient deaths | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | % of recipients | N | % of cases | % of recipients | N | % | N | % | % of wait-list | N | % of cases | % of wait-list | |
| Organ type | ||||||||||||||||
| Kidney | 32 503 | 69.5 | 470 | 78.7 | 1.4 | 124 | 26.4 | 0.4 | 4096 | 79.0 | 179 | 90.9 | 4.4 | 18 | 10.1 | 0.4 |
| SPK | 1469 | 3.1 | 19 | 3.2 | 1.3 | 4 | 21.1 | 0.3 | 145 | 2.8 | 9 | 4.6 | 6.2 | — | — | — |
| Pancreasa | 251 | 0.5 | 3 | 0.5 | 1.2 | — | — | — | 21 | 0.4 | — | — | — | — | — | — |
| Liver | 8734 | 18.7 | 64 | 10.7 | 0.7 | 13 | 20.3 | 0.1 | 325 | 6.3 | 6 | 3.0 | 1.8 | 1 | 16.7 | 0.3 |
| Heart | 2194 | 4.7 | 23 | 3.9 | 1.0 | 6 | 26.1 | 0.3 | 270 | 5.2 | — | — | — | — | — | — |
| Lung | 1318 | 2.8 | 13 | 2.2 | 1.0 | 6 | 46.2 | 0.5 | 283 | 5.5 | 2 | 1.0 | 0.7 | 1 | 50.0 | 0.4 |
| Intestinal | 121 | 0.3 | 2 | 0.3 | 1.7 | — | — | — | 17 | 0.3 | 1 | 0.5 | 5.9 | — | — | — |
| Multi-organ | 191 | 0.4 | 3 | 0.5 | 1.6 | 1 | 33.3 | 0.5 | 27 | 0.5 | — | — | — | — | — | — |
| Sex | ||||||||||||||||
| Male | 28 372 | 60.6 | 387 | 64.8 | 1.4 | 107 | 27.6 | 0.4 | 3027 | 58.4 | 121 | 61.4 | 4.0 | 15 | 12.4 | 0.5 |
| Female | 18 392 | 39.3 | 210 | 35.2 | 1.1 | 47 | 22.4 | 0.3 | 2148 | 41.4 | 76 | 38.6 | 3.5 | 5 | 6.6 | 0.2 |
| Unknown | 25 | 0.1 | — | — | — | — | — | — | 9 | 0.2 | — | — | — | — | — | — |
| Age group (y) | ||||||||||||||||
| 0-17 | 1703 | 3.6 | 3 | 0.5 | 0.2 | — | — | — | 170 | 3.3 | 3 | 1.5 | 1.8 | — | — | — |
| 18-29 | 2899 | 6.2 | 21 | 3.5 | 0.7 | — | — | — | 339 | 6.5 | 7 | 3.6 | 2.1 | 1 | 14.3 | 0.3 |
| 30-39 | 4737 | 10.1 | 40 | 6.7 | 0.8 | — | — | — | 648 | 12.5 | 21 | 10.7 | 3.2 | 1 | 4.8 | 0.2 |
| 40-49 | 7311 | 15.6 | 93 | 15.6 | 1.3 | 15 | 16.1 | 0.2 | 966 | 18.6 | 40 | 20.3 | 4.1 | 2 | 5.0 | 0.2 |
| 50-59 | 11 806 | 25.2 | 142 | 23.8 | 1.2 | 31 | 21.8 | 0.3 | 1523 | 29.4 | 61 | 31.0 | 4.0 | 6 | 9.8 | 0.4 |
| 60-69 | 11 294 | 24.1 | 195 | 32.7 | 1.7 | 67 | 34.4 | 0.6 | 1164 | 22.5 | 51 | 25.9 | 4.4 | 9 | 17.6 | 0.8 |
| 70+ | 7039 | 15.0 | 103 | 17.3 | 1.5 | 41 | 39.8 | 0.6 | 374 | 7.2 | 14 | 7.1 | 3.7 | 1 | 7.1 | 0.3 |
| Ethnicity | ||||||||||||||||
| White | 34 816 | 74.4 | 334 | 55.9 | 1.0 | 79 | 23.7 | 0.2 | 3222 | 62.2 | 68 | 34.5 | 2.1 | 7 | 10.3 | 0.2 |
| Asian | 5453 | 11.7 | 129 | 21.6 | 2.4 | 38 | 29.5 | 0.7 | 1017 | 19.6 | 58 | 29.4 | 5.7 | 6 | 10.3 | 0.6 |
