Abstract
Amyotrophic lateral sclerosis (ALS) is characterized by adult-onset progressive degeneration of upper and lower motor neurons. Increasing numbers of genes are found to be associated with ALS; among those, the first identified gene, SOD1 coding a Cu/Zn-superoxide dismutase protein (SOD1), has been regarded as the gold standard in the research on a pathomechanism of ALS. Abnormal accumulation of misfolded SOD1 in affected spinal motor neurons has been established as a pathological hallmark of ALS caused by mutations in SOD1 (SOD1-ALS). Nonetheless, involvement of wild-type SOD1 remains quite controversial in the pathology of ALS with no SOD1 mutations (non-SOD1 ALS), which occupies more than 90% of total ALS cases. In vitro studies have revealed post-translationally controlled misfolding and aggregation of wild-type as well as of mutant SOD1 proteins; therefore, SOD1 proteins could be a therapeutic target not only in SOD1-ALS but also in more prevailing cases, non-SOD1 ALS. In order to search for evidence on misfolding and aggregation of wild-type SOD1 in vivo, we reviewed pathological studies using mouse models and patients and then summarized arguments for and against possible involvement of wild-type SOD1 in non-SOD1 ALS as well as in SOD1-ALS.
Keywords: Amyotrophic lateral sclerosis, Cu/Zn-superoxide dismutase, Protein misfolding
Background
Amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disease classically characterized by loss of motor neurons in the central nervous system including motor cortex, brainstem, and spinal cord [1]. The loss of motor neurons leads to inability to control voluntary muscles and ultimately results in respiratory failure. Only two drugs, Riluzole and Edaravone, are currently available, but their therapeutic effects are limited to the extent that the survival can be extended at most a few months [2]. Together with full elucidation of the pathomechanism, therefore, development of efficient cures for this devastating disease has long been demanded.
In 1993, mutations in the gene encoding Cu/Zn-superoxide dismutase (SOD1) were first reported as a cause of ALS [3], and since then, more than 30 genes responsible for ALS have been identified [1]. A genetic cause/predisposition still remains unclear in most of ALS cases (~ 80%), and SOD1 mutations describe only approximately 3% of total ALS cases (called SOD1-ALS) [4]. Nonetheless, pathological examinations on SOD1-ALS cases provide us with important clues to understand disease mechanisms; namely, SOD1 proteins abnormally accumulate and form inclusions selectively in affected motor neurons [5]. Based upon such pathological observations, furthermore, a mechanism has been proposed where SOD1 proteins assume an abnormal conformation (or misfold) by an amino acid substitution corresponding to a pathogenic mutation, accumulate as oligomers/aggregates, and then exert toxicity to kill motor neurons [6]. Several researchers have attempted to extend the pathological roles of SOD1 misfolding in SOD1-ALS to more prevailing ALS cases, in which no mutations in the SOD1 gene are confirmed (non-SOD1 ALS). In other words, wild-type SOD1 could cause ALS when it somehow misfolds. Nonetheless, experimental results on the involvement of wild-type SOD1 in non-SOD1 ALS are not consistent among different research groups, making this issue highly controversial. In order to discuss SOD1 proteins as a potential target for the development of therapeutics to ALS, we comprehensively reviewed reports on possible roles of wild-type SOD1 in the pathology of ALS.
Misfolded forms of SOD1 as a pathological hallmark of SOD1-ALS
SOD1 is a metalloenzyme that catalyzes the disproportionation of superoxide anion into hydrogen peroxide and molecular oxygen [7]. The enzymatic activity in most of the patients with the SOD1 mutations was almost half as much as those in healthy controls [8], which had initially been considered to trigger pathological changes in ALS. Indeed, homozygous and even heterozygous knockout of the Sod1 gene in mice exhibited a wide range of phenotypes relevant to ALS such as slowly progressive motor deficits [8]. Recently, furthermore, human patients with a homozygous truncating variant c.335dupG (p.C112Wfs*11) in the SOD1 gene that leads to total absence of the enzymatic activity were reported, and the resulting phenotype was marked by progressive loss of motor abilities [9, 10]. Heterozygous carriers of the c.335dupG variant had an approximately halved SOD1 activity when compared to normal controls but appear not to develop symptoms of ALS [10]. Also, the Sod1-knockout mice did not develop ALS-like pathologies [8]; instead, overexpression of mutant SOD1 in mice reproduces ALS-like pathological changes with a significant increase in the SOD1 enzymatic activity [11]. While any reduction in the SOD1 enzymatic activity might modify the ALS pathomechanism, mutant SOD1 is considered to cause the disease not through a loss of the enzymatic activity but by a gain of new properties exerting toxicity to motor neurons.
As a pathological hallmark of SOD1-ALS, SOD1 proteins are known to abnormally accumulate in motor neurons (e.g. [5]), leading to prevailing idea that pathogenic mutant SOD1 gains toxicity through its misfolding into non-native conformations. While the abnormal accumulation of SOD1 in motor neurons does not necessarily mean the misfolding of SOD1, biophysical examinations in vitro using recombinant SOD1 proteins have strongly supported conformational changes of SOD1 by amino acid substitutions due to the pathogenic mutations. SOD1 is functionally and conformationally matured through post-translational processes including copper and zinc binding and disulfide formation [12]. The bound copper ion acts as a catalytic center, whereas the bound zinc ion and the intramolecular disulfide bond play roles in stabilizing the native structure [13–15]. Pathogenic mutations decrease the affinity of SOD1 toward the metal ions and/or the stability of the disulfide bond [16, 17], thereby disturbing the native conformation of SOD1. In other words, the post-translational maturation appears to be hampered in the mutant SOD1 proteins, resulting in an increased propensity of SOD1 to misfold into oligomers and aggregates. Indeed, in transgenic mice expressing human SOD1 with ALS-causing mutations (G37R and G93A), oral administration of a copper complex CuII (atsm) facilitates the copper binding of mutant SOD1 in their spinal cords and improves the neurological phenotype and survival [18–20]. Also, further expression of CCS, which is a copper chaperone assisting the maturation of SOD1 in vivo [21, 22], remarkably extends the survival of the transgenic mice administered with CuII (atsm) [23]. In the absence of the CuII (atsm) administration, overexpression of CCS in the transgenic mice (G37R and G93A) is known to dramatically reduce the mean survival (from 242 days to 36 days), to which mitochondrial dysfunction appears to contribute due to the perturbation of intracellular copper dynamics [24, 25]. Increased amounts of CCS would supply most of the intracellular copper ions to overexpressed mutant SOD1 proteins; therefore, the copper ions are not recruited to the other copper-requiring enzymes such as cytochrome c oxidase in mitochondria. Indeed, overexpression of CCS did not influence the disease phenotypes of the transgenic mice expressing human SOD1 with L126Z or murine SOD1 with G86R mutation [24], which are considered to be unable to bind a copper ion. Also notably, marked acceleration of disease in the transgenic mice (G93A) with CCS overexpression was not observed when the mice had an additional mutation H80G in the SOD1 (G93A) transgene [26]. This is probably because the zinc-binding in G93A-mutant SOD1 was compromised by substitution of a zinc-ligand (His80) to Gly. Given important roles of the zinc binding in conformational stabilization of SOD1 [14, 27], H80G/G93A-mutant SOD1 was not able to receive a copper ion from the overexpressed CCS. Misfolding of SOD1 proteins in vivo as well as in vitro will hence be circumvented through their post-translational maturation of SOD1, which would eventually reduce the toxicity of mutant SOD1 proteins.
Pathological roles of wild-type human SOD1 in transgenic mouse models of SOD1-ALS
Given that wild-type SOD1 is misfolded in vitro when losing the bound metal ions and/or the conserved disulfide bond [28], SOD1 could exert the disease-causing toxicity even without the pathogenic amino acid substitutions. Actually, co-expression of wild-type human SOD1 in transgenic mice expressing ALS-linked mutant human SOD1 (G37R, G85R, G93A, and L126Z) is known to accelerate the disease onset, suggesting the toxicity of wild-type human SOD1 [29–35]. Also, mice did not develop ALS-like symptoms upon expression of A4V-mutant human SOD1, but co-expression of wild-type human SOD1 in the A4V-SOD1 expressing mice did trigger the progression of ALS-like disease [29]. Taking advantage of distinct electrophoretic mobilities of wild-type and mutant SOD1 proteins (G85R and L126Z), furthermore, wild-type human SOD1 was found to accumulate as detergent-insoluble aggregates with the mutant proteins in transgenic mice [29, 31, 33, 34], while the interactions in the aggregates would not be simply a co-assembly of mutant and wild-type proteins [33]. A mechanism of disease-accelerating effects of wild-type SOD1 remains unclear, but heteromeric interactions between wild-type and mutant SOD1 appear to aggravate the aggregation and toxicity in cultured cell models [36] and have correlation with the disease severity [37]. It should be also noted that, in some studies, overexpression of wild-type human SOD1 did not affect the onset or duration of disease in mice expressing G85R-mutant human SOD1 [5] or G86R-mutant murine SOD1 [38]. Furthermore, disease-related phenotypes were not observed in transgenic mice expressing human SOD1 that has multiple mutations including those at copper and zinc binding sites (H46R/H48Q/H63G/H71R/H80R/H120G) and two free Cys residues (C6G/C111S) with an ALS-linked mutation, H43R, and co-expression of wild-type human SOD1 did not cause the disease [35]. Such apparent discrepancies would, nonetheless, indicate that expression levels of SOD1 as well as interactions between wild-type and mutant SOD1 play key roles in exerting toxicity of wild-type human SOD1.
