Hypertensive disorders of pregnancy (HDP, e.g., gestational hypertension, preeclampsia) are associated with significant maternal and fetal morbidity and mortality. Additionally, HDP are associated with heightened future risk of cardiometabolic risk factors (e.g., hypertension, type 2 diabetes [T2D]) and cardiovascular disease. Whether HDP reflect latent preceding cardiometabolic risk is unclear. Here, we tested whether genetic predisposition to cardiometabolic traits and variants related to antihypertensive medication targets are associated with HDP.
Analyses used individual-level genomic and phenotypic data from women in the UK Biobank who reported ≥1 live birth. Genetic instruments were constructed from genome-wide association studies (GWAS) external to the UK Biobank, using independent (r2<0.2, European 1000Genomes) single nucleotide polymorphisms (SNPs). Instruments for systolic (SBP) and diastolic blood pressure (DBP) each included 75 SNPs (53 in common).1 We created genetic instruments for BMI (141 SNPs),2 resting heart rate (28 SNPs),3 low-density lipoprotein cholesterol (LDL-C, 277 SNPs),4 T2D (64 SNPs),5 and ever-smoking (10 SNPs, TAG Consortium 2010), and genetic proxies for beta blockers (rs11196597, rs1801253, rs4359161), calcium channel blockers (rs7340705, rs2488136, rs1888693, rs16916914, rs10828399, rs10828452, rs12780039, rs11014170, rs7923191, rs12258967, rs1998822, rs4748474, rs2239046), and nitrates (rs13139571, rs3918226, rs891511). Individual-level polygenic risk scores (PRS) were associated with HDP using multivariable logistic regression, adjusted for enrollment age, the first 20 principal components of ancestry, and genotyping array. The Massachusetts General Hospital institutional review board approved these analyses.
Of 214,365 women (mean age 56.9 years), 2,772 (1.29%) had prior HDP. Women with prior HDP had higher adjusted SBP1 by 10.53 mmHg (P<0.001), higher adjusted DBP1 by 5.97 mmHg (P<0.001), higher heart rate (71.6 vs. 70.4 beats per minute, P<0.001), and higher BMI (28.1 vs 27.1 kg/m2, P<0.001); were less likely to have ever smoked tobacco (32.9% vs. 40.7%, P<0.001); and were more likely to have T2D (2.3% vs. 1.6%, P=0.007).
All genetic instruments had F-statistics >10 and were strongly associated with corresponding exposures among UK Biobank participants (P<2×10−16 for all), implying low risk of weak-instrument bias.
For both SBP and DBP, the OR for HDP per standard deviation (SD) of PRS was 1.22 (P<0.001) (Figure 1A). Associations with SBP PRS were similar in women with gestational hypertension (OR 1.24 per SD, 95% CI 1.13–1.35) or preeclampsia (OR 1.19 per SD, 95% CI 1.08–1.31). Genetic predisposition for increased BMI also increased HDP odds (OR 1.06 per SD; P=0.004). BP and BMI PRS were independent and additive. Genetic predisposition for increased heart rate, T2D, smoking, and LDL-C were not associated with HDP. Results were unchanged in analyses restricted to 180,846 women with white British ancestry. Two-sample Mendelian randomization using the inverse variance-weighted method yielded consistent findings (P=1.7×10−18 for SBP, P=1.7×10−4 for BMI) without evidence of horizontal pleiotropy (P=0.91 and P=0.70 for SBP and BMI, respectively, with MR-Egger intercept).
Figure 1.