| Black | 2620 | 5.6 | 95 | 15.9 | 3.6 | 27 | 28.4 | 1.0 | 612 | 11.8 | 48 | 24.4 | 7.8 | 3 | 6.3 | 0.5 |
| Other | 1287 | 2.8 | 18 | 3.0 | 1.4 | 5 | 27.8 | 0.4 | 243 | 4.7 | 17 | 8.6 | 7.0 | 2 | 11.8 | 0.8 |
| Unknown | 2613 | 5.6 | 21 | 3.5 | 0.8 | 5 | 23.8 | 0.2 | 90 | 1.7 | 6 | 3.0 | 6.7 | 2 | 33.3 | 2.2 |
| Region | ||||||||||||||||
| North West | 7372 | 15.8 | 58 | 9.7 | 0.8 | 12 | 20.7 | 0.2 | 789 | 15.2 | 15 | 7.6 | 1.9 | 1 | 6.7 | 0.1 |
| North East and Yorkshire | 5611 | 12.0 | 61 | 10.2 | 1.1 | 22 | 36.1 | 0.4 | 676 | 13.0 | 11 | 5.6 | 1.6 | 1 | 9.1 | 0.1 |
| Midlands | 8357 | 17.9 | 91 | 15.2 | 1.1 | 24 | 26.4 | 0.3 | 1007 | 19.4 | 28 | 14.2 | 2.8 | 3 | 10.7 | 0.3 |
| East of England | 5467 | 11.7 | 69 | 11.6 | 1.3 | 18 | 26.1 | 0.3 | 429 | 8.3 | 19 | 9.6 | 4.4 | — | — | — |
| London | 8458 | 18.1 | 217 | 36.3 | 2.6 | 58 | 26.7 | 0.7 | 1291 | 24.9 | 103 | 52.3 | 8.0 | 12 | 11.7 | 0.9 |
| South East | 6967 | 14.9 | 72 | 12.1 | 1.0 | 13 | 18.1 | 0.2 | 573 | 11.1 | 16 | 8.1 | 2.8 | 3 | 18.8 | 0.5 |
| South West | 4557 | 9.7 | 29 | 4.9 | 0.6 | 7 | 24.1 | 0.2 | 419 | 8.1 | 5 | 2.5 | 1.2 | — | — | — |
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus-type 2; SPK, simultaneous pancreas-kidney.
Includes islet patient.
TABLE 2.
Year of transplant and donor type in transplant recipients with a functioning graft on February 1, 2020 in England, and SARS-CoV-2 infections and subsequent mortality between February 1 and May 20, 2020
| Recipients | SARS-CoV-2 + recipients | SARS-CoV-2 + recipient deaths | ||||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | % of recipients | N | % | % of recipients | |
| Year of transplant | ||||||||
| Before 2016 | 31 281 | 66.9 | 348 | 58.3 | 1.1 | 92 | 26.4 | 0.3 |
| 2016 | 3242 | 6.9 | 46 | 7.7 | 1.4 | 15 | 32.6 | 0.5 |
| 2017 | 3497 | 7.5 | 40 | 6.7 | 1.1 | 10 | 25.0 | 0.3 |
| 2018 | 3823 | 8.2 | 42 | 7.0 | 1.1 | 13 | 31.0 | 0.3 |
| 2019 | 3942 | 8.4 | 80 | 13.4 | 2.0 | 16 | 20.0 | 0.4 |
| 2020a | 1004 | 2.1 | 41 | 6.9 | 4.1 | 8 | 19.5 | 0.8 |
| Donor type | ||||||||
| DBD | 27 207 | 58.1 | 340 | 57.0 | 1.2 | 92 | 27.1 | 0.3 |
| DCD | 8354 | 17.9 | 146 | 24.5 | 1.7 | 36 | 24.7 | 0.4 |
| Living | 11 230 | 24.0 | 111 | 18.6 | 1.0 | 26 | 23.4 | 0.2 |
Abbreviations: DBD, donation after brain death; DCD, donation after circulatory death; SARS-CoV-2, severe acute respiratory syndrome coronavirus-type 2.