Even in the absence of ALS-causing mutant SOD1, overexpression of wild-type human SOD1 alone can exert motor neuron toxicity to mice. In hemizygous transgenic mice expressing wild-type human SOD1, their lifespan was not affected, but neurodegenerative changes appeared in old age including mitochondrial vacuolization, axonal degeneration and a moderate loss of spinal motor neurons [32, 39, 40]. Upon decreasing glutathione levels, the mice developed overt motor symptoms, and their lifespan was decreased [41]. Also, spinal cord homogenates from the hemizygous wild-type human SOD1 transgenic mice were found to contain age-dependent, progressive formation of high-molecular-weight SOD1 aggregates [40, 42], which would be caused by oxidation of a unique tryptophan in SOD1 upon endoplasmic reticulum stress [42]. Furthermore, homozygous wild-type human SOD1 transgenic mice significantly increased the expression levels of wild-type human SOD1 and thereby developed ALS-like syndrome with formation of aggregated SOD1 in spinal cord and brain [43]. Even without any amino acid substitutions, therefore, wild-type human SOD1 could exert motor neuron toxicity to model animals under certain experimental conditions.
Possible involvement of wild-type SOD1 in pathological inclusions of SOD1-ALS patients
In contrast to the mouse models, pathological involvement of wild-type SOD1 is highly controversial in SOD1-ALS as well as non-SOD1 ALS patients. While most of SOD1-ALS patients express both wild-type and mutant SOD1 proteins, it is difficult to biochemically and immunohistochemically distinguish between wild-type and mutant SOD1 in tissues. In that sense, the involvement of wild-type SOD1 was examined in a SOD1-ALS patient with the G127insTGGG (G127X) mutation; such a truncated G127X-mutant SOD1 can be discriminated from the wild-type protein because of the difference in size and also of a non-native procession of the five amino acids following Gly127 in the variant [44, 45]. Wild-type SOD1 was detected in a detergent-insoluble (0.1% Nonidet P-40-insoluble) fraction of the cervical ventral horn of the G127X patient, while no control patients were examined [45] . Also, G127X patients had aggregates in glial cell nuclei of spinal cords, some of which were stained with an antibody (Chi 131–153 ab) raised against a peptide sequence absent in G127X-mutant SOD1 (Asn131 - Gln153) [46]. Those Chi 131–153 ab-positive aggregates were not stained with a G127X-mutant specific antibody directed to the non-native, C-terminal sequence of the five amino acids, suggesting pathological aggregation of wild-type SOD1 that is not co-localized with G127X-mutant proteins. As discussed later, however, even in control patients, significant amounts of wild-type SOD1 were present in the 0.1% Nonidet P-40-insoluble fraction [47]. Also, the same research group has published the paper showing that G127X-mutant but not wild-type SOD1 in the ventral horn of lumbar spinal cord of a G127X patient was sedimented by density gradient ultracentrifugation [44], implying no involvement of the wild-type protein in the mutant SOD1 aggregates. Some of the pathogenic full-length as well as truncated mutant SOD1 proteins are known to exhibit distinct electrophoretic mobilities from that of the wild-type protein [48]; therefore, more biochemical analysis on tissue samples from SOD1-ALS patients will reveal any involvement of wild-type SOD1 in the abnormal accumulation of SOD1 proteins in spinal cord.
Controversies on pathological involvement of wild-type SOD1 in non-SOD1 ALS
Also in non-SOD1 ALS cases, which are much more prevailing than SOD1-ALS, there are harsh controversies on pathological roles of wild-type SOD1. While few studies have examined the metal binding and/or disulfide status of wild-type SOD1 in ALS, the lack of such post-translational processes is expected to result in the decrease of its enzymatic activity. Indeed, SOD1 activity in brain homogenates of sporadic ALS cases was reported to be decreased [49], but another study confirmed little differences in the activity in several parts of the central nervous system between sporadic ALS cases and non-ALS controls [50]. It should be noted that only the activity but not the amount of SOD1 was compared in those previous reports; therefore, it remains to be concluded whether wild-type SOD1 becomes misfolded and enzymatically inactive under pathological conditions of ALS.
SOD1 is ubiquitously and highly (10–100 μM) expressed as a soluble protein [51–53] (Human Protein Atlas available from http://www.proteinatlas.org) and diffusedly detected in most of subcellular compartments including cytoplasm [54], mitochondria [55], nucleus [56], and endoplasmic reticulum [57]. Based upon many studies using mouse models as well as purified proteins (e.g. [14, 58]), a consensus has been reached on the significantly reduced solubility of SOD1 by ALS-causing mutations, which leads to the formation of detergent-insoluble SOD1 aggregates. It should, however, be noted that only a few studies confirmed the solubility changes of SOD1 proteins in spinal cord tissues of ALS patients (even in those of SOD1-ALS patients).
Bosco et al. prepared insoluble pellets from spinal cord homogenates in detergent-free lysis buffer, where comparable levels of SOD1 proteins were detected among a SOD1-ALS case (A4V mutation), four sporadic ALS cases, and four non-neurological controls [59]. No differences were observed in the amount of 0.1% Nonidet P-40-resistant SOD1 among two SOD1-ALS patients with the homozygous D90A mutations and two controls [47]. In contrast, when spinal cord homogenates were treated with 0.5% Nonidet P-40, significantly more amounts of SOD1 were detected in the insoluble fraction of a SOD1-ALS case (A4V mutation) than those of two familial ALS cases with unknown genetic causes, 12 sporadic ALS cases, and three controls [60]. Significantly more amounts of SOD1 were also detected in the 1% Nonidet P-40-insoluble pellets from two sporadic ALS cases (a non-SOD1 ALS and a case with C9orf72 mutation) as well as two SOD1-ALS cases (A4V and G72C mutations) than those of three Alzheimer’s disease cases and four non-neurological controls [61]. Furthermore, a filter-trap assay using a 0.22 μm cellulose acetate membrane was examined to detect SOD1 aggregates in spinal cord homogenates containing Nonidet P-40 and sodium dodecyl sulfate; wild-type SOD1 aggregates trapped on the membrane were significantly augmented in the lumbar spinal cord of sporadic ALS cases (4 positive/7 total) compared with control subjects (0 positive/6 total) [42]. It is thus possible that SOD1 proteins form detergent-insoluble aggregates in pathological conditions of ALS cases even without SOD1 mutations (Fig. 1, left), but more numbers of studies will be required for conclusions.
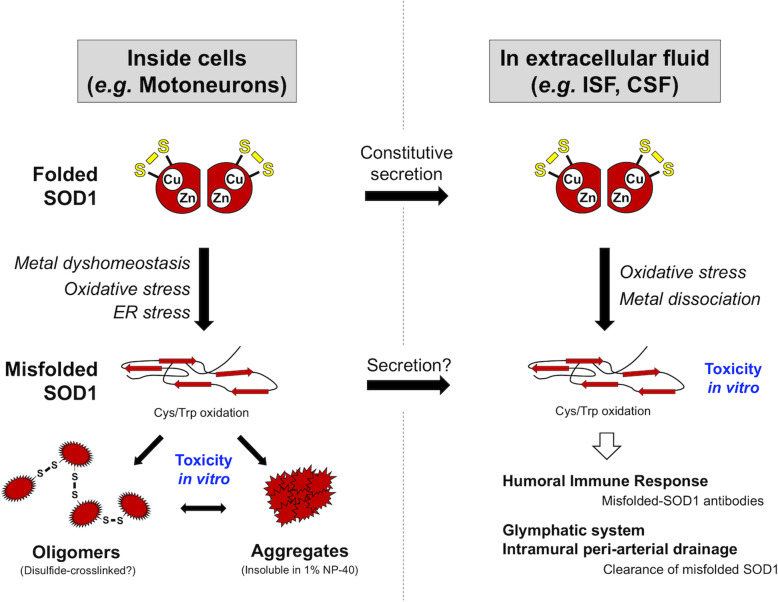
Fig. 1.
Schematic representation on possible changes of wild-type SOD1 in ALS. (Left) A natively folded SOD1 binds copper and zinc ions and forms an intramolecular disulfide bond. Pathological conditions might disrupt intracellular metal homeostasis and augment oxidative stress/ER stress, facilitating the formation of misfolded SOD1 even without any disease-causing mutations. Disulfide-crosslinked oligomers and insoluble aggregates of wild-type SOD1 have been detected in spinal cords of sporadic ALS. (Right) SOD1 has been known to constitutively secreted to extracellular fluid such as ISF and CSF, and recently, toxic wild-type SOD1 in abnormally misfolded conformations was detected in CSF of sporadic ALS. Misfolded SOD1 appears to be cleared by humoral immune response and/or glymphatic/intramural peri-arterial drainage systems, and their failure might contribute to the disease.
Given that SOD1 is highly expressed in most of intracellular compartments, an immunohistochemical method using anti-SOD1 antibodies may be suitable for detection of pathological changes occurring in wild-type SOD1 only if the protein is densely accumulated as inclusion bodies. Indeed, a subset of Lewy body-like (hyaline) inclusions in the anterior horn cells of 10 out of 20 sporadic ALS patients (albeit with no test on SOD1 mutations) were immunoreactive to anti-SOD1 antibodies, while skein-like inclusions and Bunina bodies were not [62–64]. Also, SOD1-immunoreactive inclusions were discerned against background staining in spinal cord motor neurons of a familial ALS patient without SOD1 mutation [50]. In the other study, however, no SOD1-immunoreactivity was confirmed in the hyaline inclusions of all sporadic ALS cases examined (17 cases, again with no mention about SOD1 mutations) [65]. While such a sharp discrepancy among those studies remains to be solved, different SOD1 antibodies were used for immunohistochemical analysis: a rabbit or sheep polyclonal antibody was raised against a holo form of human SOD1 in the former two studies [66], and a rabbit polyclonal antibody was raised against a SOD1 peptide corresponding to Asp124 to Lys136 in the latter [67]. These ideas are challenged by a report showing no SOD1-positive inclusions in non-SOD1 ALS cases with a rabbit polyclonal anti-SOD1 antibody or a mouse monoclonal anti-SOD1 antibody [68]. Nonetheless, misfolding of SOD1 is well expected to affect epitope availability; therefore, the choice of the antibodies is still a key factor to detect any misfolded forms of SOD1 proteins in vivo. Indeed, increasing numbers of studies have examined non-SOD1 ALS cases with conformation-specific antibodies that can discriminate misfolded SOD1 from the natively folded protein in vitro (called misfolded-SOD1 antibodies hereafter).