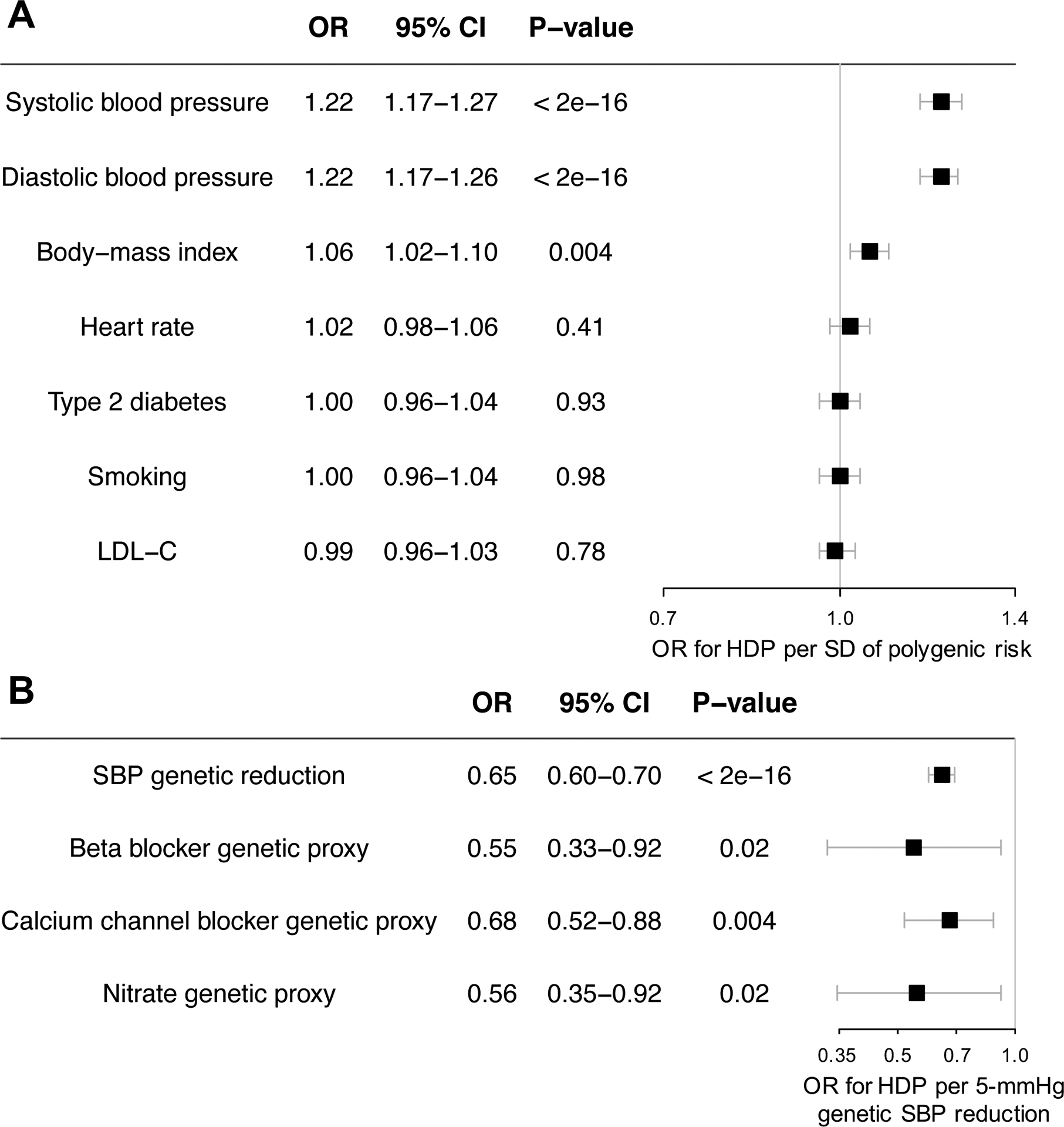
(A) Risk of hypertensive disorders of pregnancy conferred by genetic predisposition to cardiometabolic risk factors and genetically determined resting heart rate. (B) Risk reduction associated with genetic proxies for multiple blood pressure-lowering drug classes.*
Odds ratios are adjusted for age, the first 20 principal components of ancestry, and genotype array. *Risk reduction associated with antihypertensive medications is displayed per 5 mmHg of lower systolic blood pressure due to genetic variation in antihypertensive medication target pathways. OR = odds ratio; CI = confidence interval; HDP = hypertensive disorders of pregnancy; SD = standard deviation; LDL-C = low-density lipoprotein cholesterol; SBP = systolic blood pressure
We tested the effect of genetic predisposition for HDP on SBP using 205 SNPs at least nominally associated with HDP (P<5*10−4, r2<0.01) in the UK Biobank, with the outcome extracted from the 2011 International Consortium for Blood Pressure GWAS. HDP showed a nominally significant association with SBP using both the inverse variance weighted (β=+0.17 mmHg, P=0.015) and MR.RAPS (β=+0.16 mmHg, P=0.04 [Huber]; β=+0.15 mmHg, P=0.05 [Tukey]) methods.