Includes patients transplanted between February 1 and May 20, 2020 as per Figure 1.
The risks of testing positive for SARS-CoV-2 were higher for those on the wait-list than for SOT recipients. As of May 20, 2020, 197 patients (3.8%) on the wait-list and 597 (1.3%) of SOT recipients tested positive for SARS-CoV-2. However, of those testing positive, the all-cause mortality risk during the follow-up period was 10.2% (20/197) for waitlisted patients and 25.8% (154/597) for SOT recipients.
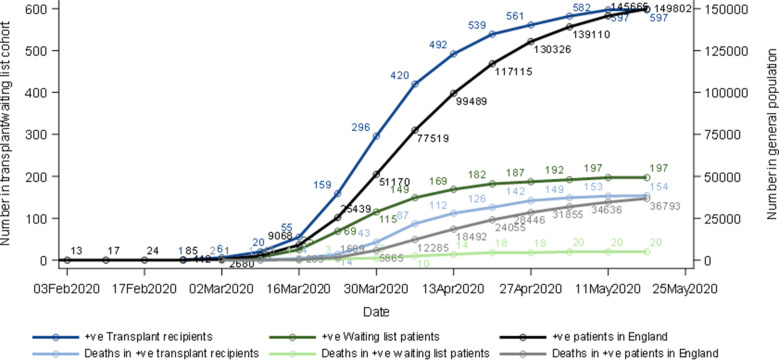
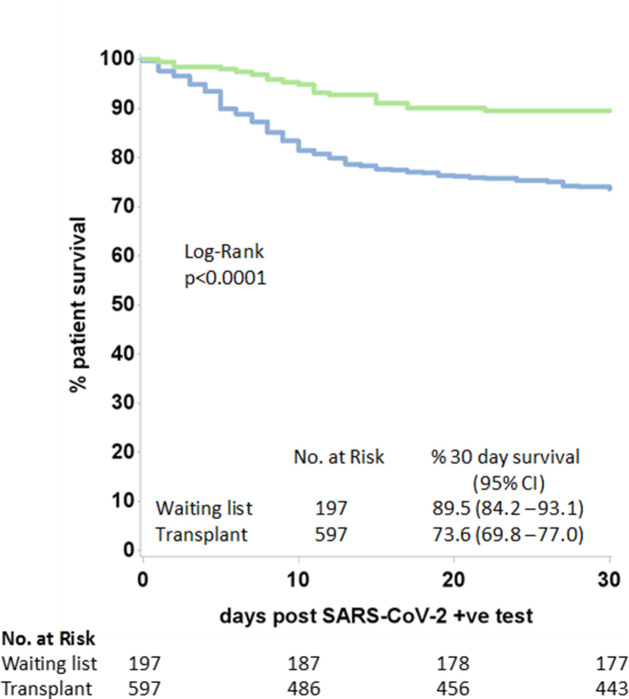
Figure 2 shows the cumulative number of SARS-CoV-2-positive patients and subsequent all-cause mortality by time, with data on the general population in England for comparison. The first SOT recipient with SARS-CoV-2-positive status was notified on March 1, 2020. After a steep rise in incidence through March and April, the disease incidence and all-cause mortality in both waitlisted and SOT recipients plateaued in early May 2020. The median (IQR) duration between the positive result and death was 10.5 (6.5-15) days for the wait-list cohort, and 7.5 (4-12) days for SOT recipients (P = .14). A Kaplan–Meier plot of patient survival from date of positive SARS-CoV-2 shows estimated 30-day survival for both patient groups (P < .0001) ( Figure 3). The median (IQR) follow-up from the date of the positive SARS-CoV-2 result to the end of the study period was 36 (13-47) and 44 (30-52) days for the waitlisted and SOT recipient groups, respectively.
FIGURE 2.