Immunohistochemical examination on non-SOD1 ALS cases with misfolded-SOD1 antibodies
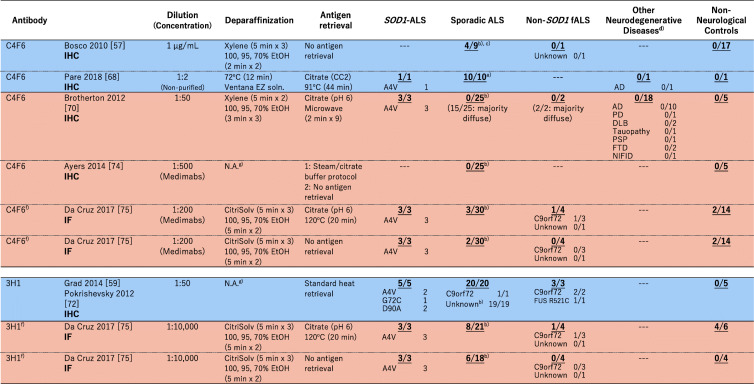
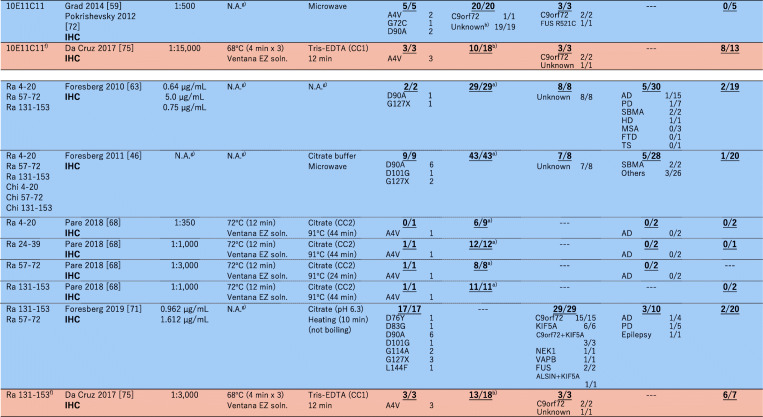
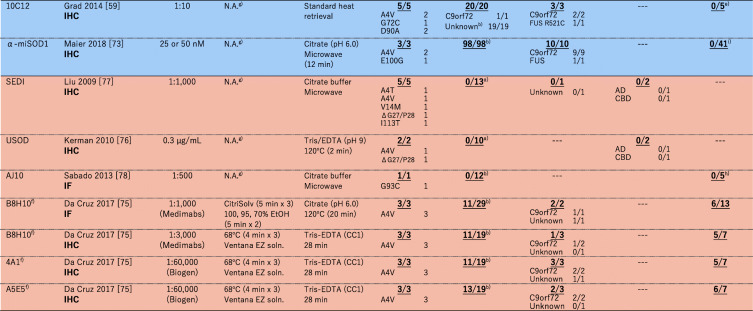
As summarized in a recent comprehensive paper [69] as well as in an excellent review [70], a number of misfolded-SOD1 antibodies have been used for examination of sporadic ALS cases, and the results are sharply divided. In this review, we performed extensive search on the previous reports describing immunohistochemical and/or immunofluorescence examinations on human spinal cord tissues with misfolded-SOD1 antibodies, which is summarized in Table 1. As colored cyan in Table 1, some studies have claimed positive immunostaining of spinal cords (motor neurons and glial cells) selectively in sporadic and familial ALS with misfolded-SOD1 antibodies [46, 50, 59, 61, 69, 73–75]. As reviewed later in detail, a misfolded-SOD1 antibody (α-miSOD1) designed based on an antibody from the healthy elderly subjects was also found to stain spinal cord of sporadic as well as familial ALS patients but not of non-neurological controls [71]. In the other studies (colored orange in Table 1), however, no difference in the staining pattern was observed between ALS and non-ALS controls [72, 74, 76–79]. Some of the misfolded-SOD1 antibodies in Table 1 (in particular, the ones reported from one research group: SEDI, USOD, AJ10, B8H10, 4A1, and A5E5) were found to immunostain spinal motorneurons in SOD1-ALS but not in non-SOD1 ALS, which might simply mean that misfolded conformations of wild-type SOD1 in non-SOD1 ALS are not the same with those of mutant SOD1 in SOD1-ALS. Immunostaining results using mouse monoclonal C4F6, 3H1, 10E11C11 and a rabbit polyclonal Ra 131–153 antibody have been reported from more than two research groups but still did not reach a consensus about the detection of misfolded SOD1 in non-SOD1 ALS cases (Table 1). Much effort has been directed to resolve those discrepancies, which could be caused by differences in experimental procedures including tissue fixation, antigen retrieval, and working concentrations of primary antibodies [69]. Indeed, antigen retrieval treatments in a citrate buffer with heat (boiling, steaming, microwave) are considered to denature SOD1 proteins, which could efficiently expose the epitope for misfolded-SOD1 antibodies [72] but appears not to describe the discrepancy on the immunohistochemical detection of misfolded SOD1 (Table 1).
Table 1.
A summary on immunohistochemical (IHC)/immunofluorescence (IF) detection of misfolded SOD1 with misfolded-SOD1 antibodies in human spinal cords. The numbers in each box represent (the number of misfolded-SOD1-positive cases)/(the number of total cases examined)
aNo SOD1 mutations were confirmed. bThere is no mention on the presence or absence of SOD1 mutations. cNo motor neurons in the five remaining cases. dAD, Alzheimer’s disease, PD Parkinson’s disease, DLB Dementia with Lewy body, PSP Progressive supranuclear palsy, FTD Frontotemporal dementia, NIFID neuronal intermediate filament inclusion disease, SBMA Spinal and bulbar muscular atrophy, HD Huntington’s disease, MSA Multiple system atrophy, TS Tuberous sclerosis, CBD Corticobasal degeneration. eThere is no mention on the non-neurological controls in the paper. fIn this review, the cases with cytoplasmic granular staining, rare round deposits, abundant round deposits, and globular inclusions are counted as misfolded-SOD1 positive, while the cases with no signal, sparse diffuse staining, and abundant diffuse staining are counted as misfoded-SOD1 negative. gNot available (no mention in the paper). hThe control cases are described as “non-ALS controls”. iIn the paper, it was described that “no or only weak immunoreactivity was observed in motor neurons of most of the 41 spinal cord tissue samples from NNC patients”
In immunohistochemical/immunofluorescence analysis of tissues, the experimental procedures/conditions are often not described in detail; in particular, a working concentration of a primary antibody is usually indicated as a dilution factor but not a concentration of the antibody in many studies. These situations prevent us from comparing the previously reported staining results in detail; based upon Table 1, however, a trend can be found that a significant dilution of the misfolded-SOD1 antibodies fails to detect non-SOD1 ALS-specific immunostaining. The antibody C4F6 is commercially available from MediMabs, and the concentration was found to be < 0.05 mg/mL in our hands. Ayers et al. [72] and Da Cruz et al. [77] have reported the absence of C4F6-positive staining in sporadic ALS cases by using the C4F6 antibody from MediMabs in 500-fold and 200-fold dilution (Table 1), which would correspond to < 0.1 and < 0.25 μg/mL of the working concentration, respectively. Instead, Bosco et al. successfully detected C4F6-positive staining with 1.0 μg/mL C4F6 in some of sporadic ALS cases but not in non-neurological controls (Table 1) [59]. Also in the three papers by Grad et al. [61], Pokrishevsky et al. [75], and Da Cruz et al. [77], we have supposed that they used the antibodies 3H1 and 10E11C11 originated from the same source for immunohistochemical examination on misfolded SOD1 (we further assumed the same concentration of the original antibody solution in their studies). Successful detection of misfolded SOD1 in ALS tissues with a lower dilution rate of the antibodies was reported by Grad et al. and Pokrishevsky et al., but Da Cruz et al. appear to have used a significantly diluted solution of the antibody and failed to detect the non-SOD1 ALS-specific, 3H1- and 10E11C11-positive staining. Furthermore, Brännström group prepared a polyclonal antibody Ra 131–153 for detection of misfolded SOD1 proteins and observed the Ra 131–153-positive immunostaining in non-SOD1 ALS as well as SOD1-ALS cases [46, 50, 69, 73]. The antibody was then distributed to the other research group and used for the immunohistochemical examination; however, the Ra 131–153-positive immunostaining was observed in not only ALS but also non-neurological control cases [77], which might be due to an antigen retrieval step using Tris-EDTA-based solution [69]. Collectively, further investigations with more quantitative, detailed descriptions on the experimental procedures (a working concentration of antibodies, in particular) will be definitely required for evaluating immunohistochemical evidence of misfolded SOD1 proteins in non-SOD1 ALS cases.