Genetic variants mimicking effects of beta blockers, calcium channel blockers, and nitrates all demonstrated reduction in HDP risk proportional to SBP effect (Figure 1B). Additionally, rs7692387-A (intron variant of GUCY1A3) was associated with HDP (OR 0.90, 95% CI 0.84–0.97, P=0.004). The association of rs7692387-A with HDP appeared disproportionately large (OR 0.21 per 5-mmHg SBP lowering; P=0.004; Pheterogeneity vs. SBP PRS = 0.037).
In a large cohort of UK women, genetic predisposition to elevated SBP, DBP, and BMI were significantly associated with HDP, implying that these cardiometabolic risk pathways are causal for development of HDP. BP and BMI may therefore represent viable preventive targets for reducing HDP risk. In the Control of Hypertension in Pregnancy Study, tight BP control decreased rates of severe gestational hypertension but not preeclampsia. However, women enrolled <24 weeks gestation derived greater benefit from tight control, implying earlier BP lowering may be required to effectively prevent HDP. Further, the protective effect of GUCY1A3 rs7692387 variation relative to BP-lowering suggests that NO signaling may be particularly protective against HDP via BP-independent pathways.
Our study has limitations. HDP prevalence in the UK Biobank is lower than population estimates, possibly due to healthy participant bias, underreporting of HDP history, or both, potentially biasing findings toward the null. Only 17 women had eclampsia. Reduced power may have hindered our ability to detect smaller associations for T2D and ever-smoking, although point estimates were entirely neutral. We lacked adequate power to perform dedicated subgroup analysis in black women, who are disproportionately affected by HDP. Genetic risk scores are not intended for the prediction of HDP and are used as tools of causal inference to gain mechanistic insights.
In conclusion, genetic predisposition to elevated BP and BMI are associated with HDP, implying causal pathways well-positioned for HDP prevention. Whether HDP risk may be reduced in at-risk women through antepartum BP-lowering and weight-loss strategies warrants prospective assessment in adequately powered randomized trials.
Sources of Funding:
Dr. Honigberg is supported by the National Institutes of Health (T32HL094301–07). Dr. Peloso and Dr. Natarajan are supported by grants from the National Heart, Lung, and Blood Institute (R01HL1427). Additionally, Dr. Natarajan is supported by grants from the National Heart, Lung, and Blood Institute (R01HL148565 and R01HL148050), Fondation Leducq (TNE-18CVD04), and a Hassenfeld award from the Massachusetts General Hospital.
Footnotes
Disclosures:
Dr. Bhatt discloses the following relationships - Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda. Dr. Natarajan reports grant support from Amgen, Apple, and Boston Scientific, as well as consulting income from Apple, all unrelated to this work. The other authors report no disclosures.
Data are available through the UK Biobank (www.ukbiobank.ac.uk). This research was conducted under UK Biobank application number 7089.
References
- 1.Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, Ntritsos G, Dimou N, Cabrera CP, Karaman I, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018;50:1412–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Setten J, Verweij N, Mbarek H, Niemeijer MN, Trompet S, Arking DE, Brody JA, Gandin I, Grarup N, Hall LM, et al. Genome-wide association meta-analysis of 30,000 samples identifies seven novel loci for quantitative ECG traits. Eur J Hum Genet. 2019;27:952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott RA, Scott LJ, Mägi R, Marullo L, Gaulton KJ, Kaakinen M, Pervjakova N, Pers TH, Johnson AD, Eicher JD, et al. An Expanded Genome-Wide Association Study of Type 2 Diabetes in Europeans. Diabetes. 2017;66:2888–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]