Cumulative number of solid organ transplant recipients and waitlisted patients with positive severe acute respiratory syndrome coronavirus-type 2 swabs and subsequent all-cause mortality in England, February 1 to May 20, 2020, compared to the general English population [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
Patient survival from the date of SARS-CoV-2-positive swab in wait-list patients and solid organ transplant recipients. CI, confidence interval; SARS-CoV-2, severe acute respiratory syndrome coronavirus-type 2 [Colour figure can be viewed at wileyonlinelibrary.com]
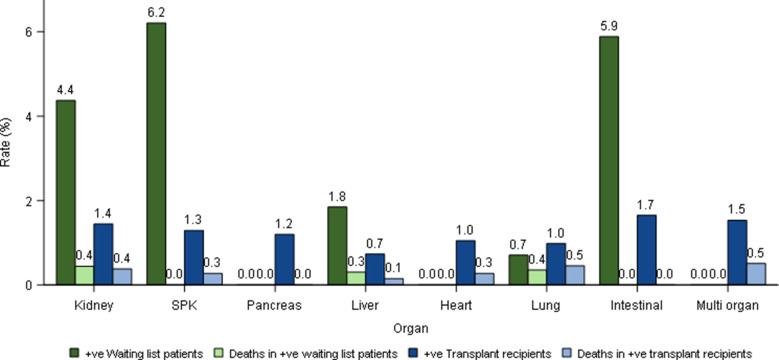
The risk of having a confirmed SARS-CoV-2 infection varied by organ type, with the highest infection rates in those waiting for simultaneous pancreas-kidney, intestinal, and kidney transplants (4.4%-6.2%) ( Figure 4). Infection rates were lower in transplanted patients (0.7%-1.7%). Despite these lower infection rates, the overall risk of becoming SARS-CoV-2 positive and then dying during the study period were similar between waitlisted and transplanted patients across different organ types (0.0%-0.5%), as mortality rates in infected patients were higher in SOT recipients. The median (IQR) age of recipients and waitlisted patients with SARS-CoV-2 was 59 (49-67) and 53 (45-62) years, respectively. The median (IQR) age of recipients and wait-list patients dying after testing positive for SARS-CoV-2 was 64.5 (58-70) and 59 (50.5-62) years, respectively.
FIGURE 4.
Risk of becoming SARS-CoV-2, severe acute respiratory syndrome coronavirus-type 2–positive and subsequent death for waiting-list and solid organ transplant recipients by organ type, February 1 to 20, May 2020. SPK, simultaneous pancreas-kidney [Colour figure can be viewed at wileyonlinelibrary.com]
Risk factors associated with the development of SARS-CoV-2 infection and subsequent mortality were examined within the SOT recipient group only, as there were insufficient cases for similar analyses to be undertaken on the wait-list cohort. SARS-CoV-2 infection was significantly more likely in transplant recipients who had received a kidney transplant (P < .0001), were older (P < .0001), nonwhite (P < .0001), lived in the London NHS region (P < .0001), had a deceased donor transplant (P < .0001), or had been transplanted in a more recent year (P < .0001). Multivariable logistic regression analysis showed that all of these variables were associated with a significantly increased risk of SARS-CoV-2 infection ( Table 3). The only variable independently associated with death in those recipients with a positive SARS-CoV-2 test was age. Compared to a reference group aged 50-59 years, odds ratios (95% confidence intervals) were 0.37 (0.19-0.72), 1.86 (1.13-3.04), and 2.37 (1.35-4.16) for recipients aged 0-49, 60-69, and 70 + years, respectively.
TABLE 3.