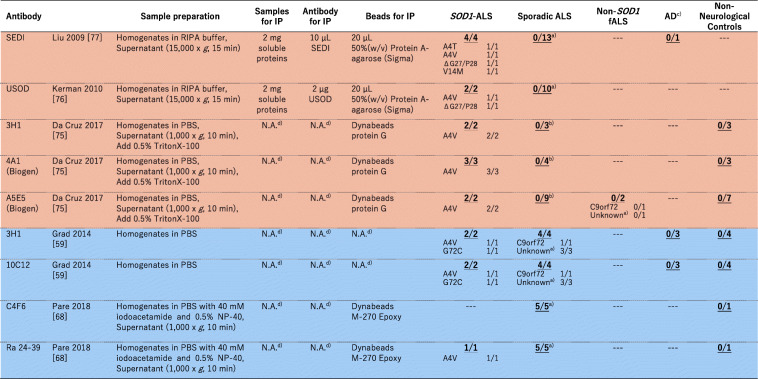
Immunoprecipitation from spinal cords of non-SOD1 ALS with misfolded-SOD1 antibodies
Immunohistochemical examinations require several harsh treatments of tissue samples (depaffinization, antigen retrieval, etc.) that can significantly affect protein conformations; therefore, the presence or absence of misfolded wild-type SOD1 proteins in tissues may not be accurately evaluated. Instead, more accurate evidence on misfolded wild-type SOD1 in ALS could be provided by immunoprecipitation (IP) from unfixed spinal cord homogenates with misfolded-SOD1 antibodies, which are summarized in Table 2. Again, experimental details required for testing reproducibility were not fully described in most of the papers, and the results were sharply divided. Mutant SOD1 in all SOD1-ALS cases examined was successfully immunoprecipitated with any of misfolded-SOD1 antibodies listed in Table 2, and wild-type SOD1 in sporadic ALS cases without SOD1 mutations was also immunoprecipitated in the studies by Grad et al. [61] and Paré et al. [69]. In contrast, the other studies by Liu et al. [78], Kerman et al. [76], and Da Cruz et al. [77] have concluded that no wild-type SOD1 proteins are immunoprecipitated from spinal cords of sporadic ALS cases with misfolded-SOD1 antibodies. Nonetheless, we note that the interpretation on the immunoprecipitation results appears somewhat different among those studies; namely, no SOD1 proteins were observed in immunoprecipitates from sporadic ALS with SEDI (Liu et al. [78]) and USOD (Kerman et al. [76]) antibodies, while the misfolded-SOD1 antibodies (3H1, 4A1, A5E5) used in the Da Cruz et al. paper did immunoprecipitate SOD1 proteins in sporadic ALS cases but also in non-neurological controls [77]. Using the 3H1 antibody, furthermore, Grad et al. were found to immunoprecipitate wild-type SOD1 from spinal cords of sporadic ALS cases but not from those of non-neurological controls [61]. Again, it is highly possible that some differences in experimental procedures influence the detection of misfolded wild-type SOD1 in sporadic ALS tissues, and much more numbers of studies with detailed description on IP methods are definitely required.
Table 2.
A summary on immunoprecipitation of misfolded SOD1 with misfolded-SOD1 antibodies from human spinal cords. The numbers in each box represent (the number of misfolded-SOD1-positive cases)/(the number of total cases examined)
aNo SOD1 mutations were confirmed. bThere is no mention on the presence or absence of SOD1 mutations. cAlzheimer’s disease. dNot available (no mention in the paper)
It is also important to note that wild-type SOD1 immunopurified with anti-SOD1 antibody from spinal cord homogenates of sporadic ALS inhibited anterograde but not retrograde fast axonal transport in the assay using isolated squid axoplasm through a mechanism possibly involving specific activation of p38 MAPK [59]. Such inhibition was no longer observed when the immunopurified SOD1 proteins were first mixed with the misfolded-SOD1 antibody C4F6 and then perfused into squid axoplasm. These results have thus supported toxic and pathogenic roles of misfolded wild-type SOD1 in sporadic ALS (Fig. 1, left).
Misfolded forms of SOD1 in cerebrospinal fluid of ALS
As described, SOD1 is localized mostly in the cytoplasm (Human Protein Atlas, see above), and the intraneuronal inclusions containing SOD1 are the pathological hallmark of SOD1-ALS [5]. Many researchers thus focused on the toxic/conformational properties of SOD1 within cells, even though SOD1 proteins were reported to be present also in the extracellular space by their active and constitutive secretion from cells (Fig. 1, upper) [80, 81]. Recently, misfolded/aggregated proteins are considered to propagate between cells, which would contribute to the pathological progression in many of neurodegenerative diseases including SOD1-ALS [82–86]. For example, premature motor neuron disease in transgenic mice expressing human SOD1 with G85R mutation is triggered by inoculation of detergent-resistant fractions of SOD1 from a SOD1-ALS patient (G127Gfs*7) into the lumbar spinal cord [83]. Also, much attention has been paid on glymphatic system [87] and intramural peri-arterial drainage pathway [88], by which misfolded/aggregated proteins in interstitial fluid (ISF) of the brain and spinal cord could be drained into cerebrospinal fluid (CSF) and then cleared [89]. Regarding SOD1-ALS, indeed, the disease duration of transgenic mice expressing ALS-linked mutant SOD1 was shortened by deletion of aquaporin-4 [90], a water channel playing central roles in the extracellular clearance through the glymphatic system [87]. Furthermore, pathologies and amyloid-β accumulation in transgenic mouse models of Alzheimer’s disease were aggravated by disrupting meningeal lymphatic vessels, which are proposed as a drain of macromolecules from ISF and CSF [91]. Therefore, SOD1 proteins that are secreted from neurons and glia and then possibly drained into CSF will be important in understanding the pathology of ALS.
Indeed, SOD1 is well known as a constituent of CSF, and amounts of SOD1 in CSF tended to increase as a function of age albeit with a low correlation coefficient (r2 = 0.1 ~ 0.2) [92–94]. In most studies, total SOD1 levels in CSF appear to be not significantly different between ALS and neurological/non-neurological controls [92–96]. Alternatively, absolute levels of SOD1 in CSF were reported to show substantial variability among individuals but with little variability in each individual over time [97]. In the same study [97], ALS cases and neurological controls were characterized by slightly higher levels of SOD1 in CSF compared to those of healthy controls; however, the amount of SOD1 in CSF did not correlate with the severity of ALS. In CSF, significant fractions of SOD1 were also reported to be N-terminally truncated, but the amount of such truncated proteins did not differ between ALS and controls, suggesting little pathological roles of the truncated SOD1 in ALS [93, 95]. In electrophoretic analysis of CSF, furthermore, neither SOD1-positive smears nor high-molecular-weight ladders were observed, indicating that detergent-resistant oligomers/aggregates were not evident in CSF of ALS [93, 95]. Based upon those reports, SOD1 in CSF appears to have no pathological roles in ALS. Nonetheless, it is quite notable that, in rats overexpressing wild-type human SOD1, half-life of the SOD1 protein was significantly longer in CSF (14.9 days) as well as in spinal cord (15.9 days) than that in liver and kidney (1.7 and 3.4 days, respectively) [98]. Also in CSF of human subjects, the turnover rate of SOD1 was found to be significantly slower (half-life: 25.0 +/− 7.4 days) than that of total proteins (half-life: 3.6 +/− 1.0 days) [98]. Accordingly, slow turnover rate of SOD1 in CSF as well as in spinal cord would allow sufficient time for SOD1 to become misfolded and to contribute to the development of pathological changes.
To test if SOD1 becomes misfolded in CSF of ALS, CSF samples from 96 ALS cases (57 sporadic ALS, 22 SOD1-ALS, 17 Non-SOD1 familial ALS) and 38 neurological controls were examined with sandwich ELISA using misfolded-SOD1 antibodies (Ra 24–39, Ra 57–72, and Ra 111–127) [94]. Signals indicating the presence of misfolded SOD1 were found in all samples, but no significant differences were confirmed between ALS with and without SOD1 mutations and also between the ALS cases combined and the controls [94]. In contrast, by using other types of misfolded-SOD1 antibodies, we recently showed that wild-type SOD1 proteins were misfolded in CSF of sporadic ALS cases as well as of a SOD1-ALS case [95]. More precisely, sandwich ELISA was performed on CSF from 21 ALS cases (20 sporadic ALS, 1 SOD1-ALS) and 40 controls by using misfolded-SOD1 antibodies (C4F6, UβB, EDI, apoSOD, 24–39 and SOD1int). Among those, C4F6, UβB, EDI, and apoSOD were found to give significantly higher signals in CSF of ALS cases compared to those of controls; in contrast, no differences were observed with 24–39 and SOD1int. It was also surprising to us that large fractions of SOD1 in CSF of sporadic ALS cases were immunoprecipitated with C4F6 antibody [95]. CSF collected from ALS patients has been known to exert toxicity toward motor-neuron like cells NSC-34 [99], and we revealed that the toxicity was alleviated by removing the misfolded SOD1 from CSF with immunoprecipitation using C4F6 antibody [95]. It is also notable that misfolded SOD1 immunoreactive to C4F6 and UβB was observed, albeit with less amount, in CSF of a subset of patients with Parkinson’s disease (PD) and progressive supranuclear palsy (PSP). Therefore, not all types of misfolded-SOD1 antibodies could detect pathological forms of wild-type SOD1 in CSF, but our study has suggested that wild-type SOD1 in CSF adopts a misfolded, toxic conformation(s) in pathological conditions of ALS and also a subset of PD and PSP. In that sense, it is important to note that levels of SOD1 in CSF of SOD1-ALS patients were reduced by oral medication with pyrimethamine [100].
Misfolding of wild-type SOD1 under oxidative environment of spinal cord and CSF
Another important issue to be solved is where SOD1 is misfolded; in other words, it remains to be tested whether SOD1 is misfolded in CSF, or misfolded SOD1 in affected spinal cord (or some other tissues) is drained into CSF (Fig. 1). As of now, we do not have an answer to this question; nonetheless, one of the notable features observed commonly in spinal cord and CSF of ALS patients is significantly elevated levels of oxidative markers, which has been summarized in an excellent review [101]. It is thus plausible that oxidative environment in the spinal cord/CSF of ALS is important to understand any pathological changes occurring in SOD1.
In accordance with this, we have detected abnormal SOD1 oligomers crosslinked via intermolecular disulfide bonds in spinal cord of SOD1-ALS cases as well as transgenic mice expressing human SOD1 with ALS mutations (G37R, G93A, and L126Z) [31, 102]. While the disulfide-crosslinked SOD1 oligomers were not evident in CSF of sporadic ALS cases and a SOD1-ALS case [95], reductant (DTT)-sensitive aggregates of wild-type SOD1 were detected in affected spinal cord of sporadic ALS cases [42]. Furthermore, Xu et al. suggested the oxidation of Cys111 in SOD1 to a sulfenic acid (−SOH) in CSF of a subset of sporadic ALS cases [103], and we also found that Cys111 was oxidized to a sulfonic acid (−SO3H) in CSF of a subset of ALS, PD, PSP, and AD cases [95]. In our experiments in vitro [104], followed by the sulfenylation of Cys111 in metal-bound SOD1 with H2O2, dissociation of the bound metal ions from the protein was found to allow another free Cys residue (Cys6) to attack the sulfenylated Cys111. SOD1 has a canonical intramolecular disulfide bond between Cys57 and Cys146; therefore, oxidation with H2O2 led to the formation of abnormal SOD1 (SOD12xS-S) with two intramolecular disulfide bonds (Cys6-Cys111 and Cys57-Cys146), and SOD12xS-S was prone to aggregation and also toxic to motor-neuron like cells NSC-34 [104].
As summarized above, Cys is considered to be the most susceptible to oxidation among amino acids and would hence be a key residue for oxidative modifications under pathological conditions. Notably, several other oxidized forms of SOD1 have been also reported in cell lines, transgenic mice, and purified SOD1 proteins. For example, SOD1 proteins with oxidized carbonyl groups were detected in lymphoblasts derived from sporadic ALS with bulbar onset [105]. SOD1 oxidized at tryptophan (Trp32) was found to accumulate in the microsomal fractions purified from spinal cord of transgenic mice expressing wild-type human SOD1 [42] and was also detected in human blood and the blood isolated from transgenic mice expressing wild-type or ALS-linked mutant human SOD1 [106]. Furthermore, several His residues as well as Trp32 are also susceptible to oxidation, which has been proposed to trigger the aggregation of SOD1 in vitro [107–110]. It, however, remains to be tested whether the His and/or Trp oxidations occur on SOD1 in ALS patients.
Misfolded SOD1 in extracellular fluid as a potential immunotherapeutic target
As reviewed above, formation of misfolded and plausibly toxic SOD1 species in extracellular fluid is well expected as a pathological change occurring in ALS cases. This could in turn open the way to alleviate the disease by removing such extracellular SOD1 proteins with the humoral immune response. Indeed, the survival of transgenic mice expressing ALS-linked mutant SOD1 was extended by vaccination with full-length misfolded SOD1 proteins [111, 112] and with peptides corresponding to the region available only in misfolded SOD1 [113, 114]. Passive immunization with several misfolded-SOD1 antibodies was also reported to be beneficial to the SOD1-ALS model mice [112, 115–117] except for one study [118]. Furthermore, sera from sporadic ALS patients were found to contain IgM antibodies reacting with misfolded SOD1 (recombinant SOD1 oxidized with 10 mM H2O2), and the sporadic ALS cases with higher levels of the IgM antibodies (n = 153) exhibited a longer survival of 6.4 years than the subjects lacking those antibodies (n = 127) [119].
Notably, Maier et al. screened human memory B cell repertoires from a large cohort of healthy elderly subjects and successfully generated a monoclonal antibody (α-miSOD1) that can react selectively with misfolded/oxidized SOD1 but not with native SOD1 [71]. Based upon the presence of B cell memory against misfolded SOD1 in a majority of those healthy elderly subjects, Maier et al. suggested that misfolding of SOD1 and the subsequent humoral immune response are frequent events in the elderly [71]. This antibody, α-miSOD1, was found to stain motor neurons of the spinal cord samples from ALS including sporadic as well as familial cases with and without SOD1 mutations, but not from non-neurological controls (Table 1) [71]. Furthermore, intracerebroventricular infusion and also intraperitoneal injections of α-miSOD1 antibody to transgenic mice expressing ALS-linked mutant human SOD1 (G37R and G93A) delayed the onset of motor symptoms and extended survival [71]. Therefore, clearance of misfolded SOD1 by utilizing the immune system would be a potential treatment for patients with sporadic as well as familial ALS; nonetheless, it should be also noted that, in sera of sporadic ALS subjects, higher levels of IgG antibodies reacting with normal wild-type SOD1 associated with a shorter survival of 4.1 years [119]. For successful immunotherapy to treat ALS, it will be critical to develop antibodies specifically recognizing toxic, misfolded SOD1 and/or to design antigens efficiently producing such antibodies.
Conclusions
While misfolding of ALS-linked mutant SOD1 has been established as a pathological change occurring in SOD1-ALS, roles of wild-type SOD1 in more prevailing non-SOD1 ALS have long been debated. Even in SOD1-ALS, involvement of wild-type SOD1 in the pathology remains obscure. As reviewed above, we performed an extensive literature search and found that a number of studies supported the presence of misfolded wild-type SOD1 in spinal cord and CSF of non-SOD1 ALS cases (Fig. 1). Nonetheless, not all studies detected misfolded wild-type SOD1 proteins in non-SOD1 ALS, possibly suggesting the importance of experimental conditions in their immunohistochemical and immunochemical detection. Also, some of misfolded-SOD1 antibodies gave positive signals in SOD1-ALS but not in non-SOD1 ALS, which may indicate distinct conformations of misfolded SOD1 between SOD1-ALS and non-SOD1 ALS. As we recently reported [95], CSF of non-SOD1 ALS contained misfolded forms of wild-type SOD1. The misfolded SOD1 in CSF was toxic to cultured cells, but it still needs to be tested whether it is a pathogenic species causing degeneration of motor neurons. Quite notably, misfolding of SOD1 could occur in the healthy elderly, and the humoral immune response to the misfolded SOD1 would be a key to prevent ALS. Consistent with beneficial results of immunization-based treatment of transgenic mouse models, therefore, immunological modulation of misfolded SOD1 in extracellular fluids such as CSF would be a promising strategy to delay onset and/or relieve symptoms of ALS.
Acknowledgements
Not applicable.
Abbreviations
- ALS
Amyotrophic lateral sclerosis
- CCS
Copper chaperone for SOD1
- CSF
Cerebrospinal fluid
- ISF
Interstitial fluid
- non-SOD1 ALS
Amyotrophic lateral sclerosis without mutations in the SOD1 gene
- PD
Parkinson’s disease
- PSP
Progressive supranuclear palsy
- SOD1
Cu/Zn-superoxide dismutase
- SOD1-ALS
Amyotrophic lateral sclerosis with mutations in the SOD1 gene
Authors’ contributions
YF and ET conducted the literature review, wrote the manuscript, read and approved the final manuscript.
Funding
This work was supported by Grants-in-Aid 16H04768 for Scientific Research (B) (to YF) and 19H05765 for Scientific Research on Innovative Areas (to YF) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and also supported by the Pharmacological Research Foundation, Tokyo (to ET).
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Yoshiaki Furukawa, Email: furukawa@chem.keio.ac.jp.
Eiichi Tokuda, Email: tokuda.eiichi@nihon-u.ac.jp.