Odds ratios and 95% confidence intervals for candidate risk factors associated with testing positive for SARS-CoV-2 infection in solid organ transplant recipients
| Odds ratio | 95% confidence interval | P value | |
|---|---|---|---|
| Organ type | |||
| Kidney | 1.00 | ||
| SPK | 1.02 | 0.64-1.65 | .9 |
| Pancreasa | 1.08 | 0.34-3.40 | .9 |
| Liver | 0.53 | 0.40-0.70 | <.001 |
| Heart | 0.94 | 0.61-1.44 | .8 |
| Lung | 0.84 | 0.48-1.47 | .5 |
| Intestinal | 1.86 | 0.45-7.71 | .4 |
| Multi-organ | 1.20 | 0.38-3.79 | .8 |
| Age group (y) | |||
| 0-17 | 0.16 | 0.05-0.49 | .002 |
| 18-29 | 0.68 | 0.43-1.07 | .09 |
| 30-39 | 0.68 | 0.47-0.97 | .03 |
| 40-49 | 1.03 | 0.79-1.35 | .8 |
| 50-59 | 1.00 | — | |
| 60-69 | 1.57 | 1.26-1.96 | <.001 |
| 70+ | 1.46 | 1.12-1.90 | .005 |
| Ethnicity | |||
| White | 1.00 | — | |
| Asian | 1.94 | 1.55-2.42 | <.001 |
| Black | 2.41 | 1.85-3.15 | <.001 |
| Other | 1.13 | 0.70-1.85 | .6 |
| Unknown | 0.71 | 0.46-1.11 | .14 |
| NHS region | |||
| London | 1.00 | — | |
| North West | 0.58 | 0.43-0.79 | .0004 |
| North East and Yorkshire | 0.43 | 0.32-0.59 | <.0001 |
| Midlands | 0.55 | 0.42-0.71 | <.0001 |
| East of England | 0.65 | 0.49-0.87 | .004 |
| South East | 0.56 | 0.42-0.74 | <.0001 |
| South West | 0.35 | 0.24-0.53 | <.0001 |
| Year of transplant | |||
| Before 2016 | 1.00 | — | |
| 2016 | 1.22 | 0.89-1.66 | .2 |
| 2017 | 0.98 | 0.70-1.36 | .9 |
| 2018 | 0.91 | 0.66-1.27 | .6 |
| 2019 | 1.60 | 1.24-2.06 | .0003 |
| 2020 | 3.65 | 2.60-5.12 | <.0001 |
| Donor type | |||
| DBD | 1.00 | — | |
| DCD | 1.09 | 0.89-1.33 | .4 |
| Living | 0.78 | 0.62-0.98 | .03 |
Abbreviations: DBD, donation after brain death; DCD, donation after circulatory death; NHS, National Health Service; SARS-CoV-2, severe acute respiratory syndrome coronavirus-type 2; SPK, simultaneous pancreas-kidney.
Includes islet recipients.
4. DISCUSSION
The COVID-19 pandemic has had a major impact on health services of many countries and disrupted most clinical programs, including organ donation and transplantation, with intensive-care facilities facing extreme pressure due to a surge of COVID-19 admissions.16, 17, 18, 19 Given the absence of definitive data on the risks of SARS-CoV-2 infections posttransplant, patients on the wait-list for a transplant and clinical teams are unsure of the relative safety of transplantation vs remaining on the wait-list. Many transplant programs are either suspended or have significantly curtailed their activity.
The analyses presented are derived from the largest and most comprehensive dataset yet available on the incidence of and outcomes after SARS-CoV-2 infection in SOT recipients during the pandemic. Furthermore, it is also the first national study from a country with significant rates of infection and deaths from COVID-19 to comprehensively examine the survival of patients on the national deceased donor wait-list over the same period, providing a more valid comparator group for risk-benefit assessment of undertaking transplantation than using general population data.4
We found that a higher proportion of waitlisted patients tested positive for SARS-CoV-2 than SOT recipients did (3.8% vs 1.3%). Since the highest proportion of patients on the list are waiting for a kidney transplant, it is possible that the inability of many hemodialysis patients to self-isolate due to requirement to travel 3 times/wk to a dialysis facility and remain in proximity to other patients and healthcare staff may contribute to a higher risk of COVID-19. After an initial rapid rise in numbers of affected patients, the number of new infections appears to have plateaued by May 2020.
While the overall risk of infection in waitlisted patients was higher, SOT recipients who tested positive for SARS-CoV-2 had a higher all-cause mortality during the follow-up period compared to waitlisted patients (25.8% vs 10.2%). Among the patients transplanted in 2020 (ie, during the pandemic), the incidence of SARS-CoV-2 infection was 4% with a subsequent risk of death of <1% within the follow-up period. Increasing age, nonwhite ethnicity, and living in London were associated with higher incidences of SARS-CoV-2 infection in SOT recipients. No patient aged <18 years is recorded as having died after confirmed SARS-CoV-2 infection. These findings are in accordance with studies of the general population in the United Kingdom.4 , 20 Other variables also associated with increasing risks of SARS-CoV-2 infection in posttransplant patients include recent transplantation, while liver transplant recipients had a reduced infection risk. These results may reflect the relatively high burden of immunosuppression in early posttransplant patients, and that recipients of liver transplants generally require less immunosuppression than other forms of SOT.