References
- 1.Andersen PM, Al-Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol. 2011;7:603–615. doi: 10.1038/nrneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- 2.Jaiswal MK. Riluzole and edaravone: a tale of two amyotrophic lateral sclerosis drugs. Med Res Rev. 2019;39:733–748. doi: 10.1002/med.21528. [DOI] [PubMed] [Google Scholar]
- 3.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. [DOI] [PubMed]
- 4.Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. 2013;14:248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- 5.Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 6.Trist B, Hilton JB, Crouch PJ, Hare DJ, Double KL. Superoxide dismutase 1 in health and disease: How a front-line antioxidant becomes neurotoxic. Angew Chem Int Ed Engl. 2020;in press. [DOI] [PMC free article] [PubMed]
- 7.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 8.Saccon RA, Bunton-Stasyshyn RK, Fisher EM, Fratta P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain. 2013;136:2342–2358. doi: 10.1093/brain/awt097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen PM, Nordstrom U, Tsiakas K, Johannsen J, Volk AE, Bierhals T, et al. Phenotype in an infant with SOD1 homozygous truncating mutation. N Engl J Med. 2019;381:486–488. doi: 10.1056/NEJMc1905039. [DOI] [PubMed] [Google Scholar]
- 10.Park JH, Elpers C, Reunert J, McCormick ML, Mohr J, Biskup S, et al. SOD1 deficiency: a novel syndrome distinct from amyotrophic lateral sclerosis. Brain. 2019;142:2230–2237. doi: 10.1093/brain/awz182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog Neurobiol. 2008;85:94–134. doi: 10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa Y, O'Halloran TV. Posttranslational modifications in Cu,Zn-superoxide dismutase and mutations associated with amyotrophic lateral sclerosis. Antioxid Redox Signal. 2006;8:847–867. doi: 10.1089/ars.2006.8.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forman HJ, Fridovich I. On the stability of bovine superoxide dismutase. The effects of metals. J Biol Chem. 1973;248:2645–2649. [PubMed] [Google Scholar]
- 14.Furukawa Y, O'Halloran TV. Amyotrophic lateral sclerosis mutations have the greatest destabilizing effect on the apo, reduced form of SOD1, leading to unfolding and oxidative aggregation. J Biol Chem. 2005;280:17266–17274. doi: 10.1074/jbc.M500482200. [DOI] [PubMed] [Google Scholar]
- 15.Stathopulos PB, Rumfeldt JA, Scholz GA, Irani RA, Frey HE, Hallewell RA, et al. Cu/Zn superoxide dismutase mutants associated with amyotrophic lateral sclerosis show enhanced formation of aggregates in vitro. Proc Natl Acad Sci U S A. 2003;100:7021–7026. doi: 10.1073/pnas.1237797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayward LJ, Rodriguez JA, Kim JW, Tiwari A, Goto JJ, Cabelli DE, et al. Decreased metallation and activity in subsets of mutant superoxide dismutases associated with familial amyotrophic lateral sclerosis. J Biol Chem. 2002;277:15923–15931. doi: 10.1074/jbc.M112087200. [DOI] [PubMed] [Google Scholar]
- 17.Tiwari A, Hayward LJ. Familial amyotrophic lateral sclerosis mutants of copper/zinc superoxide dismutase are susceptible to disulfide reduction. J Biol Chem. 2003;278:5984–5992. doi: 10.1074/jbc.M210419200. [DOI] [PubMed] [Google Scholar]
- 18.Hilton JB, Mercer SW, Lim NK, Faux NG, Buncic G, Beckman JS, et al. CuII (atsm) improves the neurological phenotype and survival of SOD1G93A mice and selectively increases enzymatically active SOD1 in the spinal cord. Sci Rep. 2017;7:42292. doi: 10.1038/srep42292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts BR, Lim NK, McAllum EJ, Donnelly PS, Hare DJ, Doble PA, et al. Oral treatment with Cu(II)(atsm) increases mutant SOD1 in vivo but protects motor neurons and improves the phenotype of a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2014;34:8021–31. [DOI] [PMC free article] [PubMed]
- 20.Soon CP, Donnelly PS, Turner BJ, Hung LW, Crouch PJ, Sherratt NA, et al. Diacetylbis(N(4)-methylthiosemicarbazonato) copper (II) (CuII (atsm)) protects against peroxynitrite-induced nitrosative damage and prolongs survival in amyotrophic lateral sclerosis mouse model. J Biol Chem. 2011;286:44035–44044. doi: 10.1074/jbc.M111.274407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culotta VC, Klomp LWJ, Strain J, Casareno RLB, Krems B, Gitlin JD. The copper chaperone for superoxide dismutase. J Biol Chem. 1997;272:23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- 22.Wong PC, Waggoner D, Subramaniam JR, Tessarollo L, Bartnikas TB, Culotta VC, et al. Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc Natl Acad Sci U S A. 2000;97:2886–2891. doi: 10.1073/pnas.040461197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams JR, Trias E, Beilby PR, Lopez NI, Labut EM, Bradford CS, et al. Copper delivery to the CNS by CuATSM effectively treats motor neuron disease in SOD(G93A) mice co-expressing the copper-chaperone-for-SOD. Neurobiol Dis. 2016;89:1–9. doi: 10.1016/j.nbd.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Son M, Fu Q, Puttaparthi K, Matthews CM, Elliott JL. Redox susceptibility of SOD1 mutants is associated with the differential response to CCS over-expression in vivo. Neurobiol Dis. 2009;34:155–162. doi: 10.1016/j.nbd.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son M, Puttaparthi K, Kawamata H, Rajendran B, Boyer PJ, Manfredi G, et al. Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc Natl Acad Sci U S A. 2007;104:6072–6077. doi: 10.1073/pnas.0610923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Son M, Srikanth U, Puttaparthi K, Luther C, Elliott JL. Biochemical properties and in vivo effects of the SOD1 zinc-binding site mutant (H80G) J Neurochem. 2011;118:891–901. doi: 10.1111/j.1471-4159.2011.07360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furukawa Y, Anzai I, Akiyama S, Imai M, Cruz FJ, Saio T, et al. Conformational disorder of the most immature Cu,Zn-superoxide dismutase leading to amyotrophic lateral sclerosis. J Biol Chem. 2016;291:4144–55. [DOI] [PMC free article] [PubMed]
- 28.Furukawa Y. Protein aggregates in pathological inclusions of amyotrophic lateral sclerosis. In: Maurer MH. Amyotrophic lateral sclerosis. InTech; 2012. p. 335–356.
- 29.Deng HX, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, et al. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci U S A. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukada K, Nagano S, Satoh M, Tohyama C, Nakanishi T, Shimizu A, et al. Stabilization of mutant cu/Zn superoxide dismutase (SOD1) protein by coexpressed wild SOD1 protein accelerates the disease progression in familial amyotrophic lateral sclerosis mice. Eur J Neurosci. 2001;14:2032–2036. doi: 10.1046/j.0953-816x.2001.01828.x. [DOI] [PubMed] [Google Scholar]
- 31.Furukawa Y, Fu R, Deng HX, Siddique T, O'Halloran TV. Disulfide cross-linked protein represents a significant fraction of ALS-associated Cu,Zn-superoxide dismutase aggregates in spinal cords of model mice. Proc Natl Acad Sci U S A. 2006;103:7148–53. [DOI] [PMC free article] [PubMed]
- 32.Jaarsma D, Haasdijk ED, Grashorn JA, Hawkins R, van Duijn W, Verspaget HW, et al. Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol Dis. 2000;7:623–43. [DOI] [PubMed]
- 33.Prudencio M, Durazo A, Whitelegge JP, Borchelt DR. An examination of wild-type SOD1 in modulating the toxicity and aggregation of ALS-associated mutant SOD1. Hum Mol Genet. 2010;19:4774–4789. doi: 10.1093/hmg/ddq408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Deng HX, Grisotti G, Zhai H, Siddique T, Roos RP. Wild-type SOD1 overexpression accelerates disease onset of a G85R SOD1 mouse. Hum Mol Genet. 2009;18:1642–1651. doi: 10.1093/hmg/ddp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prudencio M, Lelie H, Brown HH, Whitelegge JP, Valentine JS, Borchelt DR. A novel variant of human superoxide dismutase 1 harboring amyotrophic lateral sclerosis-associated and experimental mutations in metal-binding residues and free cysteines lacks toxicity in vivo. J Neurochem. 2012;121:475–485. doi: 10.1111/j.1471-4159.2012.07690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brasil AA, de Carvalho MDC, Gerhardt E, Queiroz DD, Pereira MD, Outeiro TF, et al. Characterization of the activity, aggregation, and toxicity of heterodimers of WT and ALS-associated mutant Sod1. Proc Natl Acad Sci U S A. 2019;116:25991–26000. doi: 10.1073/pnas.1902483116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi Y, Acerson MJ, Abdolvahabi A, Mowery RA, Shaw BF. Gibbs energy of superoxide dismutase Heterodimerization accounts for variable survival in amyotrophic lateral sclerosis. J Am Chem Soc. 2016;138:5351–5362. doi: 10.1021/jacs.6b01742. [DOI] [PubMed] [Google Scholar]
- 38.Audet JN, Gowing G, Julien JP. Wild-type human SOD1 overexpression does not accelerate motor neuron disease in mice expressing murine Sod1(G86R) Neurobiol Dis. 2010;40:245–250. doi: 10.1016/j.nbd.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 39.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 40.Jonsson PA, Graffmo KS, Brannstrom T, Nilsson P, Andersen PM, Marklund SL. Motor neuron disease in mice expressing the wild type-like D90A mutant superoxide dismutase-1. J Neuropathol Exp Neurol. 2006;65:1126–1136. doi: 10.1097/01.jnen.0000248545.36046.3c. [DOI] [PubMed] [Google Scholar]