The only variable independently associated with death in SOT recipients with a positive SARS-CoV-2 test was age. Increasing age has been identified as a strong risk factor for COVID-19-related deaths in other studies,3 , 4 and therefore this finding was unsurprising. Although our dataset did not adequately capture comorbidity data, it is notable that increasing rates of comorbidities do not appear to explain the apparent association between increasing age and death from SARS-CoV-2 infection, as 2 large studies from the United Kingdom show that age is an independent risk factor for death even after adjustment for multiple major comorbidities.3 , 4 Transplant clinicians will need to take these findings into account when considering the suitability of older patients to undergo transplantation in countries with a high incidence of SARS-CoV-2 infection.
This study also supports the assumption that SOT recipients are at high risk of death after SARS-CoV-2 infection, with 154 (25.8%) of 597 patients dying during the study period. Although this mortality rate is similar to that of the general UK population with SARS-CoV-2, the median age of transplant recipients with SARS-CoV-2 infection in our study was far lower (59 years vs 73 years).3
To our knowledge, the only other national transplant registry that has reported on COVID-19-related deaths identified 21 Swiss SOT recipients between March 9 and April 6 who tested positive for SARS-CoV-2.14 After a median follow-up of 33 days, 2 patients died. In Spain, Domínguez-Gil et al noted that 363 SOT recipients had developed COVID-19, but no mortality data or denominator were presented.18 Deaths on the Spanish national wait-list were reported at a rate of 1.3 per week during the pandemic (8 in total), compared to a prepandemic rate of 1.4 deaths per week, but without further information on whether those who died had COVID-19. The OpenSAFELY study included 20 130 organ transplant recipients but only 49 in-hospital deaths from COVID-19 were identified, with the general UK population as the comparator group.4
Many of the previous studies on the outcomes of SOT recipients with COVID-19 have focused on a single organ type, with the majority on kidney transplant recipients. Of those studies that have included more than 10 renal transplant patients, mortality rates varied from 6%7 to 67%.6 The largest study thus far on kidney transplant recipients reported on a voluntary Spanish registry of 868 patients on renal replacement therapy with COVID-19 between March 18 and April 11, including ≈290 renal transplant recipients.21 There were no linkages to central registries of death or notifiable disease. The mean age of all patients was 67 years, and the transplant population was younger than those on dialysis. Eighty-five percent of patients were admitted to the hospital, and 23% of patients died (18.6% of transplant recipients vs 24.9% of those on dialysis). Age was found to be a risk factor for death after COVID-19 in both transplant and dialysis patients.
Latif et al described outcomes of COVID-19 in 28 heart transplant recipients in New York City with 25% mortality,22 while a series of 13 patients from Michigan found that 2 patients (15%) died.23 Six of 23 heart transplant recipients with SARS-CoV-2 infection died in our study. Reports of COVID-19 outcomes in lung transplant recipients have been limited, with a small number of case-series of fewer than 5 patients.12 , 24 The largest series of 17 lung patients comes from 2 centers in New York City, though survival outcomes for this group are not reported.9 We found that 6 of 13 lung transplant recipients with SARS-CoV-2 died.
Similarly, mortality rates from COVID-19 in liver transplant recipients have not been widely described. An international collaborative from 21 countries reported a 23% mortality rate from COVID-19 in 39 liver transplant patients,25 while a 21% mortality rate in 24 SARS-CoV-2-positive liver recipients was identified in a survey of Italian liver transplant programs.26 Our results suggest that liver transplant recipients have a lower SARS-CoV-2 infection rate than recipients of other solid organ transplants, though larger analyses with longer follow-up are needed. To our knowledge, no COVID-19-related deaths have previously been reported in heart, lung, or liver transplant wait-list populations.
The strengths of our study include a complete national dataset of the at-risk population, accurate identification of all at-risk patients who tested positive for SARS-CoV-2, near real-time ascertainment of all-cause mortality in at-risk patients, and analysis of outcomes between waitlisted and transplanted patients. It highlights the value of routine, national, embedded data collection allowing systems to analyze comprehensive data and respond accordingly.