- 41.Killoy KM, Harlan BA, Pehar M, Helke KL, Johnson JA, Vargas MR. Decreased glutathione levels cause overt motor neuron degeneration in hSOD1(WT) over-expressing mice. Exp Neurol. 2018;302:129–135. doi: 10.1016/j.expneurol.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medinas DB, Rozas P, Martinez Traub F, Woehlbier U, Brown RH, Bosco DA, et al. Endoplasmic reticulum stress leads to accumulation of wild-type SOD1 aggregates associated with sporadic amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2018;115:8209–8214. doi: 10.1073/pnas.1801109115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graffmo KS, Forsberg K, Bergh J, Birve A, Zetterstrom P, Andersen PM, et al. Expression of wild-type human superoxide dismutase-1 in mice causes amyotrophic lateral sclerosis. Hum Mol Genet. 2013;22:51–60. doi: 10.1093/hmg/dds399. [DOI] [PubMed] [Google Scholar]
- 44.Jonsson PA, Bergemalm D, Andersen PM, Gredal O, Brannstrom T, Marklund SL. Inclusions of amyotrophic lateral sclerosis-linked superoxide dismutase in ventral horns, liver, and kidney. Ann Neurol. 2008;63:671–675. doi: 10.1002/ana.21356. [DOI] [PubMed] [Google Scholar]
- 45.Jonsson PA, Ernhill K, Andersen PM, Bergemalm D, Brannstrom T, Gredal O, et al. Minute quantities of misfolded mutant superoxide dismutase-1 cause amyotrophic lateral sclerosis. Brain. 2004;127:73–88. doi: 10.1093/brain/awh005. [DOI] [PubMed] [Google Scholar]
- 46.Forsberg K, Andersen PM, Marklund SL, Brannstrom T. Glial nuclear aggregates of superoxide dismutase-1 are regularly present in patients with amyotrophic lateral sclerosis. Acta Neuropathol. 2011;121:623–634. doi: 10.1007/s00401-011-0805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jonsson PA, Graffmo KS, Andersen PM, Marklund SL, Brannstrom T. Superoxide dismutase in amyotrophic lateral sclerosis patients homozygous for the D90A mutation. Neurobiol Dis. 2009;36:421–424. doi: 10.1016/j.nbd.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Fujisawa T, Homma K, Yamaguchi N, Kadowaki H, Tsuburaya N, Naguro I, et al. A novel monoclonal antibody reveals a conformational alteration shared by amyotrophic lateral sclerosis-linked SOD1 mutants. Ann Neurol. 2012;72:739–749. doi: 10.1002/ana.23668. [DOI] [PubMed] [Google Scholar]
- 49.Bowling AC, Schulz JB, Brown RH, Jr, Beal MF. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1993;61:2322–2325. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 50.Forsberg K, Jonsson PA, Andersen PM, Bergemalm D, Graffmo KS, Hultdin M, et al. Novel antibodies reveal inclusions containing non-native SOD1 in sporadic ALS patients. PLoS One. 2010;5:e11552. doi: 10.1371/journal.pone.0011552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 52.Thul PJ, Akesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, et al. A subcellular map of the human proteome. Science. 2017;356:eaal3321. [DOI] [PubMed]
- 53.Uhlen M, Zhang C, Lee S, Sjostedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357:eaan2507. [DOI] [PubMed]
- 54.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 55.Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria - A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem. 2001;276:38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 56.Tsang CK, Liu Y, Thomas J, Zhang Y, Zheng XF. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat Commun. 2014;5:3446. doi: 10.1038/ncomms4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mondola P, Ruggiero G, Seru R, Damiano S, Grimaldi S, Garbi C, et al. The Cu,Zn superoxide dismutase in neuroblastoma SK-N-BE cells is exported by a microvesicles dependent pathway. Brain Res Mol Brain Res. 2003;110:45–51. doi: 10.1016/s0169-328x(02)00583-1. [DOI] [PubMed] [Google Scholar]
- 58.Jonsson PA, Graffmo KS, Andersen PM, Brannstrom T, Lindberg M, Oliveberg M, et al. Disulphide-reduced superoxide dismutase-1 in CNS of transgenic amyotrophic lateral sclerosis models. Brain. 2006;129:451–464. doi: 10.1093/brain/awh704. [DOI] [PubMed] [Google Scholar]
- 59.Bosco DA, Morfini G, Karabacak NM, Song Y, Gros-Louis F, Pasinelli P, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG, et al. Copper-binding-site-null SOD1 causes ALS in transgenic mice: aggregates of non-native SOD1 delineate a common feature. Hum Mol Genet. 2003;12:2753–2764. doi: 10.1093/hmg/ddg312. [DOI] [PubMed] [Google Scholar]
- 61.Grad LI, Yerbury JJ, Turner BJ, Guest WC, Pokrishevsky E, O'Neill MA, et al. Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosome-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2014;111:3620–5. [DOI] [PMC free article] [PubMed]
- 62.Matsumoto S, Kusaka H, Ito H, Shibata N, Asayama T, Imai T. Sporadic amyotrophic lateral sclerosis with dementia and Cu/Zn superoxide dismutase-positive Lewy body-like inclusions. Clin Neuropathol. 1996;15:41–6. [PubMed]
- 63.Shibata N, Asayama K, Hirano A, Kobayashi M. Immunohistochemical study on superoxide dismutases in spinal cords from autopsied patients with amyotrophic lateral sclerosis. Dev Neurosci. 1996;18:492–498. doi: 10.1159/000111445. [DOI] [PubMed] [Google Scholar]
- 64.Shibata N, Hirano A, Kobayashi M, Sasaki S, Kato T, Matsumoto S, et al. Cu/Zn superoxide dismutase-like immunoreactivity in Lewy body-like inclusions of sporadic amyotrophic lateral sclerosis. Neurosci Lett. 1994;179:149–152. doi: 10.1016/0304-3940(94)90956-3. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe M, Dykes-Hoberg M, Culotta VC, Price DL, Wong PC, Rothstein JD. Histological evidence of protein aggregation in mutant SOD1 transgenic mice and in amyotrophic lateral sclerosis neural tissues. Neurobiol Dis. 2001;8:933–941. doi: 10.1006/nbdi.2001.0443. [DOI] [PubMed] [Google Scholar]
- 66.Asayama K, Janco RL, Burr IM. Selective induction of manganous superoxide dismutase in human monocytes. Am J Phys. 1985;249:C393–C397. doi: 10.1152/ajpcell.1985.249.5.C393. [DOI] [PubMed] [Google Scholar]
- 67.Pardo CA, Xu Z, Borchelt DR, Price DL, Sisodia SS, Cleveland DW. Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc Natl Acad Sci U S A. 1995;92:954–958. doi: 10.1073/pnas.92.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keller BA, Volkening K, Droppelmann CA, Ang LC, Rademakers R, Strong MJ. Co-aggregation of RNA binding proteins in ALS spinal motor neurons: evidence of a common pathogenic mechanism. Acta Neuropathol. 2012;124:733–747. doi: 10.1007/s00401-012-1035-z. [DOI] [PubMed] [Google Scholar]
- 69.Pare B, Lehmann M, Beaudin M, Nordstrom U, Saikali S, Julien JP, et al. Misfolded SOD1 pathology in sporadic amyotrophic lateral sclerosis. Sci Rep. 2018;8:14223. doi: 10.1038/s41598-018-31773-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rotunno MS, Bosco DA. An emerging role for misfolded wild-type SOD1 in sporadic ALS pathogenesis. Front Cell Neurosci. 2013;7:253. doi: 10.3389/fncel.2013.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maier M, Welt T, Wirth F, Montrasio F, Preisig D, McAfoose J, et al. A human-derived antibody targets misfolded SOD1 and ameliorates motor symptoms in mouse models of amyotrophic lateral sclerosis. Sci Transl Med. 2018;10:eaah3924. [DOI] [PubMed]
- 72.Ayers JI, Xu G, Pletnikova O, Troncoso JC, Hart PJ, Borchelt DR. Conformational specificity of the C4F6 SOD1 antibody; low frequency of reactivity in sporadic ALS cases. Acta Neuropathol Commun. 2014;2:55. [DOI] [PMC free article] [PubMed]
- 73.Forsberg K, Graffmo K, Pakkenberg B, Weber M, Nielsen M, Marklund S, et al. Misfolded SOD1 inclusions in patients with mutations in C9orf72 and other ALS/FTD-associated genes. J Neurol Neurosurg Psychiatry. 2019;90:861–9. [DOI] [PMC free article] [PubMed]
- 74.Brotherton TE, Li Y, Cooper D, Gearing M, Julien JP, Rothstein JD, et al. Localization of a toxic form of superoxide dismutase 1 protein to pathologically affected tissues in familial ALS. Proc Natl Acad Sci U S A. 2012;109:5505–5510. doi: 10.1073/pnas.1115009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pokrishevsky E, Grad LI, Yousefi M, Wang J, Mackenzie IR, Cashman NR. Aberrant localization of FUS and TDP43 is associated with misfolding of SOD1 in amyotrophic lateral sclerosis. PLoS One. 2012;7:e35050. doi: 10.1371/journal.pone.0035050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kerman A, Liu HN, Croul S, Bilbao J, Rogaeva E, Zinman L, et al. Amyotrophic lateral sclerosis is a non-amyloid disease in which extensive misfolding of SOD1 is unique to the familial form. Acta Neuropathol. 2010;119:335–344. doi: 10.1007/s00401-010-0646-5. [DOI] [PubMed] [Google Scholar]
- 77.Da Cruz S, Bui A, Saberi S, Lee SK, Stauffer J, McAlonis-Downes M, et al. Misfolded SOD1 is not a primary component of sporadic ALS. Acta Neuropathol. 2017;134:97–111. doi: 10.1007/s00401-017-1688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu HN, Sanelli T, Horne P, Pioro EP, Strong MJ, Rogaeva E, et al. Lack of evidence of monomer/misfolded superoxide dismutase-1 in sporadic amyotrophic lateral sclerosis. Ann Neurol. 2009;66:75–80. doi: 10.1002/ana.21704. [DOI] [PubMed] [Google Scholar]
- 79.Sabado J, Casanovas A, Hernandez S, Piedrafita L, Hereu M, Esquerda JE. Immunodetection of disease-associated conformers of mutant cu/zn superoxide dismutase 1 selectively expressed in degenerating neurons in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2013;72:646–661. doi: 10.1097/NEN.0b013e318297fd10. [DOI] [PubMed] [Google Scholar]