We acknowledge the weaknesses of this study. Although the NHSBT database holds data on SOT recipients and waitlisted patients in Wales, Scotland, and Northern Ireland, this study examined patients residing in England only, as linkages with the relevant notifiable diseases and mortality registries in other nations were not in place at the time of writing. Given the notifiable disease status, data on patients testing positive for SARS-CoV-2 were reported and collated centrally and available for analysis but data on patients testing negative were not available. Due to the relatively low number of events in the waiting-list group, multivariable analyses comparing the mortality rates between the 2 cohorts were not possible. Our analysis does not include the indication for SARS-CoV-2 testing, symptoms, hospital or intensive-care unit admissions, and treatments of COVID-19. We expect that the overwhelming majority of patients with SARS-CoV-2-positive tests had symptoms, as tests were predominantly done in hospitalized patients during the study period in the United Kingdom. As such, there was a selection bias toward sicker, symptomatic, and hospitalized patients. The study period was short, and further follow-up will be needed to determine definitive case-fatality rates. We were unable at this time to report on excess mortality in the study cohorts. Direct comparisons of SARS-CoV-2 positivity and subsequent mortality between the waitlisted and transplanted groups are limited by inherent demographic, biological, and behavioral differences (eg, age, immunosuppressive burden, comorbidities, access to testing, challenges in self-isolating in those on unit dialysis) and should be treated with caution. Finally, while we could ascertain all-cause mortality in patients testing positive for SARS-CoV-2, we were unable to access cause of death as certified. The linked dataset did not provide cause of death information. Given the restriction of access for swab testing to symptomatic hospitalized patients and short median time (10.5 days for waitlisted and 7.5 days for SOT recipients) between a positive swab result and death, COVID-19-associated mortality is inferred but not proven by cause of death as certified.
In conclusion, analyses from this large, comprehensive national dataset will enable transplant clinicians and their patients to have a more accurate risk assessment of incidence of and outcomes following SARS-CoV-2 infection, with the first-of-its-kind analysis of risk in waitlisted and SOT recipient groups. Such information will help clinical teams decide on reinstating organ transplant programs and patient selection, and will aid patient consent discussions. Future work should include longer follow-up, further case-mix adjustment and multivariable analyses, along with more detailed understanding of the impact of pharmacological and nonpharmacological interventions on SARS-CoV-2 infection and mortality rates in waitlisted patients and SOT recipients.
ACKNOWLEDGMENTS
The authors acknowledge Public Health England and NHS Digital Tracing Service for sharing data; James Thomas, Tariq Malik, Anne Marie O’Connell (PHE) and Richard Little (NHSBT) for enabling database linkages; Olive McGowan, Karen Quinn, Julie Whitney, Liz Armstrong as members of the NHSBT clinical leadership team supporting organ donation and transplantation in the United Kingdom; and the transplant multidisciplinary clinical teams and patients across the United Kingdom. This work was carried out by NHSBT, an NHS special health authority with responsibility for organ donation and transplantation in the United Kingdom. Other than the authors, no other public or private entity had any role in study design, data analysis, data interpretation, or writing of the report. LM had full access to all of the data and was responsible for merging the linked datasets in the study. All authors had final responsibility for the decision to submit for publication.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
AUTHOR CONTRIBUTIONS
RR and CC aided in the conception of the study and the statistical design of the study, and co-wrote the manuscript. LM aided in the conception of the study, designed the study, performed the statistical analyses, and critically reviewed the manuscript. IU-L, DT, JC, PF, JP, IC, LB, RB, JD, GCO, MB, JA, DH, AM, DM, DG, and JLRF aided in the conception of the study, and critically reviewed the writing of the manuscript. All authors agree to be accountable for the work and have given final approval for publication.
DATA AVAILABILITY STATEMENT
Non-identifiable data that supports the findings of this study are available from the corresponding author upon reasonable request. Any data release can only be completed after appropriate information governance clearances
Footnotes
Rommel Ravanan and Chris J. Callaghan contributed equally to the manuscript.
REFERENCES
- 1.UK Government. Coronavirus (COVID-19) in the UK. https://coronavirus.data.gov.uk/?_ga=2.107739851.893381260.1589709324-1340677483.1577435701. Accessed May 28, 2020
- 2.Office for National Statistics. Coronavirus (COVID-19) related deaths by ethnic group, England Wales: 2 March 2020 to 10 April 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/coronavirusrelateddeathsbyethnicgroupenglandandwales/2march2020to10april2020. Accessed May 28, 2020.
- 3.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The OpenSAFELY Collaborative. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. 2020. https://www.medrxiv.org/content/10.1101/2020.05.06.20092999v1.full.pdf. Accessed May 28, 2020
- 5.UK Government. Guidance on shielding and protecting people who are clinically extremely vulnerable from COVID-19. 2020. https://www.gov.uk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-CoVid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-CoVid-19. Accessed May 28, 2020
- 6.Abrishami A, Samavat S, Behnam B, Arab-Ahmadi M, Nafar M, Sanei TM. Clinical course, imaging features, and outcomes of COVID-19 in kidney transplant recipients. Eur Urol. 2020;78(2):281–286. doi: 10.1016/j.eururo.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montagud-Marrahi E, Cofan F, Torregrosa JV, et al. Preliminary data on outcomes of SARS-CoV-2 infection in a Spanish single centre cohort of kidney recipients. Am J Transplant. [published online ahead of print 2020] 10.1111/ajt.15970 [DOI] [PMC free article] [PubMed]
- 8.Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020;97:1076–1082. doi: 10.1016/j.kint.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Columbia University Kidney Transplant P Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31(6):1150–1156. doi: 10.1681/ASN.2020030375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu L, Gong N, Liu B, et al. Coronavirus Disease 2019 Pneumonia in Immunosuppressed Renal Transplant Recipients: A Summary of 10 Confirmed Cases in Wuhan, China. Eur Urol. 2020;77:748–754. doi: 10.1016/j.eururo.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koczulla RA, Sczepanski B, Koteczki A, et al. SARS-CoV-2 infection in two patients following recent lung transplantation. Am J Transplant. [published online ahead of print 2020] 10.1111/ajt.15998 [DOI] [PMC free article] [PubMed]
- 13.Akalin E, Azzi Y, Bartash R, et al. COVID-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tschopp J, L’Huillier AG, Mombelli M, et al. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. [published online ahead of print 2020] 10.1111/ajt.16062 [DOI] [PMC free article] [PubMed]
- 15.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395(10237):e95–e96. doi: 10.1016/S0140-6736(20)31040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vries A, Alwayn I, Hoek R, et al. Immediate impact of COVID-19 on transplant activity in the Netherlands. Transpl Immunol. 2020;61:101304. doi: 10.1016/j.trim.2020.101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domínguez-Gil B, Coll E, Fernández-Ruiz M, et al. COVID-19 In Spain: Transplantation In The Midst Of The Pandemic. Am J Transplant. 2020. 10.1111/ajt.15983 [DOI] [PMC free article] [PubMed]
- 19.ICNARC. ICNARC report on COVID-19 in critical care. https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports. Accessed May 28, 2020.
- 20.de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. [published online ahead of print 2020] 10.1016/S14733099(20)30371-6 [DOI] [PMC free article] [PubMed]
- 21.Sanchez-Alvarez JE, Perez Fontan M, Jimenez Martin C, et al. SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN) Nefrologia. 2020;40(3):272–278. doi: 10.1016/j.nefro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latif F, Farr MA, Clerkin KJ, et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. [published online ahead of print 2020] 10.1001/jamacardio.2020.2159 [DOI] [PMC free article] [PubMed]
- 23.Ketcham SW, Adie SK, Malliett A, et al. Coronavirus disease 2019 in heart transplant recipients in Southeastern Michigan - Case series. J Card Fail. 2020;26(6):457–461. doi: 10.1016/j.cardfail.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cozzi E, Faccioli E, Marinello S, et al. COVID-19 pneumonia in lung transplant recipients: report of two cases. Am J Transplant. [published online ahead of print 2020] 10.1111/ajt.15993 [DOI] [PMC free article] [PubMed]
- 25.Webb GJ, Moon AM, Barnes E, Barritt AS, Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5(7):643–644. doi: 10.1016/S2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agnes S, Andorno E, Avolio AW, et al. Preliminary analysis of the impact of COVID-19 outbreak on Italian liver transplant programs. Liver Transpl. [published online ahead of print 2020] 10.1002/lt.25790 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Non-identifiable data that supports the findings of this study are available from the corresponding author upon reasonable request. Any data release can only be completed after appropriate information governance clearances