- 80.Cruz-Garcia D, Brouwers N, Duran JM, Mora G, Curwin AJ, Malhotra V. A diacidic motif determines unconventional secretion of wild-type and ALS-linked mutant SOD1. J Cell Biol. 2017;216:2691–2700. doi: 10.1083/jcb.201704056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mondola P, Damiano S, Sasso A, Santillo M. The Cu, Zn superoxide dismutase: not only a dismutase enzyme. Front Physiol. 2016;7:594. doi: 10.3389/fphys.2016.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ayers JI, Diamond J, Sari A, Fromholt S, Galaleldeen A, Ostrow LW, et al. Distinct conformers of transmissible misfolded SOD1 distinguish human SOD1-FALS from other forms of familial and sporadic ALS. Acta Neuropathol. 2016;132:827–840. doi: 10.1007/s00401-016-1623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ekhtiari Bidhendi E, Bergh J, Zetterstrom P, Forsberg K, Pakkenberg B, Andersen PM, et al. Mutant superoxide dismutase aggregates from human spinal cord transmit amyotrophic lateral sclerosis. Acta Neuropathol. 2018;136:939–953. doi: 10.1007/s00401-018-1915-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grad LI, Guest WC, Yanai A, Pokrishevsky E, O'Neill MA, Gibbs E, et al. Intermolecular transmission of superoxide dismutase 1 misfolding in living cells. Proc Natl Acad Sci U S A. 2011;108:16398–16403. doi: 10.1073/pnas.1102645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Polymenidou M, Cleveland DW. The seeds of neurodegeneration: prion-like spreading in ALS. Cell. 2011;147:498–508. doi: 10.1016/j.cell.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soto C. Transmissible proteins: expanding the prion heresy. Cell. 2012;149:968–977. doi: 10.1016/j.cell.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 89.Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer's disease. Neurobiol Dis. 2016;93:215–225. doi: 10.1016/j.nbd.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watanabe-Matsumoto S, Moriwaki Y, Okuda T, Ohara S, Yamanaka K, Abe Y, et al. Dissociation of blood-brain barrier disruption and disease manifestation in an aquaporin-4-deficient mouse model of amyotrophic lateral sclerosis. Neurosci Res. 2018;133:48–57. doi: 10.1016/j.neures.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 91.Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. 2018;560:185–191. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frutiger K, Lukas TJ, Gorrie G, Ajroud-Driss S, Siddique T. Gender difference in levels of cu/Zn superoxide dismutase (SOD1) in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9:184–7. [DOI] [PubMed]
- 93.Jacobsson J, Jonsson PA, Andersen PM, Forsgren L, Marklund SL. Superoxide dismutase in CSF from amyotrophic lateral sclerosis patients with and without CuZn-superoxide dismutase mutations. Brain. 2001;124:1461–1466. doi: 10.1093/brain/124.7.1461. [DOI] [PubMed] [Google Scholar]
- 94.Zetterstrom P, Andersen PM, Brannstrom T, Marklund SL. Misfolded superoxide dismutase-1 in CSF from amyotrophic lateral sclerosis patients. J Neurochem. 2011;117:91–99. doi: 10.1111/j.1471-4159.2011.07177.x. [DOI] [PubMed] [Google Scholar]
- 95.Tokuda E, Takei Y, Ohara S, Fujiwara N, Hozumi I, Furukawa Y. Wild-type Cu/Zn-superoxide dismutase is misfolded in cerebrospinal fluid of sporadic amyotrophic lateral sclerosis. Mol Neurodegener. 2019;14:42. [DOI] [PMC free article] [PubMed]
- 96.Yoshida E, Mokuno K, Aoki S, Takahashi A, Riku S, Murayama T, et al. Cerebrospinal fluid levels of superoxide dismutases in neurological diseases detected by sensitive enzyme immunoassays. J Neurol Sci. 1994;124:25–31. doi: 10.1016/0022-510x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 97.Winer L, Srinivasan D, Chun S, Lacomis D, Jaffa M, Fagan A, et al. SOD1 in cerebral spinal fluid as a pharmacodynamic marker for antisense oligonucleotide therapy. JAMA Neurol. 2013;70:201–207. doi: 10.1001/jamaneurol.2013.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Crisp MJ, Mawuenyega KG, Patterson BW, Reddy NC, Chott R, Self WK, et al. In vivo kinetic approach reveals slow SOD1 turnover in the CNS. J Clin Invest. 2015;125:2772–2780. doi: 10.1172/JCI80705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vijayalakshmi K, Alladi PA, Sathyaprabha TN, Subramaniam JR, Nalini A, Raju TR. Cerebrospinal fluid from sporadic amyotrophic lateral sclerosis patients induces degeneration of a cultured motor neuron cell line. Brain Res. 2009;1263:122–133. doi: 10.1016/j.brainres.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 100.Lange DJ, Shahbazi M, Silani V, Ludolph AC, Weishaupt JH, Ajroud-Driss S, et al. Pyrimethamine significantly lowers cerebrospinal fluid Cu/Zn superoxide dismutase in amyotrophic lateral sclerosis patients with SOD1 mutations. Ann Neurol. 2017;81:837–48. [DOI] [PMC free article] [PubMed]
- 101.Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med. 2010;48:629–641. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 102.Tokuda E, Anzai I, Nomura T, Toichi K, Watanabe M, Ohara S, et al. Immunochemical characterization on pathological oligomers of mutant Cu/Zn-superoxide dismutase in amyotrophic lateral sclerosis. Mol Neurodegener. 2017;12:2. [DOI] [PMC free article] [PubMed]
- 103.Xu WC, Liang JZ, Li C, He ZX, Yuan HY, Huang BY, et al. Pathological hydrogen peroxide triggers the fibrillization of wild-type SOD1 via sulfenic acid modification of Cys-111. Cell Death Dis. 2018;9:67. doi: 10.1038/s41419-017-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anzai I, Tokuda E, Handa S, Misawa H, Akiyama S, Furukawa Y. Oxidative misfolding of Cu/Zn-superoxide dismutase triggered by non-canonical intramolecular disulfide formation. Free Radic Biol Med. 2020;147:187–99. [DOI] [PubMed]
- 105.Guareschi S, Cova E, Cereda C, Ceroni M, Donetti E, Bosco DA, et al. An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1. Proc Natl Acad Sci U S A. 2012;109:5074–5079. doi: 10.1073/pnas.1115402109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Taylor DM, Gibbs BF, Kabashi E, Minotti S, Durham HD, Agar JN. Tryptophan 32 potentiates aggregation and cytotoxicity of a copper/zinc superoxide dismutase mutant associated with familial amyotrophic lateral sclerosis. J Biol Chem. 2007;282:16329–16335. doi: 10.1074/jbc.M610119200. [DOI] [PubMed] [Google Scholar]
- 107.Coelho FR, Iqbal A, Linares E, Silva DF, Lima FS, Cuccovia IM, et al. Oxidation of the tryptophan 32 residue of human superoxide dismutase 1 caused by its bicarbonate-dependent peroxidase activity triggers the non-amyloid aggregation of the enzyme. J Biol Chem. 2014;289:30690–30701. doi: 10.1074/jbc.M114.586370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mulligan VK, Kerman A, Laister RC, Sharda PR, Arslan PE, Chakrabartty A. Early steps in oxidation-induced SOD1 misfolding: implications for non-amyloid protein aggregation in familial ALS. J Mol Biol. 2012;421:631–652. doi: 10.1016/j.jmb.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 109.Rakhit R, Cunningham P, Furtos-Matei A, Dahan S, Qi XF, Crow JP, et al. Oxidation-induced misfolding and aggregation of superoxide dismutase and its implications for amyotrophic lateral sclerosis. J Biol Chem. 2002;277:47551–47556. doi: 10.1074/jbc.M207356200. [DOI] [PubMed] [Google Scholar]
- 110.Zhang H, Andrekopoulos C, Joseph J, Chandran K, Karoui H, Crow JP, et al. Bicarbonate-dependent peroxidase activity of human Cu,Zn-superoxide dismutase induces covalent aggregation of protein: intermediacy of tryptophan-derived oxidation products. J Biol Chem. 2003;278:24078–24089. doi: 10.1074/jbc.M302051200. [DOI] [PubMed] [Google Scholar]
- 111.Takeuchi S, Fujiwara N, Ido A, Oono M, Takeuchi Y, Tateno M, et al. Induction of protective immunity by vaccination with wild-type apo superoxide dismutase 1 in mutant SOD1 transgenic mice. J Neuropathol Exp Neurol. 2010;69:1044–1056. doi: 10.1097/NEN.0b013e3181f4a90a. [DOI] [PubMed] [Google Scholar]
- 112.Urushitani M, Ezzi SA, Julien JP. Therapeutic effects of immunization with mutant superoxide dismutase in mice models of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2007;104:2495–2500. doi: 10.1073/pnas.0606201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu HN, Tjostheim S, Dasilva K, Taylor D, Zhao B, Rakhit R, et al. Targeting of monomer/misfolded SOD1 as a therapeutic strategy for amyotrophic lateral sclerosis. J Neurosci. 2012;32:8791–8799. doi: 10.1523/JNEUROSCI.5053-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao B, Marciniuk K, Gibbs E, Yousefi M, Napper S, Cashman NR. Therapeutic vaccines for amyotrophic lateral sclerosis directed against disease specific epitopes of superoxide dismutase 1. Vaccine. 2019;37:4920–4927. doi: 10.1016/j.vaccine.2019.07.044. [DOI] [PubMed] [Google Scholar]
- 115.Dong QX, Zhu J, Liu SY, Yu XL, Liu RT. An oligomer-specific antibody improved motor function and attenuated neuropathology in the SOD1-G93A transgenic mouse model of ALS. Int Immunopharmacol. 2018;65:413–421. doi: 10.1016/j.intimp.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 116.Ghadge GD, Kay BK, Drigotas C, Roos RP. Single chain variable fragment antibodies directed against SOD1 ameliorate disease in mutant SOD1 transgenic mice. Neurobiol Dis. 2019;121:131–137. doi: 10.1016/j.nbd.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 117.Gros-Louis F, Soucy G, Lariviere R, Julien JP. Intracerebroventricular infusion of monoclonal antibody or its derived fab fragment against misfolded forms of SOD1 mutant delays mortality in a mouse model of ALS. J Neurochem. 2010;113:1188–1199. doi: 10.1111/j.1471-4159.2010.06683.x. [DOI] [PubMed] [Google Scholar]
- 118.Broering TJ, Wang H, Boatright NK, Wang Y, Baptista K, Shayan G, et al. Identification of human monoclonal antibodies specific for human SOD1 recognizing distinct epitopes and forms of SOD1. PLoS One. 2013;8:e61210. doi: 10.1371/journal.pone.0061210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van Blitterswijk M, Gulati S, Smoot E, Jaffa M, Maher N, Hyman BT, et al. Anti-superoxide dismutase antibodies are associated with survival in patients with sporadic amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis. 2011;12:430–438. doi: 10.3109/17482968.2011.585163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.